Summary
Lung cancer remains the most common cause of cancer-related death in the United States. This study evaluated the costs of alternative diagnostic evaluations for patients with suspected non–small cell lung cancer (NSCLC). Researchers used a cost-minimization model to compare various diagnostic approaches in the evaluation of patients with NSCLC. It was less expensive to use an initial endoscopic ultrasound (EUS) with fine needle aspiration (FNA) to detect a mediastinal lymph node metastasis ($18,603 per patient), compared with combined EUS FNA and endobronchial ultrasound (EBUS) with FNA ($18,753). The results were sensitive to the prevalence of malignant mediastinal lymph nodes; EUS FNA remained least costly, if the probability of nodal metastases was <32.9%, as would occur in a patient without abnormal lymph nodes on computed tomography (CT). While EUS FNA combined with EBUS FNA was the most economical approach, if the rate of nodal metastases was higher, as would be the case in patients with abnormal lymph nodes on CT. Both of these strategies were less costly than bronchoscopy or mediastinoscopy. The pretest probability of nodal metastases can determine the most cost-effective testing strategy for evaluation of a patient with NSCLC. Pre-procedure CT may be helpful in assessing probability of mediastinal nodal metastases.
Keywords: Ultrasonography, Endobronchial ultrasound, Non-Small Cell Lung Cancer, Medical Economics
Introduction
Lung cancer remains the most common cause of cancer death in the United States. The optimal treatment for patients with non–small cell lung cancer (NSCLC) is surgical resection. Unfortunately, metastatic involvement of mediastinal lymph nodes (Stage III disease) precludes surgery in most cases; N2 disease is defined as involvement of the ipsilateral mediastinal lymph nodes, while N3 disease involves contralateral nodes. The 5-year survival rate for patients with N2 disease detectable on preoperative computed tomography (CT) is universally poor after surgical resection, ranging from 3% to 8%.[1-7] Consequently, it is crucially important to detect stage III disease, so that these patients may avoid unnecessary surgery.
Although thoracic CT is the most commonly used noninvasive staging modality of the mediastinum, it cannot always reliably differentiate between benign and malignant mediastinal nodes, as enlarged nodes may also be inflammatory, whereas normal-sized lymph nodes may contain malignancy.[8-20] Procedures that facilitate sampling of mediastinal nodes, such as endoscopic ultrasonography (EUS)–guided fine-needle aspiration (FNA), endobronchial ultrasound (EBUS) FNA, mediastinoscopy and transbronchial needle aspiration (TBNA), have become established means for tissue confirmation. EUS FNA of posterior mediastinal lymph nodes is a highly accurate modality for cytodiagnosis.[11, 21-36] Mediastinoscopy, on the other hand, offers visualization as well as tissue diagnosis of accessible lymph node stations, but is an invasive procedure, carries a substantial cost, and has a small but definite morbidity.[12, 14, 19, 35, 37-43] TBNA has been used to evaluate suspicious subcarinal, paratracheal and hilar lymph nodes,[42-53] but its blind approach is a limitation. More recently, EBUS FNA has emerged as an approach to overcome this limitation.[54-56]
This study aimed to compare the costs of alternative diagnostic approaches in modeled patients with non–small cell lung cancer (NSCLC), using a cost-minimization approach.
Methods
We used standard decision analysis software (DATA 3.5, TreeAge Software Inc, Williamstown, Mass) to construct our decision model (Fig. 1). Decision analysis uses data available in the medical literature to produce a model of possible outcomes associated with a particular disease, in order to facilitate the determination of the most economical health care strategy, among different alternatives. The model attaches costs and health outcomes to each health state, and estimates the total costs and outcomes associated with a particular health care strategy.
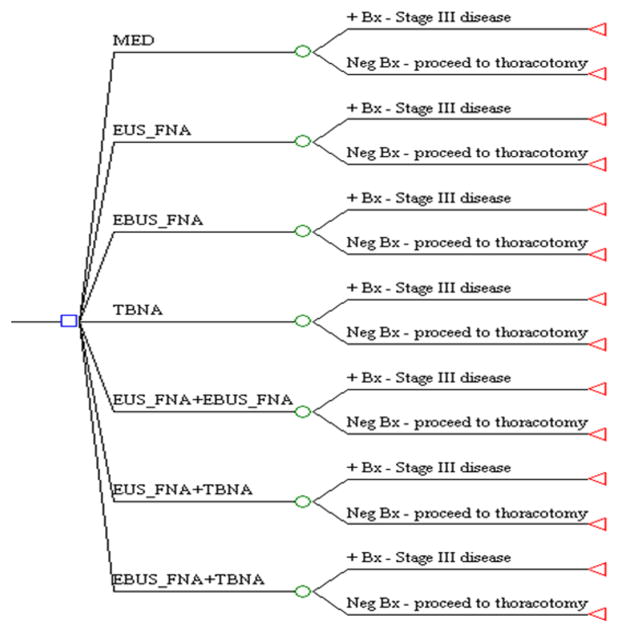
Fig. 1.
Decision tree illustrating seven diagnostic branches. Following initial diagnostic workup (CT, PET scan), the clinician is faced with seven possible sampling approaches in order to discern malignant mediastinal lymphadenopathy.
◻ = initial decision node;  = change node; ◃ = end of evaluation for that branch.
= change node; ◃ = end of evaluation for that branch.
EUS = endoscopic ultrasound; FNA = fine needle aspiration; MED = mediastinoscopy; TBNA = transbronchial needle aspiration; EBUS = endobronchial ultrasound; + Bx = biopsy yielding malignancy.
