Summary
Anchorage-dependence of cell growth is a key metastasis-suppression mechanism that is mediated by effects of integrins on growth signaling pathways [1]. The small GTPase RalA is activated in metastatic cancers through multiple mechanisms and specifically induces anchorage independence [2–4]. Loss of integrin-mediated adhesion triggers caveolin-dependent internalization of cholesterol- and sphingolipid- rich lipid raft microdomains to the recycling endosomes; these domains serve as platforms for many signaling pathways and their clearance from the plasma membrane (PM) after cell detachment suppresses growth signaling [5, 6]. Conversely, re-adhesion triggers their return to the PM and restores growth signaling. Activation of Arf6 by integrins mediates exit of raft markers from the RE but is not sufficient for return to the PM. We now show that RalA but not RalB mediates integrin-dependent membrane raft exocytosis through the exocyst complex. Constitutively active RalA restores membrane raft targeting to promote anchorage independent growth signaling. Ras-transformed pancreatic cancer cells also show RalA-dependent constitutive PM raft targeting. These results identify RalA as a key determinant of integrin-dependent membrane raft trafficking and regulation of growth signaling. They therefore define a mechanism by which RalA regulates anchorage dependence and provide a new link between integrin signaling and cancer.
Results and Discussion
Effect of Ral inhibition on cell spreading and lipid raft trafficking
When suspended cells are replated on surfaces coated with fibronectin (FN), return of rafts to the PM is required for cell spreading [6],[13]. To test the role of RalA in this process, we expressed the Ral-binding domains (RBDs) of two Ral effectors (Sec5, RLIP76) that sequester active Ral and inhibit its function [17–20]. We examined WT MEFs and, as a control, caveolin1−/− (Cav1−/−) MEFs. Since raft microdomains are not internalized after detachment in Cav1−/− MEFs [6], these cells do not require the exocytosis pathway [5]. Cells expressing these constructs (≥95% efficiency; supplementary figure 1B) were detached, held in suspension for 90 min and replated on FN. Both Sec5 and RLIP RBD inhibited spreading of WT cells and the return of GPI-linked proteins (commonly used as lipid raft markers) detected by binding of proaerolysin. By contrast, Cav1−/− cells were completely resistant (Figure. 1A, 1B, 1C). Spreading and exocytosis were, however, delayed rather than completely blocked (data not shown). The RBDs had no effect on raft endocytosis after detachment (figure 1B and supplementary figure 1A). These data suggest a role for Ral proteins in integrin-regulated raft exocytosis and cell spreading.
Figure 1. Ral inhibition delays cell spreading and raft exocytosis.
(A) WT and Cav1−/− MEFs expressing Myc-tagged Ral binding domains (RBDs) from Sec5 or RLIP were serum starved overnight, suspended for 90min and replated on FN (2 µg/ml) for 30min. Cells were fixed and stained with phalloidin-Alexa 488 and images taken. Graph: represents spread surface area/100; values are mean ± SE in µm2. (n=95 WT MEFs, n=70 Cav1−/− MEFs). Three experiments gave similar results. Suspended and re-adherent (B) WT and (C) Cav1−/− MEFs were incubated on ice with purified proaerolysin for 30min then rinsed. Bound proaerolysin was detected in cell lysate by Western blotting (WB : Aero) using tubulin as loading control (WB : Tub). RBD constructs were detected by western blotting for myc (WB: Myc). Values listed under the aerolysin blot represent band intensity normalized to tubulin relative to suspended control cells in each cell type. Blots are representative of three independent experiments. **** =p<0.00001 to * =p<0.01.
