Abstract
Aim
The primary aim of this study was to determine the safety and efficacy of the monoamine oxidase-B (MAO-B) inhibitor selegiline hydrochloride (SEL, L-Deprenyl; Eldepryl) as an aid for smoking cessation in cigarette smokers.
Methods
One hundred and one nicotine-dependent adult cigarette smokers without current psychiatric or substance use disorders participated in this 8-week randomized, double-blind, placebo-controlled trial. Participants received either SEL (5 mg bid, n=51) or placebo (PLO, n=50), in combination with brief (<10 minutes) manualized smoking cessation counseling. The main smoking outcome measures were 7-day point prevalence abstinence at end of trial (EOT), 4-week continuous smoking abstinence at end of trial (CA), and 7-day point prevalence abstinence at 6 month follow-up (6MFU). Abstinence was determined by an absence of self-reported cigarette smoking and biochemically verified by expired breath carbon monoxide and plasma cotinine levels.
Results
Rates of smoking abstinence did not differ by medication group (EOT: SEL=16%, PLO=20%, p=0.57; CA: SEL=14%, PLO=18%, p=0.56; 6MFU: SEL=12%, PLO=16%, p=0.54). Adverse events were modest and comparable between medication groups. Participants receiving SEL were more likely than those receiving PLO to report dry mouth (25.5% versus 8.2%, p<0.05).
Conclusions
Our results suggest that SEL was safe and well-tolerated by adult cigarette smokers, but did not improve smoking abstinence rates compared to PLO.
Keywords: Selegiline hydrochloride, placebo-controlled trial, smoking cessation, nicotine dependence
1. Introduction
Tobacco addiction is the most significant preventable cause of morbidity and mortality, with over 430,000 deaths in the United States resulting annually from tobacco-related disease in the United States (CDC, 2009). While effective treatments are available for smoking cessation (e.g. nicotine replacement therapies (NRTs), sustained-release bupropion, varenicline), they do not work for all smokers and a significant proportion of cigarette smokers appear to be more recalcitrant to conventional smoking cessation interventions (Hughes et al., 1996; Irvin and Brandon, 2000; Irvin et al., 2003). Thus, the development of novel and effective medications for smoking cessation based on the neurobiological mechanisms underlying nicotine dependence is a high priority (George et al., 2004; Perkins and Scott, 2008).
Monoamine oxidases (MAO's) are enzymes involved in the catabolism of monoamine neurotransmitters such as dopamine, serotonin and norephinephrine (Lewis et al., 2007). Monoamine-releasing neurons are stimulated by nicotine from cigarette smoke via activation of pre-synaptic nicotinic acetylcholine receptors (nAChRs). In addition, several clinical and neuroimaging reports suggest that cigarette smoke contains components which inhibit both monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B) isoforms. Human ex vivo studies have suggested that smokers have reduced levels of platelet MAO-A and -B activity as compared to non-smokers (for reviews see Fowler et al., 2003; George and Weinberger, 2008; Lewis et al., 2007). Since it has been observed that: 1) MAO inhibition leads to increases in synaptic monoamines which are also increased by nAChR activation; and 2) cigarette smoke possesses components which inhibit MAO isoforms, MAO inhibitors (MAOIs) have been proposed as a treatment for tobacco dependence (Berlin et al., 1995; Biberman et al, 2003; George et al, 2003; Houtsmuller et al, 2002, reviewed by George and Weinberger, 2008).
Selegiline hydrochloride (SEL, L-Deprenyl; Eldepryl®) is an irreversible (“suicide”) inhibitor of the MAO-B subtype that is predominantly located in brain and is believed to be relatively selective for MAO-B at low doses (5-10 mg daily). SEL might aid in smoking cessation by mimicking the effects of cigarette smoke on MAO (Lewis et al., 2007) and/or through inhibition of nicotine metabolism by CYP2A6 (Siu and Tyndale, 2008). A preliminary human laboratory study found that oral SEL reduced self-reported cravings for smoking (Houtsmuller et al., 2002) and a trial of oral SEL combined with transdermal nicotine patch (TNP) in 109 cigarette smokers by Biberman and colleagues (Biberman et al., 2003) found a higher level of continuous abstinence after one year with a combination of SEL and TNP (25%) as compared to TNP alone (11%). George and colleagues (George et al., 2003) conducted a pilot study comparing oral SEL (5 mg bid) to placebo (PLO) in 40 nicotine dependent adult cigarette smokers. At the end of the eight week randomized, double-blind, clinical trial, 45% of smokers treated with SEL achieved biochemically-verified smoking abstinence as compared to 15% for the PLO group (p<0.05). Cessation rates were reduced at the 6 month follow-up assessment, with point prevalence cessation rates of 20% of the SEL group versus 5% of the PLO group maintaining smoking abstinence (p=0.18). This preliminary study suggested that SEL is safe, well-tolerated, and may have efficacy for smoking cessation in nicotine-dependent cigarette smokers.
The current study was a replication of the George et al (George et al., 2003) pilot study using a larger independent sample of refractory cigarette smokers motivated to quit smoking. It was hypothesized that rates of smoking abstinence at the end of the 8-week trial and at 6-month follow-up would be higher for participants receiving SEL than PLO.
2. Methods
2.1. Subjects
To be eligible for the clinical trial, participants had to be nicotine dependent adults between the ages of 18 and 70 who smoked ≥ 15 cigarettes per day (CPD) and reported motivation to quit smoking in the following 30 days as evidenced by a score of at least 7 on the Contemplation Ladder (Biener and Adams, 1991). Nicotine dependence was defined as a score of 5 or more on the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991) and smoking was biochemically verified with baseline expired breath carbon monoxide (CO) levels of ≥10 ppm and plasma cotinine levels of ≥150 ng/ml.
