Abstract
Two kinds of Pavlovian conditioned approach behavior are possible: approach of the CS (sign-tracking) and approach of the US (goal-tracking). We hypothesized that administration of AMP would increase sign-tracking and decrease goal-tracking. However, increasing doses of AMP (up to 2.0 mg/kg) decreased measures of sign-tracking while simultaneously increasing measures of goal-tracking. Administration of AMP may shift responding from cues distant from the CS to cues closer to the CS.
Keywords: amphetamine, sign-tracking, goal-tracking, rat, addiction, Pavlovian, acute
Differential Effects of Amphetamine on Sign-Tracking and Goal-Tracking
A Pavlovian conditioned appetitive approach or autoshaping procedure typically involves presentation of a conditioned stimulus (CS) which is followed shortly afterward with presentation of an unconditioned stimulus (US) such as food. In such a procedure, the response of approaching or contacting the CS typically develops even though doing so does not affect US presentation [2], or in some cases, reduces or eliminates exposure to the appetitive US [7]. As Pavlovian conditioned responses have been implicated in the development and maintenance of drug addiction [2, 4, 6, 8, 22] understanding the fundamental mechanisms behind Pavlovian conditioning is relevant to the understanding of addiction and related phenomena.
The nature of the conditioned approach response may change in a situation in which the location of the CS and the location where the US is delivered are separate and distinct. In the case where a CS in one location indicates delivery of reinforcement in another, the subject can make two kinds of conditioned response. The first, sign-tracking, involves approach/contact of the CS, whereas the second, goal-tracking, involves approach/contact of the location where the US is to be delivered [3, 9, 10, 11].
Considerable evidence now exists that suggests psychomotor stimulants such as amphetamine (AMP) and cocaine enhance responding to Pavlovian conditioned stimuli [17,18,24]. For example, acute AMP administration enhances performance of an instrumental response which was reinforced by presentation of a conditioned stimulus [12]. It has also been demonstrated that post-training administration of amphetamine enhanced consolidation of a Pavlovian conditioned approach response [14], and that acute amphetamine exposure enhances approach to a conditioned stimulus (CS+) associated with liquid sucrose reward but not approach of a stimulus not associated with such reward (CS−), and moreover that such enhanced responding to the CS+ was accompanied by increased magnitude of responding from neurons in nucleus accumbens [23]. These previous studies, however, have employed conditioning procedures where CS and US were delivered in the same location, and thus, sign- and goal- tracking behaviors would be indistinguishable from each other. The question of interest is whether administration of a dopaminergic psychostimulant would enhance sign-tracking, goal-tracking, both or neither in a context where the two stimuli were separate from each other. Proponents of incentive sensitization theory suggest that in addiction, the addictive drug itself sensitizes natural reward systems in the brain, leading the sensitized organism to attribute powerful motivational qualities to stimuli predictive of reward [1, 13]. Such stimuli become “motivational magnets” [21, p. 128] which the organism is compelled to approach and contact. In addition it has been noted that rats with a natural propensity to sign-track at higher levels (in a study where US and CS were distal but only sign-tracking behavior was studied) show increased dopamine and DOPAC activity in relevant brain areas such as accumbal core regions [20]. Increasing dopamine activity via acute administration of AMP should have similar effects; thus, we hypothesize that AMP should increase sign-tracking behavior. To the extent that sign- and goal-tracking are incompatible behaviors, we should also see a concommitant decrease in goal-tracking.
In the present study we explored the effects of acute systemic AMP administration on these two different kinds of Pavlovian approach behavior, sign- and goal-tracking. Subjects were trained in a task in which CS and US were delivered in different locations and thus in which separate goal-tracking and sign-tracking behaviors were possible. After subjects reached a stable level of performance, they were run in the task under five different doses of amphetamine and a saline control. Based on previous studies showing acute amphetamine enhances instrumental responding for conditioned reinforcement [12, 16], and approach of the CS [23], we hypothesized that AMP would enhance sign-tracking with increasing doses (i.e. decrease latency of the behavior, increase number of responses), and, to the extent that goal-tracking and sign-tracking are competing behaviors, decrease goal-tracking (i.e. increase latency, decrease number of responses, decrease duration of response).