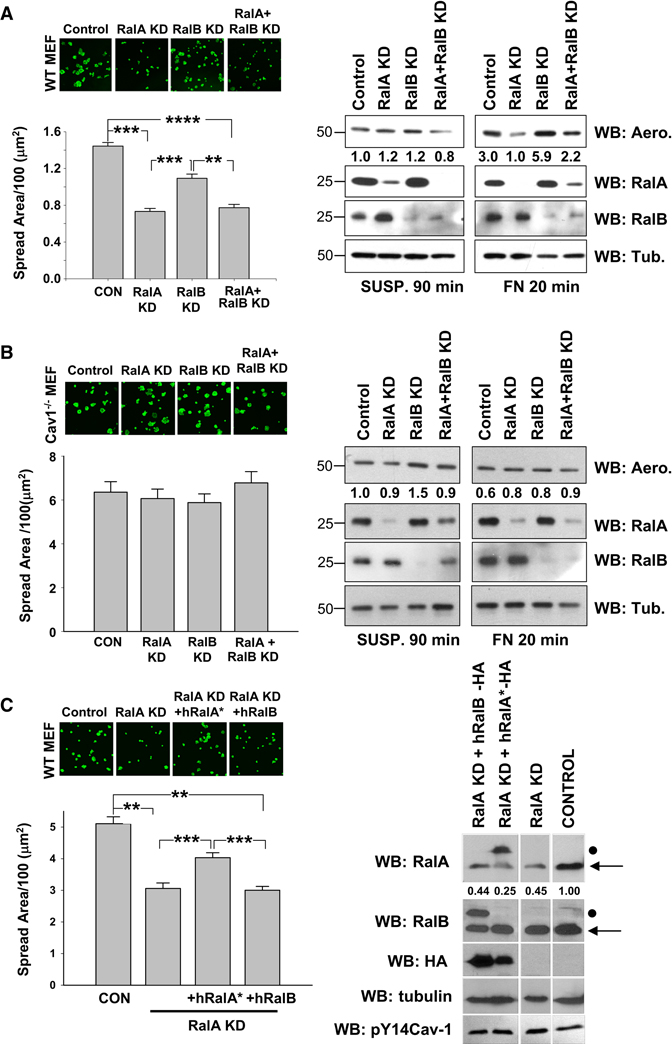
Knockdown of RalA and RalB
Next, cells were transfected with specific siRNAs for RalA and RalB. Loss of RalA (≥ 90%), but not RalB (≥ 90%), significantly delayed cell spreading and return of GPI linked proteins to the cell membrane in re-adherent WT MEFs (Figure 2A), Cav1−/− MEFs were again unaffected (Figure 2B). Loss of RalA delayed rather than completely blocked cell spreading (supplementary figure 2a), as previously observed for Arf6 inhibitors [13]. Reconstitution of Cav1−/− MEFs with WT Cav1, but not Y14F Cav1, restored membrane raft endocytosis [5] and sensitivity to RalA siRNA (supplementary figure 2B, 2C). Earlier studies reported interdependence between RalA and RalB such that loss of both restored function compared to loss of single isoforms [21, 22]. However, loss of RalA plus RalB inhibited cell spreading and membrane raft localization similarly to loss of RalA alone (figure 2A). Neither knockdown affected membrane raft endocytosis after cell detachment (supplementary figure 2D). Re-expression of siRNA-resistant hRalA* but not hRalB (supplementary figure 2E) restored spreading of RalA knockdown cells (Figure 2C). Thus, RalA, but not RalB, is required for adhesion-dependent raft membrane targeting and cell spreading.
Figure 2. Effects of Ral knockdown on cell spreading and surface rafts.
(A) WT MEFs and (B) Cav1−/− MEFs were transfected with RalA siRNA (RalA KD), RalB siRNA (RalB KD), both (RalA+ RalB KD) or control siRNA (CON). Cells were detached, held in suspension for 90min and replated on FN (2 µg/ml) for 20min. Cells were fixed and stained with phalloidin-Alexa 488 and images taken. Graph: represents spread surface area/100; values are mean ± SE in µm2. (>100 cells were analysed per experiment, 3 independent experiments gave similar results). Surface binding of proaerolysin was measured as in Fig 1 (WB : Aero) using tubulin (WB : Tub) as loading control (right panel). Values represent band intensity normalized to tubulin relative to control 90min suspended cell band intensity. RalA and RalB expression were detected by western blotting (WB : RalA, RalB) (C) RalA-depleted WT MEFs were transfected with vectors for HA tagged siRNA-resistant human RalA (hRalA*) or human RalB. Cells were treated as above and spreading quantitated. Graph: spread surface area/100; values are mean ± SE in µm2. (n=128 cells). Two independent experiments gave similar results. Quantified values represent endogenous RalA (←) band intensity normalized to tubulin. Mutant siRNA-resistant hRalA and RalB are indicated by •. **** =p<0.00001 to * =p<0.01.