Exclusion criteria included the presence of a positive urine drug screen or urine pregnancy test at baseline evaluation, evidence of alcohol or other drug abuse or dependence in the previous 6 months, meeting DSM-IV criteria for a current diagnosis of major depressive disorder, panic disorder or post-traumatic stress disorder, meeting DSM-IV criteria for a current or past diagnosis of bipolar disorder or schizophrenia, having unstable medical disorders (e.g., untreated diabetes), and an inability to give informed consent. Participants were not permitted to participate if they were taking any over-the-counter (e.g., pseudoephedrine) or prescription (e.g., methylphenidate) sympathomimetic agents, any antidepressant medication, or other medications (e.g., meperidine) which might interact with SEL. Participants were recruited from the Greater New Haven (Connecticut) community using flyers and newspaper, television, and radio advertisements. Written informed consent was obtained from all participants and the research protocols were approved by Yale Medical School's Human Investigation Committee. The trial was registered at Clinicaltrials.gov as NCT00129311.
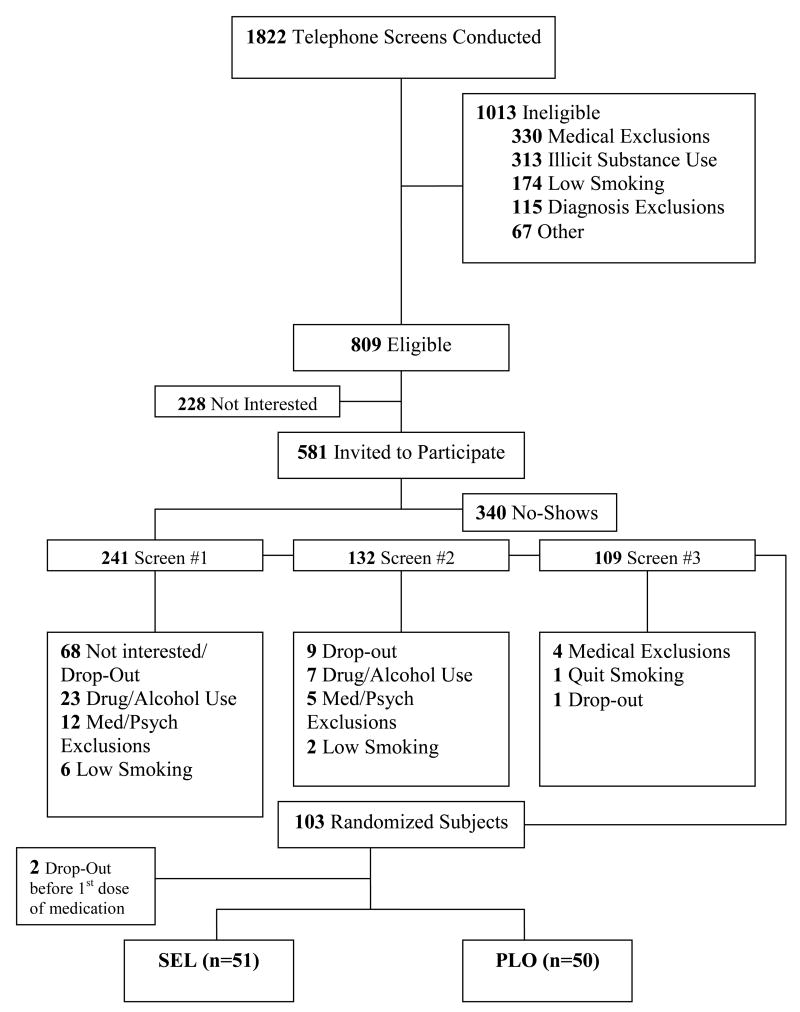
Figure 1 presents a CONSORT flowchart of participant recruitment. Participants were enrolled between October 2004 and August 2008. A total of 1,822 adult smokers were screened by phone and 581 were determined to be eligible for an in-person screening. Subsequently, 241 smokers attended the first screening appointment which established general eligibility for the study by the research assistants. Two subsequent screening appointments by study clinicians and psychiatrists assessed diagnostic and medical eligibility. The presence of past and current psychiatric disorders and alcohol or other drug abuse or dependence over the previous 6 months was assessed using The Structured Clinical Interview for DSM-IV (First et al., 1997). Major reasons for exclusion during the screening process (n=138) included psychiatric and medical exclusions (n=21), positive urine drug screens or signs of recent alcohol or drug abuse/dependence (n=30), low smoking level or nicotine dependence (n=8), and withdrawal of informed consent (n=81). One hundred and three participants were randomized to receive either active study medication or PLO (Figure 1). Two participants did not receive any study medication due to medical exclusions that arose after their screening appointments and before the appointments at which they would have taken the first dose of medication. Therefore, data from the 101 randomized smokers who received at least one dose of study medication are reported as the intention-to-treat study sample.
Figure 1.
Recruitment flowchart for the selegiline for smoking cessation clinical trial. Key words: SEL, selegiline; PLO, placebo
2.2. Study Medications
Participants were randomly assigned to receive either SEL (n=51) or matching PLO (n=50) with gender as a stratification variable. Connecticut Mental Health Center research pharmacists encapsulated 5 mg SEL hydrochloride capsules in blue 00 opaque capsules; PLO capsules contained the lactose matrix only. Medications were dispensed to subjects on a weekly basis with medication bottles with MEMS VI smart caps. Both participants and research staff were blinded to study medication assignment.
Medication dosing duration and schedule was designed to replicate the procedure used in the pilot study by George and colleagues (George et al., 2003). Participants received 9 weeks of medication with a maximum dose of 10 mg/day. Participants began study medication on the first day of the trial (Day 1) at 5 mg po once daily. The dose of medication was increased to 5 mg po twice daily on Day 8. All participants were asked to attempt to quit smoking at the beginning of the third week of the study (Day 15; target quit date, TQD). Study medications were tapered at 5 mg po once daily over a one-week period during the ninth week of the study. Compliance to study medication was assessed using MEMS VI smart caps.
2.3. Procedures
The study consisted of ten weekly appointments during which participants received a week's worth of study medication, completed measures of smoking, mood, and adverse events, and received an individual brief (<10 minute) smoking cessation counseling session from the Mayo Clinic's “Smoke Free and Living It” manual (MayoClinic, 2000). Participants also completed measures of nicotine dependence, withdrawal, and depression at Weeks 1, 4, and 9. The participants' Target Quit Date (TQD) was the beginning of the third week of the study (Week 3) and the trial endpoint was at the beginning of the ninth week of the study (End of Trial, EOT; Week 9). Participants were asked to attend a follow up appointment at the end of Week 9 (their 10th appointment) to assess any problems stopping the study medication and an in-person follow up assessment of their smoking six months after their EOT appointment.