Subjects were six male Sprague-Dawley rats (Charles River, Wilmington, MA, approximately 400 gm at the start of the study). Subjects were reduced to 90% of their free-feeding weights and maintained at that weight by daily weighing and feeding. Animals were housed individually in a temperature- and ventilation-controlled environment with free access to water under a reversed 12 hr light/dark cycle (lights off at 8:00 AM). Subjects maintained good health throughout the experiment. All animal care and protocols were in accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. Public Health Service and approved by the Animal Care and Use Committee of the University of Pennsylvania. Behavioral sessions were conducted in Med Associates operant chambers, (30 × 40 × 30 cm), with sidewalls made of aluminum and front and back walls made of plexiglass. Each chamber was equipped with a liquid food receptacle built into one wall, with a small well attached to a syringe pump, and infrared photocells to detect head entries. A stimulus light (1.5 cm in diameter) was located at the back of the receptacle, 6 cm above the well at the top of the receptacle. A retractable response lever, requiring approximately 5 g of downward force to operate, was located immediately to the right of the active receptacle. The infrared photocells and lever were located far enough from each other that simultaneous activation of both would be difficult if not impossible for subjects to achieve. The chamber was also equipped with red and white houselights which were illuminated at the beginning of the procedure and which remained on until the end of the session.
Subjects were run in two sessions per day at the beginning of the experiment. At the beginning of the session, subjects were given three magazine training sessions during which 40 reinforcers were delivered on a 1-min FT schedule. The US employed was 0.25 ml of a 10% sucrose solution. US delivery was indicated by the presentation of three clicks from a clicker device (3/sec), accompanied by three simultaneous flashes of the stimulus light. Subjects then began Pavlovian conditioning. Each daily session consisted of 40 CS-US pairings (VT-90s). At the beginning of each trial, the lever located to the right of the food receptacle was extended and remained extended for 4 sec. At the end of the 4 sec period the lever was retracted, and simultaneously, a reinforcer was delivered. The topography of the Pavlovian conditioned response included lever-CS directed approach followed by grasping and gnawing of the lever, recorded as lever-presses when such behavior resulted in the closing of the lever’s microswitch (SIGN-CR). Presses were recorded but the occurrence of a lever-press did not affect reinforcer presentation. The total number of SIGN-CRs during stimulus presentation was recorded by the computer. Total number of head entries into the food receptacle during stimulus presentation (GOAL-CRs) was also recorded. A separate measure, consisting of number of trials during which at least one response occurred, was computed for both SIGN-CRs and GOAL-CRs. After the session subjects were returned to their home cages and fed.
The first two sessions employed a CS-US interval of 4 sec. During the third, a 6 sec interval was employed, and during the fourth session, the interval was lengthened to 8 sec and remained at 8 sec throughout the rest of the experiment. Sessions were run twice a day until the eighth session and thereafter, where only one session was run per day. 15 min before the ninth, tenth, eleventh, and twelfth sessions, subjects were given an injection of saline in order to acclimate them to the injection procedure. After injection, subjects were placed in the chamber for 15 minutes before the procedure began; however, the procedure was otherwise identical to before. Thereafter, subjects were given twelve additional sessions of testing. Every other day, the subjects were given an injection of AMP (0.25, 0.5, 1.0, 1.5 or 2.0 mg/kg) or saline (injection volume of 1 ml/kg for all injections) and placed in the chamber for 15 minutes before the session began. Drug sessions were otherwise identical to initial training. Each day of AMP or saline administration was followed by a day of training but no injections, so that all injections were separated by at least 48 hours. The order of the treatments was different for each subject, administered in a Latin-square design.
Because it was possible that AMP might increase locomotion generally and total number of head entries specifically (which then might bias GOAL-CR and UR measures), a head-entry per second measure was computed for CS, US, and ITI periods (during the 8 sec CS presentation, during the 7.6 sec US presentation, and during the remaining time in the session). If AMP merely increased total number of head entries without specifically increasing GOAL-CRs, we should see ITI, GOAL-CR, and UR measures all increase. However, if AMP specifically increased GOAL-CRs and not head entries during ITI, the ITI measure should not be significantly different with different doses of AMP, while the GOAL-CR measure should be significant greater with increasing doses of AMP.