Activation of RalA and RalB
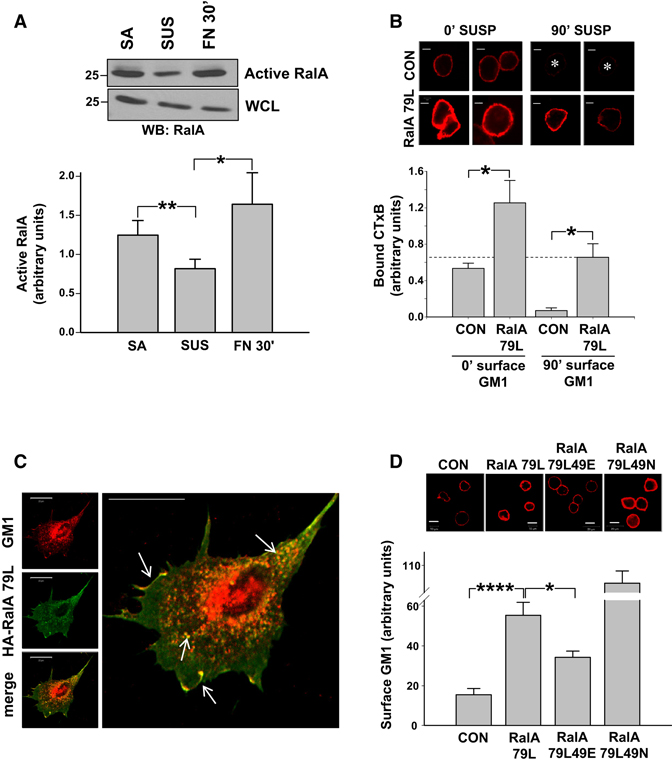
Next, we measured the effect of cell adhesion to FN on Ral activation using pull down assays. RalA activity decreased by about 40% after detachment and recovered completely on re-adhesion (Figure 3A), whereas RalB activity was unaffected (supplementary Figure 3A). Thus, rapid and specific adhesion-dependent activation of RalA correlates with its stimulation of raft exocytosis.
Figure 3. Adhesion-dependent RalA activation promotes raft plasma membrane localization.
(A) WT MEFs stably adherent (SA), suspended for 90min (SUS), or replated on FN (10 µg/ml) for 30min (FN30’) were assayed for active RalA using GST-Sec5 RBD beads and Western blotting for RalA (WB: RalA). Graph: Active RalA bands were quantified and normalized to WCL. Values are means ± SE, n=5. (** =p<0.01, * =p<0.05) (B) Untransfected (CON) and HA-RalA79L-transfected WT MEFs were serum starved overnight, detached, held in suspension for 0min or 90min, and surface labeled with CTxB-Alexa 594 (GM1-CTxB). Cells were then fixed and images taken. Graph: Values are mean surface intensity ± SE, n=10, representative of two independent experiments. *p < 0.01. Scale bar represents 5 µm. (C) HA tagged RalA79L-expressing WT MEFs surface labeled with CTxB-Alexa594 were suspended and replated on FN (2 µg/ml) for 30min. Cells were fixed and stained for HA. Arrows indicated overlap in vesicles and membrane ruffles. Scale bar represents 20 µm. (D) Untransfected (CON) MEFs and MEFs expressing HA-RalA79L, RalA79L 49E or RalA79L 49N were serum starved overnight, detached and held in suspension for 90min. Cells were then surface labeled with CTxB-Alexa 594 (GM1-CTxB), fixed and images taken. Graph: Values are mean surface intensity ± SE, n=14, representative of two independent experiments. **** =p<0.00001 to * =p<0.01. Scale bar represents 10 µm.