2.4. Measures
2.4.1. Demographics and Smoking History
Participants were asked to report demographic information and information about their smoking history at baseline. Participants completed a 7-day timeline follow-back (TLFB; Sobell et al., 1988) as a self-report measure of alcohol consumption.
2.4.2. Measures of Nicotine Dependence, Withdrawal, and Depression
Nicotine dependence was assessed using the 6-item FTND (Heatherton et al., 1991; range 0-10). Symptoms of tobacco withdrawal were assessed using the 8-item Minnesota Nicotine Withdrawal Scale (M-NWS; Hughes and Hatsukami, 1986); range 0-32). The Tiffany Questionnaire of Smoking Urges (T-QSU; Tiffany and Drobes, 1991) was administered to evaluate urges to smoke in response to positive (Factor 1) or negative (Factor 2) reinforcement (range 1-7). Depressive symptoms were rated using the Beck Depression Inventory (BDI; Beck et al., 1988) and the Hamilton Depression Rating Scale (HDRS; Hamilton, 1960).
2.4.3. Measures of Smoking Consumption
At baseline and weekly appointments, participants completed a 7-day TLFB (Sobell et al., 1988) as a self-report measure of smoking. Objective measures to assess smoking consumption included expired breath CO level determination (Bedfont EC50 Microsmokerlyzer II, Kent, UK) and plasma cotinine levels (a metabolite of nicotine). Venous plasma for cotinine levels at baseline and EOT were determined by reversed-phase high performance liquid chromatography procedures adapted from Hariharan and colleagues (Hariharan et al., 1988).
2.4.4. Determination of Smoking Abstinence
Trial endpoint 7-day point prevalence smoking abstinence (EOT), continuous abstinence during the last 4 weeks of the trial (CA), and 7-day point prevalence smoking abstinence at 6-month follow up (6MFU) were determined by an absence of self-reported cigarettes smoking and biochemically verified by an expired breath CO level < 10 ppm and plasma cotinine level <15 ng/ml (Benowitz et al., 2002). It should be noted that a plasma cotinine level of <25 ng/ml was used to determine abstinence during a brief period during the study due to variations in laboratory assay analysis procedures during the course of the trial. A smoking lapse was defined as taking at least one puff of a cigarette by self-report and relapse to smoking was defined as smoking at least 7 consecutive days measured by self-report and confirmed through CO levels.
2.4.5. Measures of Adverse Events
Adverse events were assessed using the Systematic Assessment for Treatment-Emergent Events (SAFTEE; Levine and Schooler, 1992), a structured interview conducted by research staff. The SAFTEE included a list of 33 study specific events (e.g., dry mouth, dizziness) that, if endorsed, were rated in severity from “minimal” to “very severe.” Each event was coded as “present” or “absent” for each participant. An event was judged to be “present” 1) if that event was absent at baseline and then was endorsed at any time during the study or 2) if that event was present at baseline and the severity of that event increased at any point during the study.
2.4.6. Measures of Medication Compliance
Compliance to medication was assessed by self-report and pill counts, and was confirmed using the Medication Event Monitoring System (MEMS) SmartCap V electronic medication compliance system (Advanced Analytic Research on Drug Exposure (AARDEX) Corporation, Union City, CA). For each week of the study, the percentages of doses taken were calculated based on the maximum number of doses participants were instructed to take and the number of doses taken as shown by the MEMS data. In most cases, participants were expected to take 7 doses of medication during week 1 (qd dosing) and 14 doses of medications during weeks 2-8 (bid dosing). If a participant missed an appointment, they were encouraged to reschedule their appointment as quickly as possible to minimize the number of medication doses missed. In the event of a missed appointment, medication compliance was calculated based on the expected number of doses to be taken between 1) their last regular appointment and the rescheduled appointment and 2) the rescheduled appointment and their next regular appointment. In the event of a discrepancy, MEMS data were used to calculate the percentages.
2.5. Statistical Analysis
Using outcome data from the pilot study (George et al., 2003) to calculate effect sizes (φs=0.23-0.33) and estimate attrition, a power analysis determined that there would be sufficient power (>80%) to demonstrate significant main effects of SEL on EOT abstinence and CA with enrollment of 70 participants (35 in each medication group) and on 6MFU abstinence with 150 participants (75 in each medication group). A final target enrollment of 200 was determined to account for 25% participant attrition at the 6MFU assessment. Details about screening and recruitment were presented above and all analyses below were performed on the finale randomized sample of 101 participants.
Comparison of demographic and clinical characteristics, compliance with study medication, adequacy of medication blinding, days from TQD to lapse, days from lapse to relapse, and adverse events between the two medication groups were examined by Chi-square and independent samples t-tests. Kaplan-Meier survival curve analysis (Bland and Altman, 1998) was used to compare treatment retention for participants receiving SEL versus PLO. Smoking abstinence outcomes (EOT, CA, and 6MFU) for the overall sample and by gender were assessed using Fisher's Exact Tests. The study included normally distributed psychometric scales for depression and smoking urges that were measured at multiple times across the course of the trial. These were examined with Hierarchical Linear Models (HLM) to determine whether the slopes of the outcomes varied by medication condition. The trial also included two biological outcomes (CO and plasma cotinine levels) that were found to be non-normally distributed and could not be analyzed by HLM because of the violation of the normality assumption. Accordingly, participants' scores were dichotomized at each assessment when the measure was collected and slopes of the logits were analyzed with Hierarchical General Linear Models (HGLM), a more general form of HLM that can handle binary variables using the Bernoulli distribution. An “intention-to-treat” approach for all analyses was used, and subjects who were lost during the trial or at 6-month follow-up assessment were counted as smoking (Hughes et al., 2003). Statistical analyses were performed using SPSS v.16.0 software for PC (SPSS Inc., Chicago, IL). Statistical tests were two-tailed and differences were considered significant when p<0.05.