A cost-minimization analysis, which assumes that competing diagnostic strategies have equivalent outcomes, is the most appropriate form of economic analysis to use in this setting. The benefit of each diagnostic test lies in their respective abilities to detect malignant mediastinal lymphadenopathy, i.e. to detect stage III disease. Detecting stage III disease is useful because it prevents subjecting the patient to a more expensive procedure (thoracotomy) to achieve the same endpoint. Recognizing that:
There is a well-defined outcome in each arm of our decision model (detection of stage III disease),
The long-term outcomes (i.e., measure of effectiveness) are equivalent in each model arm, i.e., the survival of all patients with stage III disease is similar regardless of how the disease extent was diagnosed, and
The downstream costs of medical care in patients with stage III disease are similar in all arms, once the extent of disease has been established,
This ensures that a cost minimization approach is most appropriate. We assumed that each of the FNA techniques has perfect specificity; i.e., no false-positive FNA results. Therefore, no patient would mistakenly forgo potentially life-saving treatment, and each strategy would be equally effective in clinical patient outcomes.
Patient model
The model assumed a patient diagnosed as having either (1) verified NSCLC or (2) suspected NSCLC, based on a primary pulmonary mass with or without enlarged mediastinal lymph nodes detected by CT. The 7 main branches of the tree represent the management options: (1) mediastinoscopy with biopsy of any visualized lymph nodes; (2) EUS FNA of any visualized lymph nodes; (3) EBUS FNA of visualized lymph nodes; (4) TBNA biopsy of any lymph nodes seen on CT; (5) combined EUS FNA and EBUS FNA; (6) combined EUS FNA and TBNA; (7) combined EBUS FNA and TBNA. Pathologic evidence of benign nodal tissue on FNA prompted thoracotomy, which provided a view to surgical resection. The false-negative rates of each FNA procedure determined the likelihood of malignant disease being found at surgery. A positive biopsy at sampling confirmed Stage III disease and precluded thoracotomy.
Assumptions
The model is based on a series of assumptions:
Patients referred for mediastinoscopy, EUS FNA, EBUS FNA and TBNA are clinically similar.
All patients underwent initial luminal bronchoscopy; only patients in the TBNA study arms underwent bronchoscopic FNA.
All patients have undergone CT and PET scanning prior to invasive staging in order to guide further management.
The aim of each procedure is to detect nodal metastases. However, there is no reason to preferentially favor performance of one procedure over another.
Detection of mediastinal nodal metastases signifies stage III disease and precludes thoracotomy.
For calculation of pathology interpretation costs, one cytology sample was acquired in patients undergoing TBNA; two cytology samples were acquired in patients undergoing EUS FNA, EBUS FNA or mediastinoscopy while three separate cytology samples were acquired in patients undergoing combination procedures.
The following procedure-related complication rates requiring hospitalization were assumed: EUS FNA and EBUS FNA and TBNA, all 0.5%,[23-36, 43-59], mediastinoscopy, 2%[35, 37-42] and thoracic surgery, 8%.[60]
EUS FNA/EBUS FNA/TBNA combination procedure sensitivities vary linearly with changes in sensitivity of the individual component procedures.
Because 50-60% of patients undergoing mediastinoscopy with an indication of NSCLC in our institution do so as hospital inpatients, 50% inpatient plus 50% outpatient reimbursement rates were used to represent the direct costs of mediastinoscopy.
The total cost of a procedure-related complication was calculated using the diagnosis related group (DRG) for hospitalization of a patient with NSCLC.
The positive predictive value of a cytologic finding of malignancy is 100%.
From a Bayesian perspective, the initial FNA test result and the subsequent histologic examination of tissue from mediastinoscopy with biopsy are independent tests when used sequentially for diagnostic purposes in the sense that the two tests are independent given disease state.
Baseline costs
The base-case analysis took the payer's perspective expressed in US dollars, based on 2007 Medicare reimbursement rates. Direct medical costs were estimated from Medicare ambulatory patient classification (APC) payments (combined professional plus facility fee) for hospital-based outpatient procedures. Inpatient hospital facility fees were calculated as the amount Medicare pays, based on assignment to a DRG, for a patient with a diagnosis of NSCLC. In the case of hospitalization required for a procedure-related complication, the facility fee component was calculated using the DRG for hospitalization for a post-procedure complication. This amount remains constant regardless of the nature of the complication, e.g. hemorrhage, infection, perforation. The professional fee component was calculated using the CPT codes for the initial consultation, daily physician visit, and discharge consultation. The total cost for each procedure is obtained from the following formula: [(Cost of procedure without complications) (1 – Complication rate)] + [(Cost of procedure with complications) (Complication rate)]. Taking into account the procedure complications in this way provides a precise estimation of costs involved. Costs for outpatient visits and hospitalizations are illustrated in Table I.
Table I.