Active RalA Promotes Raft Exocytosis in Nonadherent Cells
We next examined the effects of constitutively active RalA on localization of lipid raft components in non-adherent cells. Activated, fast-cycling RalA 79L expressed at ≥ 95% transfection efficiency (supplementary figure 3B) somewhat increased surface GM1 levels immediately after detachment (≈ 2.6 fold at 0min in suspension) (Figure 3B) or when adherent (data not shown). Strikingly, after 90min in suspension, controls cells showed nearly complete loss of surface raft components whereas RalA 79L expressing cells showed only a ≈2-fold decrease, retaining ≈10-fold higher levels than control cells (Figure 3B). Total GM1 and GPI linked protein levels were unaffected by RalA 79L (supplementary figure 3C). Surface rafts in suspended RalA 79L-expressing cells were actively endocytosed but a smaller fraction (≅ 38%) of the total labeled GM1 was perinuclear in nonadherent cells compared to untransfected cells (≅ 58%) (supplementary figure 3D), supporting increased exocytosis as the cause for elevated surface raft levels. GTPase-deficient active RalA V23 also supported membrane raft exposure in non-adherent cells, whereas RalB V23 did not (data not shown).
Active RalA-expressing WT MEFs replated on FN showed enhanced localization of GM1-labeled vesicles to the cell periphery at early times (supplementary figure 3D) and spread faster (data not shown) than control cells. In cells pre-labeled with CTxB, RalA 79L strongly co-localized with the lipid raft marker in distinct intracellular vesicles and lamellipodia during respreading (Figure 3C) (Pearson’s coefficient = 0.89 ± 0.015). Taken together, these data support the idea that activated RalA triggers exocytosis of raft microdomains.
RalA effector site mutants
Active RalA regulates exocyst assembly to drive exocytic vesicles to the PM [23]. As a first test for involvement of the exocyst, we compared effects of RalA79L to its 49E mutant that does not bind the exocyst, and the 49N mutant that binds exocyst subunits but does not bind other effectors. Whereas 49N increased surface GM1 levels in suspended cells better than the parent activated RalA79L, 49E was significantly less effective (Figure 3D). All constructs were expressed at comparable levels (supplementary figure 3E). Surface GM1 levels were unchanged in Cav1−/− MEFs (data not shown). Thus, the promotion of membrane rafts exocytosis by active RalA 79L correlates with its exocyst interaction.
Role of the exocyst in membrane raft targeting
We then examined Sec5, an exocyst component and RalA effector that localizes to the PM and is required for exocyst function [20, 23–25]. Knockdown of Sec5 in WT MEFs delayed cell spreading on FN (Figure 4A) and decreased surface GPI levels (Figure 4B), whereas Cav1−/− MEFs were unaffected (supplementary figure 4A, 4B). Next, Sec5-depleted cells were reconstituted with WT Sec5 or the T11A mutant that does not bind RalA [25, 26]. WT but not T11A Sec5 restored cell spreading and raft exocytosis after replating of knockdown cells (Figures 4C and 4D). Sec5 knockdown also disrupted PM targeting of raft microdomains in suspended cells by active RalA 79L (Supplementary Figure 4C). During replating, Sec5-GFP co-localized with surface rafts (GM1) at PM ruffles (Figure 4E), unlike a GFP control (Supplementary Figure 4D). Together, these results strongly implicate the RalA-exocyst complex in adhesion-dependent membrane targeting of raft microdomains.
Figure 4. Sec5 and RalA Regulate Membrane Raft Targeting and Signaling.