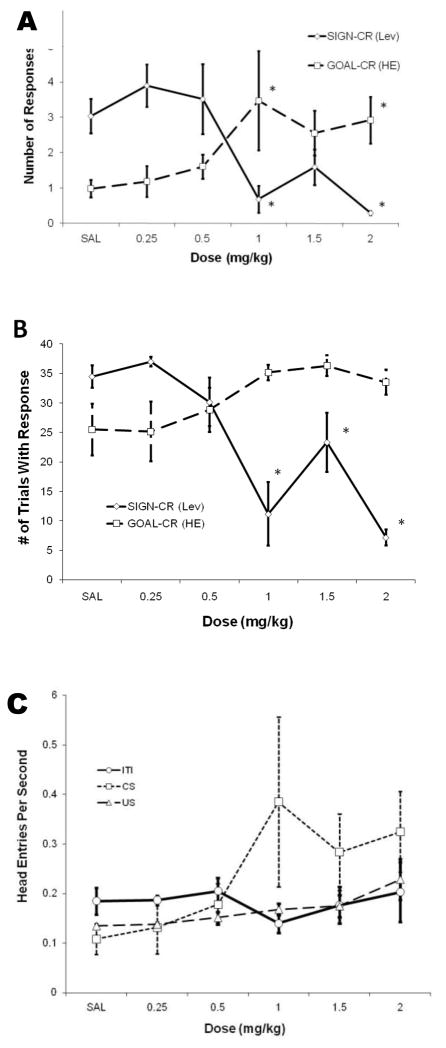
It was found that the effects of AMP were in the opposite direction than predicted, with SIGN-CRs decreasing rather than increasing, and with GOAL-CRs increasing rather than decreasing. Fig. 1A shows number of responses for both kinds of conditioned approach behavior. Within-subjects ANOVA indicated a significant effect of dose for number of SIGN-CR, F(5,25)=7.34, p<.05, and for number of GOAL-CRs, F(5,25)=2.74, p<.05. A within-subjects LSD procedure comparing the different AMP doses with saline revealed that number of SIGN-CRs was significantly different at doses of 1.0 mg/kg, t(5)=−2.95, p<.05, and 2.0 mg/kg, t(5)= −3.45, p<.05, and that the number of GOAL-CRs was significantly different in at doses of 1.0 mg/kg, t(5)=2.88, p<.05, and 2.0 mg/kg, t(5)=2.24, p<.05. Figure 1B shows number of trials with at least one SIGN-CR or GOAL-CR. Within-subjects ANOVA indicated a significant effect of dose for this measure of SIGN-CR, F(5,25)=12.52, p<.05; however, the F-value for GOAL-CRs just missed significance, F(5,25)=2.57, ns. A within-subjects LSD procedure comparing the different AMP doses with saline revealed that number of trials with at least one SIGN-CRs was significantly different at doses of 1.0 mg/kg, t(5)= −2.34, p<.05, 1.5 mg/kg, t(5)= −2.35, p<.05, and 2.0 mg/kg, t(5)= −3.52, p<.05. Performance on non-drug days was not found to be significantly different than performance seen on saline test days on any measure, suggesting no drug carryover effects on performance.
Figure 1.
Effects of five different doses of amphetamine and saline control on SIGN-CR and GOAL-CR behavior. Significant differences between saline and individual amphetamine doses are indicated by *, p<.05. A) Number of responses as a function of dose. B) Number of trials during which at least one response occurred. C) Head entries per second for intertrial interval periods (ITI), during the conditioned stimulus (CS), and during presentation of the unconditioned stimulus (US), as a function of amphetamine dose.
Fig. 1C shows mean number of head entries per second during ITI, CS, and US, for the six different drug conditions. AMP did not influence number of head entries per second during these different time periods similarly. Number of head entries during ITI did not show a large increase with increasing dose of AMP and actually dropped relative to saline at the 1.0 mg/kg dose (though not significantly). By contrast, number of head entries per second during the CS increased dramatically with increasing doses of AMP, while number of head entries during US showed a similar (though not as dramatic) increase. Within-subjects ANOVA showed a nonsignificant effect of dose on head entries per second during ITI, F(5,25)=.652, ns, a significant effect of dose on head entries per second during CS, F(5, 25)=2.706, p<.05, and a significant effect of dose on head entries per second during US, F(5,25) = 3.725, p<.05. Thus, the increase in head entries seen under AMP was confined to CS and US periods and did not represent a general increase in the number of head entries under drug.