Medicare-Based Reimbursement Rates for Outpatient (Combined Professional and Facility Fees) and Inpatient (Diagnosis Related Group [DRG]) Hospital Procedures in US dollars*
| Procedure | CPT Code | Prof + Fac Fee ($) | DRG |
|---|---|---|---|
| Bronchoscopy w/o TBNA | 31622 | 560 | |
| EUS FNA | 43242 | 480 | |
| EBUS FNA | 31620 | 1,711 | |
| TBNA | 31629 | 1,430 | |
| MED (outpatient)** | 39400 | 1,842 | |
| MED (outpatient) – professional fee (anesthesia)** | 1,196 | ||
| MED (inpatient)** | 6,624 | ||
| TRC | 16,913 | ||
| Cytology | 88173 | 27 | |
| Hospitalization for FNA complication*** | 24,456 | ||
| Hospital admission*** | 99222 | 290 | |
| Hospital care × 1 day*** | 99231 | 105 | |
| Hospital discharge*** | 99238 | 170 |
based on 2007 Medicare Fee Schedule
direct cost of mediastinoscopy was based on 50% inpatient plus 50% outpatient reimbursement rates assuming that half of patients undergoing mediastinoscopy do so as outpatients and half as inpatients
management of FNA complication was assumed to require hospital admission (day 1), observation for 1 day (day 2) and discharge (day 3), i.e. a 3-day hospitalization. When a procedure related complication occurs, the original facility fee is lost, i.e. only the original professional fee remains which is added to the cost of the hospitalization
EUS = endoscopic ultrasound; FNA = fine needle aspiration; MED = mediastinoscopy; TBNA = transbronchial needle aspiration; EBUS = endobronchial ultrasound; TRC = thoracotomy
Direct costs were used in preference to charges or total costs because direct costs reflect true resource utilization better and tend to be more generalizable. Indirect health and institutional costs, such as the cost to society for lost work, quality of life, and institutional administration or maintenance of buildings or costs involved in the original diagnosis were not included. Discounting was not performed as the diagnostic evaluation only lasts several days.
Parameter values
The performance characteristics of EUS FNA, EBUS FNA, TBNA and all combinations of these tests for the base case analysis were obtained from the only prospective comparison of these tests published to date.[57] To take into account the uncertainty in parameter values, these were varied through the range of their 95% confidence intervals from this study to assess their impact on the final result. (Table II) Performance characteristics of mediastinoscopy were obtained from studies of patients with known or suspected NSCLC without distant metastases published in the peer-reviewed medical literature.
Table II.
Baseline values for performance characteristics of diagnostic modalities
| Variable Sensitivity | Baseline probability (range, %) |
|---|---|
| EUS FNA | 69% (53-82%)[57] |
| EBUS FNA | 69% (53-82%)[57] |
| TBNA | 36% (22-52%)[57] |
| EUS FNA+EBUS FNA | 93% (81-99%)[57] |
| EUS FNA+TBNA | 79% (63-90%)[57] |
| EBUS FNA+TBNA | 76% (61-88%)[57] |
| MED | 95% (70-95%)[12,14,19,35,37-42] |
| Prevalence of MMLN | 30% (0-50%)[57] |
EUS = endoscopic ultrasound; FNA = fine needle aspiration; EBUS = endobronchial ultrasound; TBNA = transbronchial needle aspiration; MMLN = malignant mediastinal lymph nodes; MED = mediastinoscopy
Sensitivity analysis
By performing a sensitivity analysis, we determined whether changing the probability of an event occurrence altered the favored decision strategy. One-way sensitivity analysis of the variables in Table II was performed to determine the optimal management strategy. Two-way sensitivity analysis was also performed by simultaneously varying the probability of 2 variables where appropriate. When changing a variable led to a different strategy being least costly, the variable was deemed sensitive to variation. We varied the prevalence of nodal malignancy through a broad range of possible values (5-50%) to assess the most economical outcome in a wide variety of clinical settings.
Results
Base-case analysis
For the base-case, initial EUS FNA biopsy was the most economical strategy ($18,603) compared with the other options: EBUS FNA ($19,828), TBNA ($21,136), mediastinoscopy ($20,157), combined EUS FNA/EBUS FNA ($18,753), combined EUS FNA/TBNA ($18,838) and combined EBUS FNA/TBNA ($20,260).
Sensitivity analysis
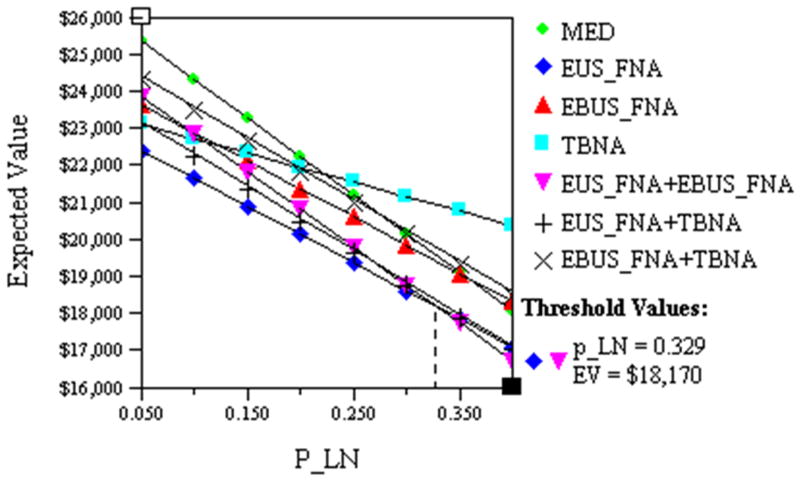
One-way sensitivity analyses showed initial EUS FNA remained the least costly option provided the probability of lymph node metastases was <32.9%, cost $18,170 (Fig. 2); above this probability combined EUS FNA/EBUS FNA was the most economical approach.
Fig. 2.
This figure illustrates the impact of varying the prevalence of malignant mediastinal lymph nodes (P_LN) on the cost of patient management. The costs of EUS FNA (◆) and EUS FNA+EBUS FNA (▼) are equivalent ($18,170) when P_LN is 32.9%.