(A) WT MEFs transfected with control siRNA, Sec5 siRNA oligo #1 (Sec5-1) or # 2 (Sec5-2) were suspended for 90 min and replated on FN (2 µg/ml) for 20min. Cells were fixed and stained with phalloidin-Alexa 488. Graph: spread surface area/100; values are mean ± SE in µm2 (n=75 cells), representative of three independent experiments. (B) Re-adherent control and Sec5 siRNA oligo # 2 treated cells (Sec5-2) were incubated on ice with purified proaerolysin for 30min. Bound proaerolysin was detected in cell lysates by Western blotting (WB:Aero). Sec5, RalA and RalB expression was confirmed by western blotting (WB). Values represent band intensity normalized to RalB levels relative to control (C) WT MEFs expressing Sec5 siRNA oligo #2 (Sec5 KD) were transfected with Myc- WT Sec5 (WT Sec5) or Myc -T11A Sec5 (T11A Sec5). Cells were suspended for 90 min, replated on FN (2 µg/ml) for 20min, fixed and stained with phalloidin-Alexa 488. Graph: spread surface area/100; values are mean ± SE in µm2 (n=65 cells), representative of three independent experiments. (D) Re-adherent cells were incubated on ice with purified proaerolysin for 30min. Bound proaerolysin detected in cell lysate by Western blotting (WB : Aero) using tubulin (WB : tub.) as a loading control. Graph: Aerolysin normalized to tubulin, values are mean ± S.E from three independent experiments. (A–D) ****p < 0.00001; ***p < 0.0001. (E) WT Sec5-GFP (Sec5-GFP) expressing WT MEFs were suspended for 90min, and replated on FN (2 µg/ml) for 20min. Cells were chilled, stained with CTxB-Alexa 594 (Surface GM1), and fixed. (F–G) WT MEFs untransfected (CON) or transfected with HA-RalA79L (RalA 79L) were kept suspended or adherent for 8hours without serum then treated with 10% serum for 30min. Cells were lysed and Western blotted for (F) active (pSer473 Akt) and total Akt (Akt) (G) Phospho-p44/p42 and total Erk (Erk). Phosphorylated Akt and Erk were normalized to total protein. Graph: Values are means ± SE, n=4 (pAkt) and n=3 (pErk). (H) MiaPaCa2 cells (CON) in which RalA (RalAi) and RalB (RalBi) were stably depleted were analyzed by western blotting (WB) for the indicated proteins. (I) Control (CON), RalA (RalAi) and RalB (RalBi) knockdown MiaPaCa2 cells were maintained adherent or suspended for 4hours with serum. Cells were chilled, and proaerolysin surface labeling measured as in Fig 1., Bands were quantified and normalized to tubulin; Graph shows proaerolysin binding in suspended cells relative to adherent cells for each condition. Values are means ± SE, n=3. (F–I) **p < 0.01; *p < 0.05.
Active RalA induces anchorage independent signaling
Integrin-mediated membrane targeting of raft microdomains regulates anchorage-dependent PI3-kinase/Akt and Ras/Erk signaling [6][5]. Thus, active RalA should increase the activity of these pathways in suspended cells. In cells treated with serum for 30 min, active RalA had no effect on Erk or Akt in adherent cells, but substantially increased Akt (≈ 3.8 fold) and Erk (≈ 2.8 fold) activation in suspended cells relative to controls (Figure 4F,G). Thus, surface rafts correlate with Erk and Akt activation.
K-Ras activates RalA, which promotes anchorage-independent growth in pancreatic and other cancers [4], including human pancreatic MiaPaCa2 cells [3]. Knock down of RalA but not RalB in these cells reduced surface GPI linked protein levels in suspended cells (Figure 4H,I). These knockdowns did not affect Cav1, pY14Cav1, Arf6 levels (Figure 4H) or GM1 endocytosis (supplementary figure 4E). These results support the role of RalA in controlling membrane raft exocytosis and their PM localization to promote anchorage independence in human cancer cells.
Conclusions
Previous studies showed that upon detachment of anchorage-dependent cells from the substratum, membrane rafts are endocytosed through caveolae and transported to the recycling endosome [13]. Upon replating, domains are exocytosed by a caveolin-independent, Arf6- and microtubule-dependent pathway that promotes cell spreading. Adhesion-dependent activation of Arf6, while necessary, is not sufficient to deliver raft microdomains to the PM. Thus, there must be an additional adhesion-dependent step.
We now show that adhesion-dependent activation of RalA is also required for exocytosis. Inhibiting RalA function (Sec5 RBD, RLIP RBD, siRNA) significantly delayed cell spreading and PM delivery of raft components in WT MEFs. Expression of active RalA promoted PM raft localization in non-adherent cells. RalB was neither activated by adhesion nor affected spreading nor raft exocytosis. Thus, the pathway is specifically mediated by RalA. Differential activation of RalA and RalB during cytokinesis by isoform-specific RalGEFs has also been described [27]. Integrins may therefore activate a RalA-specific GEF.