3. Results
3.1. Baseline Demographic and Smoking Characteristics
Baseline demographic and smoking variables for the overall sample and by medication group are presented in Table 1. There were no significant differences between SEL and PLO groups on most baseline demographic, smoking, and clinical variables (all ps>0.05). The PLO group had a higher baseline cotinine level than the SEL group (t =2.1, df=92, p<0.05) although the groups did not differ on CPD or CO level (ps>0.05).
Table 1.
Baseline demographic and smoking variables for overall sample (n=101) and participants receiving Selegiline (SEL, n=51) and placebo (PLO, n=50).
| Total Sample n= 101 | SEL n=51 | PLO n=50 | p-values | |
|---|---|---|---|---|
| Age (years) | 47.4 ± 12.0 | 48.5 ± 11.0 | 46.2 ± 13.0 | p = 0.33 |
| % Women | 50.5 | 49.0 | 52.0 | p = 0.84 |
| % Minority | 12.9 | 13.7 | 12.0 | p = 0.80 |
| Education (years) | 14.0 ± 2.1 | 13.8 ± 2.0 | 14.2 ± 2.2 | p = 0.34 |
| Cigarettes Per Day | 22.4 ± 8.1 | 22.2 ± 7.1 | 22.5 ± 9.1 | p = 0.82 |
| Age of Smoking Onset | 16.3 ± 5.6 | 16.2 ± 6.9 | 16.3 ± 4.1 | p = 0.89 |
| Duration of smoking (years) | 30.7 ± 12.6 | 31.6 ± 11.6 | 29.8 ± 13.6 | p = 0.46 |
| Smoking pack/years | 35.0 ± 22.3 | 35.1 ± 15.9 | 35.0 ± 25.6 | p = 0.99 |
| Previous quit attempts | 6.2 ± 7.5 | 6.9 ± 8.7 | 5.3 ± 6.2 | p = 0.29 |
| Expired breath CO level (ppm) | 24.2 ± 9.20 | 24.5 ± 9.4 | 23.9 ± 9.1 | p = 0.74 |
| FTND score | 6.3 ± 1.6 | 6.4 ± 1.5 | 6.1 ± 1.6 | p = 0.31 |
| Plasma Cotinine (ng/ml) | 307 ± 120 | 281 ± 96 | 332 ± 136 | p < 0.05 |
| Contemplation Ladder | 7.1 ± 0.3 | 7.1 ± 0.3 | 7.1 ± 0.3 | p = 0.97 |
| % History of MDD | 27.7 | 29.4 | 26.0 | p = 0.70 |
| % Alcohol Consumers | 46.5 | 45.1 | 48.0 | p = 0.29 |
| Average Alcohol Consumption (standard drinks/day) | 0.3 ± 0.5 | 0.3 ± 0.4 | 0.3 ± 0.5 | p = 0.78 |
| BDI score | 4.6 ± 3.8 | 5.0 ± 4.2 | 4.2 ± 3.4 | p = 0.31 |
| HDRS 17 items | 2.1 ± 2.0 | 2.1 ± 2.0 | 2.1 ± 2.1 | p = 0.93 |
| HDRS 21 items | 2.2 ± 2.2 | 2.3 ± 2.2 | 2.1 ± 2.2 | p = 0.76 |
Key: SEL, selegiline; PLO, placebo; CO, carbon monoxide; FTND, Fagerström Test for Nicotine Dependence; MDD, major depressive disorder; BDI, Beck Depression Inventory; HDRS, Hamilton Depression Rating Scale
Notes: Contemplation Ladder range = 1-10. Significance of comparisons between groups based on independent samples t tests except for use of chi-square tests for outcomes expressed in percentages.
3.2. Retention of Participants in the Clinical Trial and at Follow-up
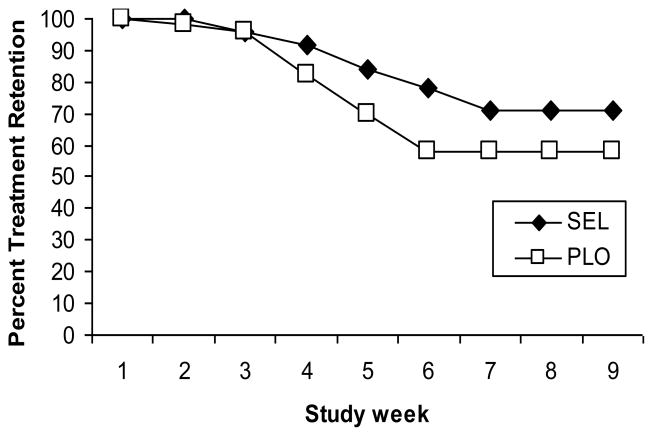
Kaplan-Meier survival curve analysis suggested that participant retention was not significantly different in the SEL group as compared to the PLO group (Figure 2, Log Rank test χ2=1.00, df=1, p=0.32). Total weeks in treatment during the trial did not significantly differ by medication group although there was a trend for participants in the SEL group to complete more appointments than participants in the PLO group (SEL 7.9 ± 2.1, PLO 7.0 ± 2.8; p=0.08). Overall, half of the participants completed the 6MFU assessment (51%) and there was no significant difference in the number of participants completing the 6MFU by medication group (SEL n=23, PLO n=28; χ2=1.13, df=1, p=0.29).
Figure 2.
Treatment retention in selegiline (SEL, n=51) versus placebo (PLO, n=50) groups during the 8-week trial. Key words: SEL, selegiline; PLO, placebo
3.3. Compliance with Study Medication
Nineteen participants in the SEL group stopped study medication during the trial including 12 participants who dropped out of the study and 7 participants who stopped study medication but continued to attend appointments (to complete assessments and receive smoking cessation counseling). Twenty-five participants in the PLO group stopped taking study medication including 19 who dropped out of the study and 6 who stopped study medications but continued to attend appointments. There were no significant differences between medication groups for the number of participants who stopped study medication (χ2=1.67, df=1, p=0.20).