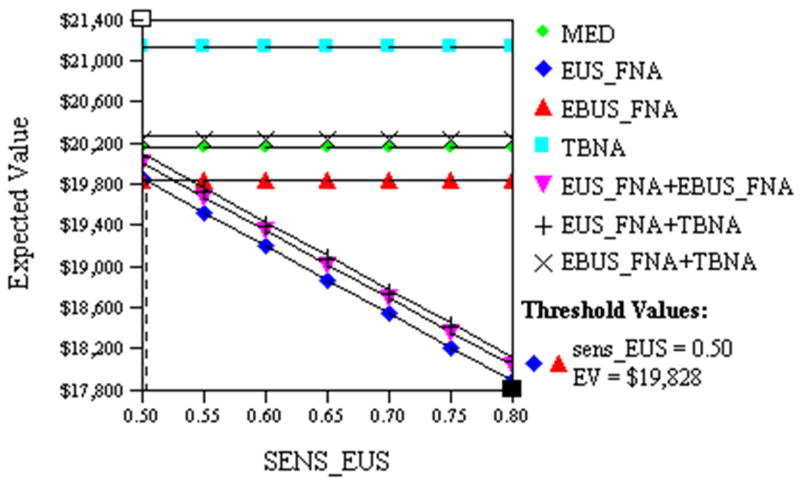
EUS FNA sensitivity and EBUS FNA sensitivity were also varied. EUS FNA remained the least costly if its sensitivity exceeded 50%, cost $19,828 (Fig. 3), this probability was lower than our lowest plausible sensitivity. EBUS FNA was least costly if its sensitivity exceeded 71.3%, cost $18,603.
Fig. 3.
This figure illustrates the impact of varying the sensitivity of EUS FNA (SENS_EUS) on the cost of patient management. The costs of EUS FNA (◆) and EBUS FNA (▲) are equivalent ($19,828) when SENS_EUS is 50%.
Two-way sensitivity analyses were also performed. Throughout a plausible range for EUS FNA sensitivity (55-80%), EUS FNA remained the least costly strategy as long as the probability of lymph node metastases was <32%; above this, the combination EUS FNA/EBUS FNA was the preferred option. Throughout a plausible range for EBUS FNA sensitivity (55-80%), EUS FNA again remained the least costly strategy as long as the probability of lymph node metastases <40%; above this, the combination EUS FNA/EBUS FNA was the preferred option as long as its sensitivity was >65%. As the sensitivity of this combined procedure increased, the threshold value of nodal involvement that defined EUS FNA/EBUS FNA superiority declined accordingly. Again, because EUS FNA and EBUS FNA performance alone rarely vary in isolation, the global FNA sensitivity along with malignant node prevalence were varied. Throughout all FNA sensitivities, EUS FNA was the preferred option with malignant nodal prevalence <32%; above this, combination EUS FNA/EBUS FNA became the approach of choice.
Discussion
This economic analysis simulates the clinical scenario of a patient with known or suspected NSCLC. The validity of any model and its conclusions can only be verified by prospective trials. For this reason, we relied on the majority of our parameter values from the only prospective trial to date comparing the performance of EUS FNA, EBUS FNA, TBNA and combinations of these tests.[57] The findings illustrate that the least costly approach of investigating these patients is predicated on the pre-test probability of malignant mediastinal lymph nodes; below a malignant nodal probability of 32.9%, initial EUS FNA is the preferred option. However, above this probability combined approach with EUS FNA and EBUS FNA is optimal.
How relevant is our decision model in facilitating patient management? The medical literature demonstrates that 22% to 30% of patients with NSCLC have enlarged mediastinal lymph nodes detectable on CT at the time of presentation.[53-58] In the presence of enlarged nodes on CT, the pre-test probability of malignant nodal involvement raises to 60-70%,[58] while in the absence of visualized nodes on CT, the pre-test probability of malignant nodal involvement declines to 10-30%.[59] Therefore, the findings of this economic analysis illustrate that the clinician can tailor their approach depending on the CT findings. Enlarged mediastinal nodes on CT would favor initial combined EUS FNA and EBUS FNA while absence of nodes on CT favors initial EUS FNA alone.
Several limitations of this study are noteworthy. As with any decision analysis model, it is of most use when considered as a guide in patient management rather than a substitute for sound clinical judgment. For example, in the setting of a very high pre-test probability of mediastinal nodal malignancy, it may be most appropriate to repeat a minimally invasive technique (e.g., EUS FNA) after one negative result prior to proceeding to thoracotomy. Second, the robustness of any model depends on the assumptions used. In this model, the specificity of all sampling procedures is considered perfect. In clinical practice, the unlikely event of obtaining a false positive biopsy result would lead to erroneous over-staging of a patient, thereby depriving a potentially resectable patient of a chance at curative surgery. This is a theoretical limitation of all the sampling modalities (EUS FNA, TBNA, EBUS FNA and mediastinoscopy). However, for practical purposes, the possibility of imperfect specificity (i.e., the interpretation of a benign cytology specimen as malignant) was considered to be exceedingly low and did not justify confirmatory thoracotomy in all patients with a positive FNA result. Related to the analysis, this means that the essential impact to the model of any deviation from perfect specificity is negligible. That is, in terms of the model the expected cost calculated as the product of the small, but nearly zero, probability of a false positive times the expected consequent cost, a large value if the false positive occurred, would be negligible.
Our cost analysis was based on estimate of accuracy from our own clinical trial, one of the only which has directly compared EUS, EBUS, and bronchoscopy. Our accuracy estimates are somewhat lower than other published meta-analyses.[57, 61, 62] In order to ensure that our results are robust, we performed the cost analysis across a wide range of accuracy estimates for EUS and EBUS and found that the conclusions held within the range of all published values.