The exocyst subunit Sec5 is required for cell spreading and raft exocytosis after replating or after RalA activation in suspended cells. Sec5 binding to RalA is required since Ral-binding deficient Sec5 T11A did not restore cell spreading after Sec5 knockdown. RalA was reported to bind Sec5 better than RalB and to localize to the exocyst during cytokinesis [27]. We also observed localization of Sec5 with RalA and raft markers at the PM during cell spreading. These findings strongly suggest that RalA mediates adhesion-dependent exocytic raft trafficking through the exocyst.
Active RalA-mediated PM localization of raft microdomains in suspended cells promotes anchorage-independent growth signaling (Erk, Akt activation). RalA can be activated in cancer by over-expression, oncogenic Ras, Aurora kinase, loss of tumor suppressors (eg. merlin) or viral oncogenes [2–4, 10,11]. These data therefore reveal a mechanism by which activated RalA can promote anchorage-independence. Further work will be required to determine how integrin-mediated adhesion activates RalA, to characterize the molecular determinants of the exocytosis pathway, and further understand the role of this pathway in human cancer.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by USPHS grant RO1 GM47214 to MAS and training grant 5T32-HL007284 to NB. MW was supported by RO1 CA71443 and the Robert Welch Foundation. We thank Chunming Zhu for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schwartz MA, Assoian RK. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci. 2001;114:2553–2560. doi: 10.1242/jcs.114.14.2553. [DOI] [PubMed] [Google Scholar]
- 2.Ryu CH, Kim SW, Lee KH, Lee JY, Kim H, Lee WK, Choi BH, Lim Y, Kim YH, Hwang TK, et al. The merlin tumor suppressor interacts with Ral guanine nucleotide dissociation stimulator and inhibits its activity. Oncogene. 2005;24:5355–5364. doi: 10.1038/sj.onc.1208633. [DOI] [PubMed] [Google Scholar]
- 3.Lim KH, O'Hayer K, Adam SJ, Kendall SD, Campbell PM, Der CJ, Counter CM. Divergent roles for RalA and RalB in malignant growth of human pancreatic carcinoma cells. Curr Biol. 2006;16:2385–2394. doi: 10.1016/j.cub.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Lim KH, Baines AT, Fiordalisi JJ, Shipitsin M, Feig LA, Cox AD, Der CJ, Counter CM. Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell. 2005;7:533–545. doi: 10.1016/j.ccr.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 5.del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WB, Grande-Garcia A, Anderson RG, Schwartz MA. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.del Pozo MA, Alderson NB, Kiosses WB, Chiang H-H, Anderson RGW, Schwartz MA. Integrins Regulate Rac Targeting by Internalization of Membrane Domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- 7.Weber GF. Molecular mechanisms of metastasis. Cancer Lett. 2008;270:181–190. doi: 10.1016/j.canlet.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz MA. Integrins, oncogenes, and anchorage independence. J Cell Biol. 1997;139:575–578. doi: 10.1083/jcb.139.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SC, Oxford G, Baras AS, Owens C, Havaleshko D, Brautigan DL, Safo MK, Theodorescu D. Expression of ral GTPases, their effectors, and activators in human bladder cancer. Clin Cancer Res. 2007;13:3803–3813. doi: 10.1158/1078-0432.CCR-06-2419. [DOI] [PubMed] [Google Scholar]
- 10.Wu JC, Chen TY, Yu CT, Tsai SJ, Hsu JM, Tang MJ, Chou CK, Lin WJ, Yuan CJ, Huang CY. Identification of V23RalA-Ser194 as a critical mediator for Aurora-A-induced cellular motility and transformation by small pool expression screening. J Biol Chem. 2005;280:9013–9022. doi: 10.1074/jbc.M411068200. [DOI] [PubMed] [Google Scholar]