Compliance with study medication, as confirmed by MEMS data, was generally high. Participants receiving SEL took an average of 94% of their doses of medication and participants receiving PLO took an average of 91% of their doses of medication while engaged in treatment. Compliance with study medication decreased slightly over the course of the study (SEL week 1, 100%, SEL week 9, 90%; PLO week 1, 98%, PLO week 9, 86%; F=5.9, df=7,38, p<0.001) and did not differ by medication group (F=1.3, df=7,38, p=0.27).
3.4. Adequacy of Study Medication Blinding
Participants were asked at the end of the trial to rate whether they believed they had received SEL or PLO. Similarly, research staff involved in the weekly smoking cessation counseling sessions (AHW, ER) rated which study medication they believed each participant to have received. Thirty-seven percent of participants in the SEL group correctly identified that they received SEL and 56% of participants in the PLO group correctly identified that they received PLO (χ2=2.46, df=1, p=0.12). Ratings of the research staff were similar: raters correctly identified 38% of participants receiving SEL and 46% of participants receiving PLO (χ2=0.58, df=1, p=0.45).
3.5. Smoking Cessation Outcomes
At the end of trial, 18 participants (18%) had EOT abstinence from smoking while 16 participants (16%) had CA during the last four weeks of the study as confirmed by self-report, CO, and cotinine levels. Overall, 18 participants (18%) were abstinent after six months. There were no significant difference in the rates of smoking abstinence by medication group at EOT (SEL 8/51, PLO 10/50; χ2=0.32, df=1, p=0.57), the last four weeks of the trial (CA; SEL 7/51, PLO 9/50; χ2=0.35, df=1, p=0.56), or at 6MFU (SEL 6/51, PLO 8/50; χ2=0.38, df=1, p=0.54).
The percentage of participants who quit smoking on their TQD and lapsed to smoking at some point before EOT (SEL 29.4%, PLO 28.0%) or who were not able to quit on their TQD (SEL 58.8%, PLO 54.0%) did not differ by medication group (χ2=0.78, df=2, p=0.68). There were also no differences by medication group for the number of days from the TQD to smoking lapse for participants who lapsed during the study (SEL M=7.5, SD=13.9, PLO M=5.1, SD=5.1; t=0.79, df=27, p=0.43). There was a non-significant trend for the participants taking SEL to have a longer number of days from smoking lapse to smoking relapse (SEL M=14.3, SD=13.9, PLO M=5.7, SD=5.2; t=1.83, df=10, p=0.10).
3.6. Smoking Cessation Outcomes by Gender
Because female smokers have less success with some smoking cessation treatments (e.g., TNP; Perkins, 2001), smoking outcomes were examined for gender differences within medication groups. While fewer male participants receiving SEL quit smoking, there were no significant gender differences in abstinence rates for EOT (SEL male 11.5%, SEL female 20.0%, χ2=0.69, df=1, p=0.41, phi=0.12; PLO male 20.8%, PLO female 19.2%, χ2=0.02, df=1, p=0.89, phi=0.02), CA (SEL male 7.7%, SEL female 20%, χ2=1.63, df=1, p=0.21, phi=0.18; PLO male 16.7%, PLO female 19.2%, χ2=0.06; df=1, p=0.81, phi=0.03), or 6MFU (SEL male 8.0%, SEL female 16.0%, χ2=0.85, df=1, p=0.36, phi=0.13; PLO male 20.8%, PLO female 15.4%, χ2=0.25, df=1, p=0.62, phi=0.07). Further analysis showed no significant differences when comparing medication groups within gender and no medication group by gender interactions in EOT abstinence (all ps>0.05).
3.7. Effects of SEL on CO, CPD, nicotine dependence, withdrawal, cravings, and symptoms of depression (Table 2)
Table 2.
Hierarchical Linear Model and Hierarchical General Linear Model Analyses of Slopes of Time-Varying Outcomes as a Function of Medication Condition
| Outcome | Coefficient | SE | t | p-values |
|---|---|---|---|---|
| Cigarettes per Week | -0.032 | 0.04 | -0.78 | 0.44 |
| CO Level | -0.033 | 0.08 | -0.41 | 0.68 |
| Plasma Cotinine Level | -0.160 | 0.11 | -1.42 | 0.16 |
| FTND | -0.029 | 0.07 | -0.43 | 0.67 |
| M-NWS Total Score | 0.010 | 0.12 | 0.09 | 0.93 |
| TQSU Factor 1 | -0.056 | 0.09 | -0.60 | 0.55 |
| TQUS Factor 2 | -0.017 | 0.03 | -0.66 | 0.51 |
| BDI | 0.078 | 0.08 | 0.98 | 0.33 |
| HDRS 17 items | 0.023 | 0.06 | 0.39 | 0.70 |
| HDRS 21 items | 0.027 | 0.06 | 0.43 | 0.67 |
Key: SE, standard error; CO, carbon monoxide; FTND, Fagerström Test for Nicotine Dependence; M-NWS, Minnesota Nicotine Withdrawal Scale; TQSU, Tiffany Questionnaire of Smoking Urges; BDI, Beck Depression Inventory; HDRS, Hamilton Depression Rating Scale
The effect of SEL on indices of smoking (CPD, CO), nicotine dependence (FTND), withdrawal (M-NWS), cravings (TQSU) and symptoms of depression (BDI, HDRS) over the course of the study were analyzed. There was a significant effect of time for CPD (F=24.7, df=8,47, p<0.001), CO (F=21.0, df=8,49, p<0.001), FTND (F=82.9, df=2,68, p<0.001), M-NWS (F=10.8, df=2,67, p<0.001), and cravings (Factor 1 F=8.3, df=2,67, p<0.01; Factor 2 F=11.69, df=2,68, p<0.001) with all indices decreasing over the course of the study. Symptoms of depression showed a similar decrease over the course of the study (BDI F=4.07, df=2,68, p<0.001; HDRS-17 item F=9.37, df=2,66, p<0.001; HDRS-21 item F=8.77, df=2,66, p<0.001). As shown in Table 2, groups did not differ significantly in slopes on any of the outcomes examined by HLM or HGLM suggesting that changes were similar for participants regardless of medication group for indices of smoking, nicotine dependence, cravings, and depression. When analyses were repeated just for participants with confirmed EOT abstinence (SEL n=8, PLO n=10), results for the smoking measures did not substantially change. Changes in depression scores were no longer statistically significant in the analyses of the small sample of abstainers.