Although both EUS FNA and EBUS FNA were modeled as separate choices, the favorable performance of EUS FNA and EBUS FNA in combination, illustrates the complementary nature of these 2 modalities. The incremental sensitivity of both in combination over each individually reflects the differing abilities of both tests to detect lymph nodes in differing stations of the mediastinum. EUS functions best at accurately detecting mediastinal lymphadenopathy in the subcarinal (station 7), aortopulmonary window (station 5), left paratracheal (station 4L), and paraesophageal (station 8) regions[24] while its ability to adequately visualize the upper anterior lymph node stations (1 through 3 and 4R) is compromised due to interfering tracheal air. Conversely, EBUS has excellent ability in detecting the upper anterior nodal stations.[54-57] This complementary nature of EUS and EBUS in providing views of all portions of the mediastinum between them, translates into lower expense when evaluating patients with a higher pre-test probability of nodal involvement. In the future, it may be possible to identify subgroups of patients that only require a single procedure. This would even further reduce to cost of endoscopic staging and improve cost-effectiveness.
Our study is limited to an analysis of invasive staging procedures. Other groups have previously analysis the cost effectiveness of non-invasive staging including CT and PET and generally found it to be cost effective[63].
Finally, there is increasing evidence that genomic characterization of lung cancer, particularly, K-ras and EGFR status, may serve as a guide to therapy. EUS-FNA is well positioned to obtain tissue suitable for DNA and RNA extraction suitable and thus has the capacity to provide this valuable information.[64]
The utility of nodal sampling techniques in patients with known or suspected NSCLC includes their impact on clinical decision making and also their conversion of a major inpatient surgery to a minimally invasive outpatient procedure in an appropriate subset of patients. Because the preferred choice of initial test (EUS FNA or combined EUS FNA and EBUS FNA) is highly sensitive to pre-test probability of malignant mediastinal lymph nodes, detection of enlarged nodes on thoracic CT is an important element in guiding further test selection.
Acknowledgments
We would like to thank Diane Morell for editorial review of this manuscript.
Role of the Funding Source: None.
Footnotes
Conflict of Interest Statement: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andre F, Grunenwald D, Pignon JP, Dujon A, Pujol JL, Brichon PY, Brouchet L, Quoix E, Westeel V, Le Chevalier T. Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol. 2000;18:2981–2989. doi: 10.1200/JCO.2000.18.16.2981. [DOI] [PubMed] [Google Scholar]
- 2.Jassem J, Skokowski J, Dziadziuszko R, Jassem E, Szymanowska A, Rzyman W, Roszkiewicz A. Results of surgical treatment of non-small cell lung cancer: validation of the new postoperative pathologic TNM classification. J Thorac Cardiovasc Surg. 2000;119:1141–1146. doi: 10.1067/mtc.2000.105825. [DOI] [PubMed] [Google Scholar]
- 3.Okada M, Tsubota N, Yoshimura M, Miyamoto Y, Matsuoka H. How should interlobar pleural invasion be classified? Prognosis of resected T3 non-small cell lung cancer. Ann Thorac Surg. 1999;68:2049–2052. doi: 10.1016/s0003-4975(99)01172-8. [DOI] [PubMed] [Google Scholar]
- 4.Okada M, Tsubota N, Yoshimura M, Miyamoto Y, Matsuoka H. Prognosis of completely resected pN2 non-small cell lung carcinomas: What is the significant node that affects survival? J Thorac Cardiovasc Surg. 1999;118:270–275. doi: 10.1016/S0022-5223(99)70217-5. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki K, Nagai K, Yoshida J, Nishimura M, Takahashi K, Nishiwaki Y. The prognosis of surgically resected N2 non-small cell lung cancer: the importance of clinical N status. J Thorac Cardiovasc Surg. 1999;118:145–153. doi: 10.1016/S0022-5223(99)70153-4. [DOI] [PubMed] [Google Scholar]
- 6.Shah SS, Goldstraw P. Combined pulmonary and thoracic wall resection for stage III lung cancer. Thorax. 1995;50:782–784. doi: 10.1136/thx.50.7.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vansteenkiste JF, De Leyn PR, Deneffe GJ, Stalpaert G, Nackaerts KL, Lerut TE, Demedts MG. Survival and prognostic factors in resected N2 non-small cell lung cancer: a study of 140 cases. Leuven Lung Cancer Group. Ann Thorac Surg. 1997;63:1441–1450. doi: 10.1016/s0003-4975(97)00314-7. [DOI] [PubMed] [Google Scholar]
- 8.Colice GL. Chest CT for known or suspected lung cancer. Chest. 1994;106:1538–1550. doi: 10.1378/chest.106.5.1538. [DOI] [PubMed] [Google Scholar]