- 11.Sablina AA, Chen W, Arroyo JD, Corral L, Hector M, Bulmer SE, DeCaprio JA, Hahn WC. The tumor suppressor PP2A Abeta regulates the RalA GTPase. Cell. 2007;129:969–982. doi: 10.1016/j.cell.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaus K, Le Lay S, Balasubramanian N, Schwartz MA. Integrin-mediated adhesion regulates membrane order. J Cell Biol. 2006;174:725–734. doi: 10.1083/jcb.200603034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balasubramanian N, Scott DW, Castle JD, Casanova JE, Schwartz MA. Arf6 and microtubules in adhesion-dependent trafficking of lipid rafts. Nat Cell Biol. 2007;9:1381–1391. doi: 10.1038/ncb1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mostov KE, Verges M, Altschuler Y. Membrane traffic in polarized epithelial cells. Curr Opin Cell Biol. 2000;12:483–490. doi: 10.1016/s0955-0674(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 15.Wu H, Rossi G, Brennwald P. The ghost in the machine: small GTPases as spatial regulators of exocytosis. Trends Cell Biol. 2008;18:397–404. doi: 10.1016/j.tcb.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodemann BO, White MA. Ral GTPases and cancer: linchpin support of the tumorigenic platform. Nat Rev Cancer. 2008;8:133–140. doi: 10.1038/nrc2296. [DOI] [PubMed] [Google Scholar]
- 17.Jullien-Flores V, Mahe Y, Mirey G, Leprince C, Meunier-Bisceuil B, Sorkin A, Camonis JH. RLIP76, an effector of the GTPase Ral, interacts with the AP2 complex: involvement of the Ral pathway in receptor endocytosis. J Cell Sci. 2000;113(Pt 16):2837–2844. doi: 10.1242/jcs.113.16.2837. [DOI] [PubMed] [Google Scholar]
- 18.Moskalenko S, Henry DO, Rosse C, Mirey G, Camonis JH, White MA. The exocyst is a Ral effector complex. Nat Cell Biol. 2002;4:66–72. doi: 10.1038/ncb728. [DOI] [PubMed] [Google Scholar]
- 19.Rosario M, Paterson HF, Marshall CJ. Activation of the Ral and Phosphatidylinositol 3' Kinase Signaling Pathways by the Ras-Related Protein TC21 10.1128/MCB.21.11.3750-3762.2001. Mol. Cell. Biol. 2001;21:3750–3762. doi: 10.1128/MCB.21.11.3750-3762.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugihara K, Asano S, Tanaka K, Iwamatsu A, Okawa K, Ohta Y. The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nat Cell Biol. 2002;4:73–78. doi: 10.1038/ncb720. [DOI] [PubMed] [Google Scholar]
- 21.Camonis JH, White MA. Ral GTPases: corrupting the exocyst in cancer cells. Trends Cell Biol. 2005;15:327–332. doi: 10.1016/j.tcb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Oxford G, Owens CR, Titus BJ, Foreman TL, Herlevsen MC, Smith SC, Theodorescu D. RalA and RalB: Antagonistic relatives in cancer cell migration. Cancer Research. 2005;65:7111–7120. doi: 10.1158/0008-5472.CAN-04-1957. [DOI] [PubMed] [Google Scholar]
- 23.Moskalenko S, Tong C, Rosse C, Mirey G, Formstecher E, Daviet L, Camonis J, White MA. Ral GTPases regulate exocyst assembly through dual subunit interactions. J Biol Chem. 2003;278:51743–51748. doi: 10.1074/jbc.M308702200. [DOI] [PubMed] [Google Scholar]
- 24.Hsu SC, TerBush D, Abraham M, Guo W. The exocyst complex in polarized exocytosis. Int Rev Cytol. 2004;233:243–265. doi: 10.1016/S0074-7696(04)33006-8. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Li G, Sugita S. RalA-exocyst interaction mediates GTP-dependent exocytosis. J Biol Chem. 2004;279:19875–19881. doi: 10.1074/jbc.M400522200. [DOI] [PubMed] [Google Scholar]
- 26.Fukai S, Matern HT, Jagath JR, Scheller RH, Brunger AT. Structural basis of the interaction between RalA and Sec5, a subunit of the sec6/8 complex. Embo J. 2003;22:3267–3278. doi: 10.1093/emboj/cdg329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cascone I, Selimoglu R, Ozdemir C, Del Nery E, Yeaman C, White M, Camonis J. Distinct roles of RalA and RalB in the progression of cytokinesis are supported by distinct RalGEFs. EMBO J. 2008;27:2375–2387. doi: 10.1038/emboj.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.