It is evident upon examination of our results that AMP did not influence both forms of conditioned responding examined in our study in a similar fashion. Moreover, the effect of AMP was the opposite of what had previously been hypothesized. Sign-tracking decreased dramatically with increasing doses of AMP, while goal-tracking increased. Under non-drug conditions, sign-tracking was high and goal-tracking was low; under AMP these conditions reversed themselves. However, our results add value to the distinction between “sign-tracking” and “goal-tracking” behaviors as they may be expressed in addiction, and suggest that such different forms of conditioned approach behavior may be governed by different systems in the brain. The hypothesis that sign-tracking would be enhanced was based on the hypothesis that acute AMP would enhance responding directed at a conditioned stimulus, as was the case in [23]. However, that study employed a CS located immediately next to the site of US delivery and measured CS and US responses the same way via head entries into a food receptacle, and thus conditioned responses could not be separated into sign-tracking and goal-tracking since all conditioned responses were directed at the same area. Thus, the results of the current study do not contradict those of the previous study but rather address a different kind of conditioning situation.
The assumption has always been that enhanced conditioned responding by AMP would result from the increased salience of the conditioned stimulus and a corresponding increase in approach to that stimulus; however, many previous studies have confounded approach of the CS with approach of the US delivery area. Here, where the two were located separately, AMP enhanced activity directed at the US delivery area and decreased CS approach and contact. This does not mean that AMP in some way decreased the salience or meaning of the conditioned stimulus to the AMP-infused rats - indeed, were that the case, we should have seen both kinds of conditioned responding be reduced in strength. The finding in the literature has consistently been that dopaminergic psychostimulants such as AMP increase conditioned responding. That assumption was borne out in our study - goal-tracking was increased - although the specific hypothesis that that enhancement would involve increased approach of the conditioned stimulus itself was not.
One methodological concern regarding interpretation of our results might be that acute amphetamine administration might affect conditioned responding simply by creating appetite suppression. If this were the case, we should find that subjects would be less willing to consume the sucrose reinforcer and show shorter head entry latencies during presentation of the US. However, this was not the case; some subjects showed increased latencies with increasing doses of AMP, but some showed decreased latencies with increasing doses, and there was no overall significant difference by dose (data not presented for space considerations). Thus, it seems unlikely that the changes in behavior seen were due to AMP producing suppression of appetite.
Interestingly enough, another recent study demonstrated that sensitization to amphetamine causes a shift in the activity of ventral pallidum neurons from preferential coding of a reward-distal (in time) audio CS to a preferential coding of a reward-proximal audio CS cue [19]. Thus, in addition to increasing responses to cues located closely in space to US delivery, psychomotor stimulants may also increase responding to cues located closely in time to US delivery. This is in addition to sensitization shifting responding from spatially distal cues to spatially proximal cues, much as was seen in the current study with acute administration of AMP [15]. The overall effect of engaging the brain’s natural reward systems with dopaminergic agents, either through sensitization or acute administration, may be more complex than making conditioned stimuli “motivational magnets.”
It should also be noted the effect of AMP on sign- and goal-tracking might be quite different in a different procedure, as in the current experiment the two behaviors were only examined under one set of experimental circumstances. It is not clear what effect AMP would have on sign- and goal-tracking in, for example, a procedure where sign was spatially more distal from US delivery (e.g. where the lever was on the other side of the chamber) – that is, a procedure where goal-tracking would be expected to predominate and sign-tracking should be rare and/or weak in terms of expression. Given AMP’s tendency to increase low-probability behaviors and decrease high-probability behaviors, it is possible that in such a procedure sign-tracking would increase and goal-tracking would decrease. If this were so, it would argue against the notion that AMP decreases sign-tracking and increases goal-tracking. This possibility cannot be discounted given the design of the current experiment. It would be also very interesting to determine what effects, if any, acute AMP exposure would have on conditioned approach behavior in other species and other situations in which sign- and goal-tracking are differentially prevalent [5]. In summary, we have found that acute systemic administration of AMP enhances conditioned approach to the site of US delivery, not the CS itself, in a situation where CS and US are located in different areas.