- 9.Boiselle PM, Patz EF, Jr, Vining DJ, Weissleder R, Shepard JA, McLoud TC. Imaging of mediastinal lymph nodes: CT, MR, and FDG PET. Radiographics. 1998;18:1061–1069. doi: 10.1148/radiographics.18.5.9747607. [DOI] [PubMed] [Google Scholar]
- 10.Webb WR, Gatsonis C, Zerhouni EA, Heelan RT, Glazer GM, Francis IR, McNeil BJ. CT and MR imaging in staging non-small cell bronchogenic carcinoma: report of the Radiologic Diagnostic Oncology Group. Radiology. 1991;178:705–713. doi: 10.1148/radiology.178.3.1847239. [DOI] [PubMed] [Google Scholar]
- 11.Gress FG, Savides TJ, Sandler A, Kesler K, Conces D, Cummings O, Mathur P, Ikenberry S, Bilderback S, Hawes R. Endoscopic ultrasonography, fine-needle aspiration biopsy guided by endoscopic ultrasonography, and computed tomography in the preoperative staging of non-small-cell lung cancer: a comparison study. Ann Intern Med. 1997;127:604–612. doi: 10.7326/0003-4819-127-8_part_1-199710150-00004. [DOI] [PubMed] [Google Scholar]
- 12.Goldstraw P, Kurzer M, Edwards D. Preoperative staging of lung cancer: accuracy of computed tomography versus mediastinoscopy. Thorax. 1983;38:10–15. doi: 10.1136/thx.38.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKenna RJ, Jr, Libshitz HI, Mountain CE, McMurtrey MJ. Roentgenographic evaluation of mediastinal nodes for preoperative assessment in lung cancer. Chest. 1985;88:206–210. doi: 10.1378/chest.88.2.206. [DOI] [PubMed] [Google Scholar]
- 14.Patterson GA, Ginsberg RJ, Poon PY, Cooper JD, Goldberg M, Jones D, Pearson FG, Todd TR, Waters P, Bull S. A prospective evaluation of magnetic resonance imaging, computed tomography, and mediastinoscopy in the preoperative assessment of mediastinal node status in bronchogenic carcinoma. J Thorac Cardiovasc Surg. 1987;94:679–684. [PubMed] [Google Scholar]
- 15.Aronchick JM. CT of mediastinal lymph nodes in patients with non-small cell lung carcinoma. Radiol Clin North Am. 1990;28:573–581. [PubMed] [Google Scholar]
- 16.Glazer GM, Orringer MB, Gross BH, Quint LE. The mediastinum in non-small cell lung cancer: CT-surgical correlation. AJR Am J Roentgenol. 1984;142:1101–1105. doi: 10.2214/ajr.142.6.1101. [DOI] [PubMed] [Google Scholar]
- 17.McLoud TC, Bourgouin PM, Greenberg RW, Kosiuk JP, Templeton PA, Shepard JA, Moore EH, Wain JC, Mathisen DJ, Grillo HC. Bronchogenic carcinoma: analysis of staging in the mediastinum with CT by correlative lymph node mapping and sampling. Radiology. 1992;182:319–323. doi: 10.1148/radiology.182.2.1732943. [DOI] [PubMed] [Google Scholar]
- 18.Lewis JW, Jr, Pearlberg JL, Beute GH, Alpern M, Kvale PA, Gross BH, Magilligan DJ., Jr Can computed tomography of the chest stage lung cancer? Yes and no. Ann Thorac Surg. 1990;49:591–595. doi: 10.1016/0003-4975(90)90306-q. discussion 595-596. [DOI] [PubMed] [Google Scholar]
- 19.Staples CA, Muller NL, Miller RR, Evans KG, Nelems B. Mediastinal nodes in bronchogenic carcinoma: comparison between CT and mediastinoscopy. Radiology. 1988;167:367–372. doi: 10.1148/radiology.167.2.3357944. [DOI] [PubMed] [Google Scholar]
- 20.Steinert HC, Hauser M, Allemann F, Engel H, Berthold T, von Schulthess GK, Weder W. Non-small cell lung cancer: nodal staging with FDG PET versus CT with correlative lymph node mapping and sampling. Radiology. 1997;202:441–446. doi: 10.1148/radiology.202.2.9015071. [DOI] [PubMed] [Google Scholar]
- 21.Kondo D, Imaizumi M, Abe T, Naruke T, Suemasu K. Endoscopic ultrasound examination for mediastinal lymph node metastases of lung cancer. Chest. 1990;98:586–593. doi: 10.1378/chest.98.3.586. [DOI] [PubMed] [Google Scholar]
- 22.Hawes RH, Gress F, Kesler KA, Cummings OW, Conces DJ., Jr Endoscopic ultrasound versus computed tomography in the evaluation of the mediastinum in patients with non-small-cell lung cancer. Endoscopy. 1994;26:784–787. doi: 10.1055/s-2007-1009106. [DOI] [PubMed] [Google Scholar]
- 23.Giovannini M, Seitz JF, Monges G, Perrier H, Rabbia I. Fine-needle aspiration cytology guided by endoscopic ultrasonography: results in 141 patients. Endoscopy. 1995;27:171–177. doi: 10.1055/s-2007-1005657. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen BH, Vilmann P, Folke K, Jacobsen GK, Krasnik M, Milman N, Hancke S. Endoscopic ultrasonography and real-time guided fine-needle aspiration biopsy of solid lesions of the mediastinum suspected of malignancy. Chest. 1996;110:539–544. doi: 10.1378/chest.110.2.539. [DOI] [PubMed] [Google Scholar]
- 25.Silvestri GA, Hoffman BJ, Bhutani MS, Hawes RH, Coppage L, Sanders-Cliette A, Reed CE. Endoscopic ultrasound with fine-needle aspiration in the diagnosis and staging of lung cancer. Ann Thorac Surg. 1996;61:1441–1445. doi: 10.1016/0003-4975(95)00052-6. discussion 1445-1446. [DOI] [PubMed] [Google Scholar]
- 26.Vilmann P. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of lymph nodes. Gastrointest Endosc. 1996;43:S24–29. doi: 10.1016/s0016-5107(96)81510-0. [DOI] [PubMed] [Google Scholar]