3.8. Adverse Events (Table 3)
Table 3.
Percentage of participants receiving Selegiline (SEL, n=51) and placebo (PLO, n=50) who reported adverse events.
| Adverse Event | SEL n=51 | PLO n=50 | p-values |
|---|---|---|---|
| Headache | 47.1% | 42.9% | 0.67 |
| Drowsiness | 39.2% | 32.7% | 0.49 |
| Increased Appetite | 39.2% | 24.5% | 0.12 |
| Frequent Night Awakenings | 33.3% | 28.6% | 0.61 |
| Dry Mouth | 25.5% | 8.2% | < 0.05 |
| Early Morning Awakenings | 23.5% | 20.4% | 0.71 |
| Difficulty Falling Asleep | 21.6% | 10.2% | 0.12 |
| Flu-like Symptoms | 21.6% | 8.2% | 0.06 |
| Nausea/Vomiting | 21.6% | 18.4% | 0.69 |
| Anxiety | 17.6% | 18.4% | 0.93 |
| Decreased Appetite | 17.6% | 16.3% | 0.86 |
| Irregular/Rapid heartbeat | 17.6% | 6.1% | 0.08 |
| Constipation | 13.7% | 12.2% | 0.83 |
| Dizziness | 13.7% | 16.3% | 0.72 |
| Akathisia | 9.8% | 6.1% | 0.50 |
| Poor Memory | 7.8% | 4.1% | 0.43 |
Key: SEL, selegiline; PLO, placebo
SEL was well-tolerated and there were few significant differences in adverse event ratings by medication group (Table 3). More participants receiving SEL reported dry mouth in comparison to participants receiving PLO (SEL 13/51, PLO 4/50, p<0.05) and there was a trend for more participants receiving SEL than PLO to report flu-like symptoms (SEL 11/51, PLO 4/50, p=0.06) and an irregular or rapid heartbeat (SEL 9/51; PLO 5/50; p=0.08). The most commonly reported events were headaches (39/101), drowsiness (31/101), and increased appetite (27/101), and there were no significant differences in the report of these three events by medication group (ps>0.05).
4. Discussion
This study was conducted as a larger replication of the initial pilot study by George and colleagues (George et al., 2003) which found that the MAO-B inhibitor SEL increased smoking cessation outcomes compared to PLO at the end of an 8-week medication period. While oral SEL hydrochloride was safe and well-tolerated with few significant side effects, SEL did not improve smoking cessation rates over PLO at the end of the 8-week trial or at the 6MFU. Our findings are in contrast to previous studies that suggested SEL would improve smoking cessation rates in adult smokers (Biberman et al., 2003; George et al., 2003) but are consistent with a multisite trial of the patch form of SEL which recently reported preliminary results suggesting no benefit of SEL over PLO for smoking cessation (Evaluation of Selegiline Transdermal System for Smoking Cessation: Preliminary Results of NIDA's 246 Subject, Multi-Site Trial, Elbert Glover, Ph.D., Society for Research on Nicotine and Tobacco (SRNT), Dublin Ireland, April 2009). The current findings are also in contrast to a study that found a trend toward increased CO-confirmed abstinence of the MAO-A inhibitor moclobemide (Berlin et al., 1995) and a second study which found higher rates of EOT and CA with the MAO-B inhibitor lazabemide compared to placebo (Berlin et al., 2002). It should be noted that the samples in early studies of MAOIs were typically small and underpowered to determine differences between medication groups. In addition, the study of lazabemide was terminated early due to concerns about liver toxicity. It is not clear why SEL did not improve smoking cessation outcomes as hypothesized; however, the reinforcing effects of MAOIs and differences in subject characteristics in comparison to our previous trial (George et al., 2003) may have contributed to lack of observed differences between SEL and PLO, and are discussed in more detail below.
Preclinical research on animal behavior in response to SEL and other MAOIs has been similarly mixed. Studies have reported increased self administration of nicotine with the MAOIs tranylcypromine (Guillem et al., 2005; Villegier et al., 2007), phenelzine (Guillem et al., 2005; Wooters and Bardo, 2007), clorgyline (Guillem et al., 2006), and norharmane (Guillem et al., 2006). However, Guillem and colleagues (Guillem et al., 2006) found that SEL did not increase self administration of nicotine. Nonetheless, a recent study found that nicotine withdrawal-induced place aversion in rats was more persistent in animals pretreated with tranylcypromine or phenelzine (Guillem et al., 2008). Preclinical research findings on the effects of SEL on other substances have been mixed, with SEL potentiating the effects of cocaine (Schiffer et al., 2003) but not morphine (Grasing and He, 2005). While it has been hypothesized that MAOIs would be useful for smoking cessation by mimicking the effects of smoking, these preclinical studies suggest that MAOIs may augment the effects of nicotine. Extrapolating from these animal laboratory studies, it is possible that MAOIs may make it more difficult to quit smoking by enhancing the reinforcing effects of cigarettes. One study (Houtsmuller et al., 2002) suggested that 2 week administration of SEL decreased cravings for and satisfaction from cigarettes, and George et al. (George et al., 2003) reported that there was a trend toward a reduction T-QSU Factor 1 (Desire to Smoke) ratings with SEL treatment (p=0.09). These effects were not confirmed in the present trial. Additional research, including human laboratory studies of the effects of SEL on cravings, withdrawal, and smoking behavior is needed to determine how MAO-B inhibitors affect the ability to abstain from smoking. For example, recent research found that SEL is a mechanism-based inhibitor of CYP2A6 and both SEL and its metabolites desmethylselegiline and L-amphetamine enhanced the inhibition of nicotine metabolism in humans and mice (Siu and Tyndale, 2008).