Acknowledgments
This research was supported by NIDA Grants to Charles O’Brien and Laura Peoples. We thank Alexis Simpson for technicial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 2.Brown PL, Jenkins HM. Autoshaping of the pigeon’s keypeck. J Exp Anal Behav. 1968;11:1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns M, Domjan M. Topography of spatially directed conditioned responding: Effects of context and trial duration. J Exp Psychol Anim Behav Process. 2001;27:269–278. [PubMed] [Google Scholar]
- 4.Day JJ, Carelli RM. The nucleus accumbens and pavlovian reward learning. Neuroscientist. 2001;13:148–159. doi: 10.1177/1073858406295854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domjan M, Lyons R, North NC, Bruell J. Sexual Pavlovian conditioned approach behavior in male Japanese quail (coturnix coturnix japonica) J Comp Psychol. 1986;100:413–421. [PubMed] [Google Scholar]
- 6.Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behavior. Brain Res Revs. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- 7.Hearst E, Jenkins HM. Sign-tracking: The stimulus-reinforcer relation and directed action. Austin, TX: Psychonomic Society; 1974. p. 49. [Google Scholar]
- 8.O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug use: Can they explain compulsion? J Psychopharmacology. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 9.Leslie JC, Boakes RA, Linaza J, Ridgers A. Autoshaping using visual stimuli in the rat. Psychological Record. 1979;29:523–546. [Google Scholar]
- 10.Papini MR, Brewer M. Response competition and the trial-spacing effect in autoshaping with rats. Learn Motiv. 1994;25:201–215. [Google Scholar]
- 11.Purdy JE, Roberts AC, Garcia CA. Sign tracking in cuttlefish (Sepia officinalis) J Comp Psychol. 1999;113:443–449. doi: 10.1037/0735-7036.113.4.443. [DOI] [PubMed] [Google Scholar]
- 12.Robbins TW. The acquisition of responding with conditioned reinforcement: effects of pipradrol, methylphenidate, d-amphetamine, and nomifensine. Psychopharmacology. 1978;58:79–87. doi: 10.1007/BF00426794. [DOI] [PubMed] [Google Scholar]
- 13.Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 14.Simon NW, Setlow B. Post-training amphetamine administration enhances memory consolidation in appetitive Pavlovian conditioning: Implications for drug addiction. Neurobiol Learn Mem. 2006;86:305–10. doi: 10.1016/j.nlm.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Simon NW, Setlow B. Effects of prior amphetamine exposure on approach strategy in appetitive Pavlovian conditioning in rats. Psychopharmacology. 2009;202:699–709. doi: 10.1007/s00213-008-1353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor JR, Horger BA. Enhanced responding for conditioned reward produced by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacology. 1999;142:31–40. doi: 10.1007/s002130050859. [DOI] [PubMed] [Google Scholar]
- 17.Taylor JR, Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of Pavlovian conditioned approach behavior: Differential effects of cocaine, d-amphetamine, and 3, 4-methylenedioxymeth-amphetamine (“Ecstacy”) Biological Psychiatry. 2001;50:137–143. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- 18.Taylor JR, Robbins TW. Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology. 1984;84:405–412. doi: 10.1007/BF00555222. [DOI] [PubMed] [Google Scholar]
- 19.Tindell AJ, Berridge KC, Zhang J, Pecina S, Aldridge JW. Ventral pallidal neurons code incentive motivation: Amplification by mesolimbic sensitization and amphetamine. Eur J Neurosci. 2005;22:2617–2634. doi: 10.1111/j.1460-9568.2005.04411.x. [DOI] [PubMed] [Google Scholar]
- 20.Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Individual differences in Pavlovian autoshaping of lever pressing in rats predict stress-induced corticosterone release and mesolimbic levels of monoamines. Pharmacol Biochem Behav. 2000;65:509–517. doi: 10.1016/s0091-3057(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 21.Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomie A, Silberman Y, Williams K, Pohorecky L. Pavlovian autoshaping procedures increase plasma corticosterone levels in rats. Pharmacology Biochem Beh. 2002;72:507–513. doi: 10.1016/s0091-3057(01)00781-x. [DOI] [PubMed] [Google Scholar]
- 23.Wan X, Peoples LL. Amphetamine exposure enhances accumbal responses to reward-predictive stimuli in a pavlovian conditioned approach task. Neuropsychopharm. 2008;32:1346–57. doi: 10.1523/JNEUROSCI.1071-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: Increased cue-triggered “wanting” for sucrose reward. Journal of Neuroscience. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]