- 27.Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–1095. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 28.Fritscher-Ravens A, Petrasch S, Reinacher-Schick A, Graeven U, Konig M, Schmiegel W. Diagnostic value of endoscopic ultrasonography-guided fine-needle aspiration cytology of mediastinal masses in patients with intrapulmonary lesions and nondiagnostic bronchoscopy. Respiration. 1999;66:150–155. doi: 10.1159/000029357. [DOI] [PubMed] [Google Scholar]
- 29.Williams DB, Sahai AV, Aabakken L, Penman ID, van Velse A, Webb J, Wilson M, Hoffman BJ, Hawes RH. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut. 1999;44:720–726. doi: 10.1136/gut.44.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiersema MJ, Hassig WM, Hawes RH, Wonn MJ. Mediastinal lymph node detection with endosonography. Gastrointest Endosc. 1993;39:788–793. doi: 10.1016/s0016-5107(93)70266-7. [DOI] [PubMed] [Google Scholar]
- 31.Ikenberry S, Gress F, Savides T, Hawes R. Fine-needle aspiration of posterior mediastinal lesions guided by radial scanning endosonography. Gastrointest Endosc. 1996;43:605–610. doi: 10.1016/s0016-5107(96)70200-6. [DOI] [PubMed] [Google Scholar]
- 32.Wiersema MJ, Hawes RH, Tao LC, Wiersema LM, Kopecky KK, Rex DK, Kumar S, Lehman GA. Endoscopic ultrasonography as an adjunct to fine needle aspiration cytology of the upper and lower gastrointestinal tract. Gastrointest Endosc. 1992;38:35–39. doi: 10.1016/s0016-5107(92)70327-7. [DOI] [PubMed] [Google Scholar]
- 33.Rex DK, Tarver RD, Wiersema M, O'Conner KW, Lappas JC, Tabatowski K. Endoscopic transesophageal fine needle aspiration of mediastinal masses. Gastrointest Endosc. 1991;37:465–468. doi: 10.1016/s0016-5107(91)70781-5. [DOI] [PubMed] [Google Scholar]
- 34.Wiersema MJ, Kochman ML, Chak A, Cramer HM, Kesler KA. Real-time endoscopic ultrasound-guided fine-needle aspiration of a mediastinal lymph node. Gastrointest Endosc. 1993;39:429–431. doi: 10.1016/s0016-5107(93)70122-4. [DOI] [PubMed] [Google Scholar]
- 35.Serna DL, Aryan HE, Chang KJ, Brenner M, Tran LM, Chen JC. An early comparison between endoscopic ultrasound-guided fine-needle aspiration and mediastinoscopy for diagnosis of mediastinal malignancy. Am Surg. 1998;64:1014–1018. [PubMed] [Google Scholar]
- 36.Fritscher-Ravens A, Sriram PV, Bobrowski C, Pforte A, Topalidis T, Krause C, Jaeckle S, Thonke F, Soehendra N. Mediastinal lymphadenopathy in patients with or without previous malignancy: EUS-FNA-based differential cytodiagnosis in 153 patients. Am J Gastroenterol. 2000;95:2278–2284. doi: 10.1111/j.1572-0241.2000.02243.x. [DOI] [PubMed] [Google Scholar]
- 37.Ginsberg RJ. Evaluation of the mediastinum by invasive techniques. Surg Clin North Am. 1987;67:1025–1035. doi: 10.1016/s0039-6109(16)44340-9. [DOI] [PubMed] [Google Scholar]
- 38.Barendregt WB, Deleu HW, Joosten HJ, Berg W, Janssen JP. The value of parasternal mediastinoscopy in staging bronchial carcinoma. Eur J Cardiothorac Surg. 1995;9:655–658. doi: 10.1016/s1010-7940(05)80113-7. [DOI] [PubMed] [Google Scholar]
- 39.Coughlin M, Deslauriers J, Beaulieu M, Fournier B, Piraux M, Rouleau J, Tardif A. Role of mediastinoscopy in pretreatment staging of patients with primary lung cancer. Ann Thorac Surg. 1985;40:556–560. doi: 10.1016/s0003-4975(10)60348-7. [DOI] [PubMed] [Google Scholar]
- 40.McNeill TM, Chamberlain JM. Diagnostic anterior mediastinotomy. Ann Thorac Surg. 1966;2:532–539. doi: 10.1016/s0003-4975(10)66614-3. [DOI] [PubMed] [Google Scholar]
- 41.Gossot D, Toledo L, Fritsch S, Celerier M. Mediastinoscopy vs thoracoscopy for mediastinal biopsy. Results of a prospective nonrandomized study. Chest. 1996;110:1328–1331. doi: 10.1378/chest.110.5.1328. [DOI] [PubMed] [Google Scholar]
- 42.Brynitz S, Struve-Christensen E, Borgeskov S, Bertelsen S. Transcarinal mediastinal needle biopsy compared with mediastinoscopy. J Thorac Cardiovasc Surg. 1985;90:21–24. [PubMed] [Google Scholar]
- 43.Wang KP, Terry P, Marsh B. Bronchoscopic needle aspiration biopsy of paratracheal tumors. Am Rev Respir Dis. 1978;118:17–21. doi: 10.1164/arrd.1978.118.1.17. [DOI] [PubMed] [Google Scholar]
- 44.Harrow EM, Oldenburg FA, Jr, Lingenfelter MS, Smith AM., Jr Transbronchial needle aspiration in clinical practice. A five-year experience. Chest. 1989;96:1268–1272. doi: 10.1378/chest.96.6.1268. [DOI] [PubMed] [Google Scholar]
- 45.Schenk DA, Bower JH, Bryan CL, Currie RB, Spence TH, Duncan CA, Myers DL, Sullivan WT. Transbronchial needle aspiration staging of bronchogenic carcinoma. Am Rev Respir Dis. 1986;134:146–148. doi: 10.1164/arrd.1986.134.1.146. [DOI] [PubMed] [Google Scholar]