A number of smoking cessation studies have reported differential response to pharmacological treatments by gender. Female smokers, as compared to male smokers, respond less well to TNP (Perkins, 2001; Perkins et al., 2008) but may respond better to monoaminergic agents like clonidine (Glassman et al., 1993). Although both male and female smokers show improved treatment outcomes with bupropion compared to placebo, women had a more difficult time quitting smoking whether they received bupropion or placebo (Scharf and Shiffman, 2004). These findings highlight the importance of examining outcomes by gender during the testing of novel treatments for smoking (see also Dickerson et al., 2009). In this study, consistent with the pilot study (George et al., 2003), there were no overall gender differences in quit rates within medication groups in this study; however, it appears that fewer men receiving SEL quit smoking as compared to women receiving SEL. It is not entirely clear why men would respond more poorly to a dopamine-enhancing medication, although sex-related differences in limbic circuitry structure and function have been observed and may represent a potential mechanism (Cahill, 2006). Examining the differential effect of SEL on cravings, withdrawal, and smoking by gender in laboratory studies would be useful direction of future research to determine whether male and female smokers respond differently to MAOIs.
A number of limitations of this study exist. Compared to rates reported in the pilot study (SEL 45%, PLO 15%; George et al., 2003), EOT abstinence rates in the current study were much lower in the SEL group (16%) and slightly higher in the PLO group (20%). In addition, the placebo response rate in the current study was slightly higher than the average placebo rate in recent reviews of behavioral and medication treatments for nicotine dependence (∼11-14%; Fiore et al., 2008). Because the study had a large number of inclusion and exclusion criteria, only 5.7% of people who were screened by phone and 41.9% of people who were screened in person were eventually randomized into the study. It should be noted that these percentages, while low, are consistent with the randomization rates of other recent trials of pharmacological treatments for nicotine dependence (e.g., Schnoll et al., 2008; Steinberg et al., 2009).
In addition, participants in the study were primarily Caucasian smokers with high levels of education and were free from current psychiatric or substance use disorders, which may limit the generalizability of our findings to other groups of smokers. Further, participants had to report motivation to quit smoking in the next month, had to attend a minimum of three screening appointments over a month (∼5-6 total hours of time), and had to complete intensive assessment batteries. These were not requirements of the pilot study (George et al., 2003). Despite reporting a high level of motivation to quit, participants in this study were nicotine dependent refractory smokers who had tried to quit smoking approximately 6 times before entering the study and had been smoking for 30 years, on average. Reviews of both pharmacological and behavioral smoking cessation trials for adult smokers have reported decreases in abstinence rates over time (Irvin and Brandon, 2000; Irvin et al., 2003) suggesting that smokers have become a more difficult group to treat.
Finally, our power to detect differences in smoking abstinence was decreased due to issues related to both recruitment and retention of participants. Although the recruitment goal was to enroll 200 participants, only 101 participants were eligible to be randomized after conducting over 1800 phone screens. In addition, there were a significant number of participants in both medication groups who discontinued treatment during the study (35% of the randomized sample), and only half of the participants completed the 6MFU assessment. While power analysis suggested that 101 participants would provide sufficient power for the EOT and CA analyses, our analysis of the 6MFU data required a greater number of participants (n=150) and therefore was underpowered. Aspects of the medication, recruitment, our sample, treatment retention, and the study design may all have contributed to lack of significant differences in quit rates between participants receiving SEL and PLO in the present study.
In this moderately-sized trial, oral SEL as an adjunct to brief behavioral treatment did not improve smoking quit rates as compared to PLO. Researchers are currently working to test novel medications that works on a variety of neurotransmitter systems implicated in smoking, including MAOIs (George, 2006). It is crucial to continue working to develop novel medication treatments for nicotine dependence in order to improve smoking cessation outcomes and reduce the enormous harmful health and economic costs to persons and society.
Footnotes
The Clinicaltrials.gov Registration No. for this trial, “Usefulness of Selegiline as an Aid to Quit Smoking,” is NCT00129311.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- Benowitz NL, Jacob P, III, Ahijevich K, Jarvis MJ, Hall S, LeHouzec J, Lichenstein E, Henningfield J, Tsoh J, Hurt RD, Velicer W. Biochemical verification of tobacco use and cessation. Report from the SRNT Subcommittee on Biochemical Verification. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Berlin I, Aubin HJ, Pedarriosse AM, Rames A, Lancrenon S, Lagrue G, Lazabemide in Smoking Cessation Study Investigators Lazabemide, a selective, reversible monoamine oxidase B inhibitor, as an aid to smoking cessation. Addiction. 2002;97:1347–1354. doi: 10.1046/j.1360-0443.2002.00258.x. [DOI] [PubMed] [Google Scholar]
- Berlin I, Said S, Spreux-Varoquaux O, Launay JM, Olivares R, Millet V, Lucrubier Y, Puech AJ. A reversible monoamine oxidase A inhibitor (moclobemide) facilitates smoking cessation and abstinence in heavy, dependent smokers. Clin Pharmacol Ther. 1995;58:444–452. doi: 10.1016/0009-9236(95)90058-6. [DOI] [PubMed] [Google Scholar]
- Biberman R, Neumann R, Gerber Y. A randomized controlled trial of oral selegiline plus nicotine skin patch compared with placebo plus nicotine skin patch for smoking cessation. Addiction. 2003;98:1403–1407. doi: 10.1046/j.1360-0443.2003.00524.x. [DOI] [PubMed] [Google Scholar]
- Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Survival probabilities (the Kaplan-Meier Method) BMJ; 1998. pp. 317–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–491. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- CDC. Annual smoking-attributable mortality, years of potential life lost, and economic costs---United States, 1998-2007. Morbidity and Mortality World Report (MMWR) 2009;58:221–226. [PubMed] [Google Scholar]