- 46.Harrow E, Halber M, Hardy S, Halteman W. Bronchoscopic and roentgenographic correlates of a positive transbronchial needle aspiration in the staging of lung cancer. Chest. 1991;100:1592–1596. doi: 10.1378/chest.100.6.1592. [DOI] [PubMed] [Google Scholar]
- 47.Carlin BW, Harrell JH, 2nd, Fedullo PF. False-positive transcarinal needle aspirate in the evaluation of bronchogenic carcinoma. Am Rev Respir Dis. 1989;140:1800–1802. doi: 10.1164/ajrccm/140.6.1800. [DOI] [PubMed] [Google Scholar]
- 48.Utz JP, Patel AM, Edell ES. The role of transcarinal needle aspiration in the staging of bronchogenic carcinoma. Chest. 1993;104:1012–1016. doi: 10.1378/chest.104.4.1012. [DOI] [PubMed] [Google Scholar]
- 49.Wagner ED, Ramzy I, Greenberg SD, Gonzalez JM. Transbronchial fine-needle aspiration. Reliability and limitations. Am J Clin Pathol. 1989;92:36–41. doi: 10.1093/ajcp/92.1.36. [DOI] [PubMed] [Google Scholar]
- 50.Wang KP, Brower R, Haponik EF, Siegelman S. Flexible transbronchial needle aspiration for staging of bronchogenic carcinoma. Chest. 1983;84:571–576. doi: 10.1378/chest.84.5.571. [DOI] [PubMed] [Google Scholar]
- 51.Shure D, Fedullo PF. Transbronchial needle aspiration of peripheral masses. Am Rev Respir Dis. 1983;128:1090–1092. doi: 10.1164/arrd.1983.128.6.1090. [DOI] [PubMed] [Google Scholar]
- 52.Blainey AD, Curling M, Green M. Transbronchial aspiration of subcarinal lymph nodes. Br J Dis Chest. 1988;82:149–154. doi: 10.1016/0007-0971(88)90035-6. [DOI] [PubMed] [Google Scholar]
- 53.Schenk DA, Bryan CL, Bower JH, Myers DL. Transbronchial needle aspiration in the diagnosis of bronchogenic carcinoma. Chest. 1987;92:83–85. doi: 10.1378/chest.92.1.83. [DOI] [PubMed] [Google Scholar]
- 54.Shannon JJ, Bude RO, Orens JB, Becker FS, Whyte RI, Rubin JM, Quint LE, Martinez FJ. Endobronchial ultrasound-guided needle aspiration of mediastinal adenopathy. Am J Respir Crit Care Med. 1996;153:1424–1430. doi: 10.1164/ajrccm.153.4.8616576. [DOI] [PubMed] [Google Scholar]
- 55.Plat G, Pierard P, Haller A, Hutsebaut J, Faber J, Dusart M, Eisendrath P, Sculier JP, Ninane V. Endobronchial ultrasound and positron emission tomography positive mediastinal lymph nodes. Eur Respir J. 2006;27:276–281. doi: 10.1183/09031936.06.00139204. [DOI] [PubMed] [Google Scholar]
- 56.Herth FJ, Rabe KF, Gasparini S, Annema JT. Transbronchial and transoesophageal (ultrasound-guided) needle aspirations for the analysis of mediastinal lesions. Eur Respir J. 2006;28:1264–1275. doi: 10.1183/09031936.00013806. [DOI] [PubMed] [Google Scholar]
- 57.Wallace MB, Pascual JM, Raimondo M, Woodward TA, McComb BL, Crook JE, Johnson MM, Al-Haddad MA, Gross SA, Pungpapong S, Hardee JN, Odell JA. Minimally invasive endoscopic staging of suspected lung cancer. Jama. 2008;299:540–546. doi: 10.1001/jama.299.5.540. [DOI] [PubMed] [Google Scholar]
- 58.Wallace MB, Silvestri GA, Sahai AV, Hawes RH, Hoffman BJ, Durkalski V, Hennesey WS, Reed CE. Endoscopic ultrasound-guided fine needle aspiration for staging patients with carcinoma of the lung. Ann Thorac Surg. 2001;72:1861–1867. doi: 10.1016/s0003-4975(01)03205-2. [DOI] [PubMed] [Google Scholar]
- 59.Wallace MB, Ravenel J, Block MI, Fraig M, Silvestri G, Wildi S, Schmulewitz N, Varadarajulu S, Roberts S, Hoffman BJ, Hawes RH, Reed CE. Endoscopic ultrasound in lung cancer patients with a normal mediastinum on computed tomography. Ann Thorac Surg. 2004;77:1763–1768. doi: 10.1016/j.athoracsur.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 60.Solaini L, Prusciano F, Bagioni P, di Francesco F, Solaini L, Poddie DB. Video-assisted thoracic surgery (VATS) of the lung: analysis of intraoperative and postoperative complications over 15 years and review of the literature. Surg Endosc. 2008;22:298–310. doi: 10.1007/s00464-007-9586-0. [DOI] [PubMed] [Google Scholar]
- 61.Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines. Chest. (2nd) 2007;132:202S–220S. doi: 10.1378/chest.07-1362. [DOI] [PubMed] [Google Scholar]
- 62.Micames CG, McCrory DC, Pavey DA, Jowell PS, Gress FG. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and metaanalysis. Chest. 2007;131:539–548. doi: 10.1378/chest.06-1437. [DOI] [PubMed] [Google Scholar]
- 63.Alzahouri K, Lejeune C, Woronoff-Lemsi MC, Arveux P, Guillemin F. Cost-effectiveness analysis of strategies introducing FDG-PET into the mediastinal staging of non-small-cell lung cancer from the French healthcare system perspective. Clin Radiol. 2005;60:479–492. doi: 10.1016/j.crad.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 64.Wallace MB, Block MI, Gillanders W, Ravenel J, Hoffman BJ, Reed CE, Fraig M, Cole D, Mitas M. Accurate molecular detection of non-small cell lung cancer metastases in mediastinal lymph nodes sampled by endoscopic ultrasound-guided needle aspiration. Chest. 2005;127:430–437. doi: 10.1378/chest.127.2.430. [DOI] [PubMed] [Google Scholar]