- Dickerson DL, Leeman RF, Mazure CM, O'Malley SS. The inclusion of women and minorities in smoking cessation clinical trials: A systematic review. Am J Addict. 2009;18:21–28. doi: 10.1080/10550490802408522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ. Treating Tobacco Use and Dependence: 2008 Update. U.S. Department of Health and Human Services; Rockville, MD: 2008. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. American Psychiatric Press; Washington, D.C.: 1997. [Google Scholar]
- Fowler JS, Logan J, Wang GJ, Volkow ND. Monoamine oxidase and cigarette smoking. NeuroToxicology. 2003;24:75–82. doi: 10.1016/s0161-813x(02)00109-2. [DOI] [PubMed] [Google Scholar]
- George TP, editor. Medication Treatments for Nicotine Dependence. Taylor & Francis; Boca Raton, Florida: 2006. [Google Scholar]
- George TP, O'Malley SS. Current pharmacological treatments for nicotine dependence. Trends Pharmacol Sci. 2004;25:42–48. doi: 10.1016/j.tips.2003.11.003. [DOI] [PubMed] [Google Scholar]
- George TP, Vessicchio JC, Termine A, Jatlow PI, Kosten TR, O'Malley SS. A preliminary placebo-controlled trial of selegiline hydrochloride for smoking cessation. Biol Psychiatry. 2003;53:136–143. doi: 10.1016/s0006-3223(02)01454-3. [DOI] [PubMed] [Google Scholar]
- George TP, Weinberger AH. Monoamine oxidase inhibition: Potential for developing tobacco pharmacotherapies. Clinical Pharmacology and Therapeutics. 2008;83:619–621. doi: 10.1038/sj.clpt.6100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman AH, Covey LS, Dalack GW, Stetner F, Rivelli SK, Fleiss J, Cooper TB. Smoking cessation, clonidine, and vulnerability to nicotine among dependent smokers. Clin Pharmacol Ther. 1993;54:670–679. doi: 10.1038/clpt.1993.205. [DOI] [PubMed] [Google Scholar]
- Grasing K, He S. Effects of high-dose selegiline on morphine reinforcement and precipitated withdrawal in dependent rats. Behav Pharmacol. 2005;16:1–13. doi: 10.1097/00008877-200502000-00001. [DOI] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, Stinus L. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci. 2005;25:8593–8600. doi: 10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, Stinus L. Monoamine oxidase A rather than monoamine oxidase B inhibition increases nicotine reinforcement in rats. Eur J Neurosci. 2006;24:3532–3540. doi: 10.1111/j.1460-9568.2006.05217.x. [DOI] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Koob GF, Cador M, Stinus L. Monoamine oxidase inhibition dramatically prolongs the duration of nicotine withdrawal-induced place aversion. Biol Psychiatry. 2008;63:158–163. doi: 10.1016/j.biopsych.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan M, VanNoord T, Greden JF. A high-performance liquid chromatographic method for routine simultaneous determination of nicotine and cotinine in plasma. Clin Chem. 1988;34:724–729. [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Houtsmuller EJ, Thornton JA, Stitzer ML. Effects of selegiline (L-deprenyl) during smoking and short-term abstinence. Psychopharmacology. 2002;163:213–220. doi: 10.1007/s00213-002-1152-9. [DOI] [PubMed] [Google Scholar]
- Hughes JR. The future of smoking cessation therapy in the United States. Addiction. 1996;91:1797–1802. doi: 10.1046/j.1360-0443.1996.911217974.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein D, Richmond RL, Swan GE. Measurement of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- Irvin JE, Brandon TH. The increasing recalcitrance of smokers in clinical trials. Nicotine Tob Res. 2000;2:79–84. doi: 10.1080/14622200050011330. [DOI] [PubMed] [Google Scholar]
- Irvin JE, Hendricks PS, Brandon TH. The increasing recalcitrance of smokers in clinical trials II: Pharmacotherapy trials. Nicotine & Tobacco Res. 2003;5:27–35. doi: 10.1080/1462220031000070534. [DOI] [PubMed] [Google Scholar]
- Levine J, Schooler NR. General versus specific inquiry with SAFTEE. J Clin Psychopharmacol. 1992;12:3–10. doi: 10.1097/00004714-199212000-00017. [DOI] [PubMed] [Google Scholar]
- Lewis A, Miller JH, Lea RA. Monoamine oxidase and tobacco dependence. NeuroToxicology. 2007;28:182–195. doi: 10.1016/j.neuro.2006.05.019. [DOI] [PubMed] [Google Scholar]
- MayoClinic. Smoke Free and Living It. Mayo Clinic; Rochester, MN: 2000. [Google Scholar]
- Perkins KA. Smoking cessation in women: Special considerations. CNS Drugs. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Stitzer M, Fonte CA, Briski JL, Scott JA, Chengappa KN. Development of procedures for early screening of smoking cessation medications in humans. Clin Pharmacol Ther. 2008a;84:216–221. doi: 10.1038/clpt.2008.30. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008b;10:1245–1251. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- Scharf D, Shiffman S. Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of bupropion SR. Addiction. 2004;99:1462–1269. doi: 10.1111/j.1360-0443.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- Schiffer WK, Azmoodeh M, Gerasimov M, Volkow ND, Fowler JS, Dewey SL. Selegiline potentiates cocaine-induced increases in rodent nucleus accumbens dopamine. Synapse. 2003;48:35–38. doi: 10.1002/syn.10183. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Wileyto EP, Pinto A, Leone F, Gariti P, Siegel S, Perkins KA, Dackis C, Heitjan DF, Berrettini W, Lerman C. A placebo-controlled trial of modafinil for nicotine dependence. Drug Alcohol Depend. 2008;98:86–93. doi: 10.1016/j.drugalcdep.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu ECK, Tyndale RF. Selegiline is a mechanism-based inactivator of CYP2A6 inhibiting nicotine metabolism in humans and mice. J Pharmacol Exp Ther. 2008;324:992–999. doi: 10.1124/jpet.107.133900. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. Brit J Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Steinberg MB, Greenhaus S, Schmelzer AC, Bover MT, Foulds J, Hoover DR, Carson JL. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150:447–454. doi: 10.7326/0003-4819-150-7-200904070-00004. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Addiction. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Villegier AS, Lotfipour S, McQuown SC, Belluzzi JD, Leslie FM. Tranylcypromine enhancement of nicotine self-administration. Neuropharmacology. 2007;52:1415–1425. doi: 10.1016/j.neuropharm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Bardo MT. The monoamine oxidase inhibitor phenelzine enhances the discriminative stimulus effect of nicotine in rats. Behav Pharmacol. 2007;18:601–608. doi: 10.1097/FBP.0b013e3282eff0d5. [DOI] [PubMed] [Google Scholar]