Abstract
Background & Aims
Di-α hydroxy bile salt, sodium chenodeoxycholate (CDC), and bile acid (BA) binding have unclear effects on colonic transit in health and disease.
Methods
We performed 2 randomized, double-blind, placebo-controlled studies. In healthy volunteers (20/group), we evaluated effects of oral placebo, 500 mg, or 1000 mg of CDC (delayed-release, each given for 4 days) on gastrointestinal and colonic transit. A second trial compared the effects of colesevelam (1.875g, twice daily) vs placebo in 24 patients (12/group) with diarrhea-predominant irritable bowel syndrome (IBS-D) on transit, daily bowel frequency and consistency, and colonic mucosal permeability. Serum fasting 7αC4 was measured to screen for BA malabsorption. Effects of treatments on transit were compared using analysis of covariance with body mass index (BMI) and 7αC4 as covariates.
Results
In healthy volunteers, CDC significantly accelerated colonic transit (at 24h and 48h, p=0.01 and p<0.0001, respectively), increased stool frequency, ease of passage (both p<0.001), and evacuation (p=0.02), and decreased stool consistency (p<0.001). Four of the 24 IBS-D patients had increased serum 7αC4. In IBS-D, colesevelam modestly affected overall colonic transit (24h, p=0.22). Emptying of the ascending colon took an average 4 hours longer in patients given colesevelam compared to placebo; treatment effect was associated with baseline serum 7αC4 (p=0.0025). Colesevelam was associated with greater ease of stool passage (p=0.048) and somewhat firmer stool consistency (p=0.12). No effects on mucosal permeability or safety were identified.
Conclusions
Sodium chenodeoxycholate in health and colesevelam in IBS-D patients have opposite effects on colonic transit and fecal parameters.
Keywords: chenodeoxycholate; colesevelam, permeability; 7αC4; FGF-19, malabsorption
INTRODUCTION
Bile acids have a variety of physiologic functions, and are actively reabsorbed (up to 95%) in the terminal ileum [1]. Disruption of the enterohepatic circulation of bile acids due to ileal disease (e.g., Crohn’s or radiation ileitis) or idiopathic bile acid malabsorption (BAM) causes chronic diarrhea (see systematic summary [2]). Conjugated and non-conjugated bile acids induce secretion in the human colon [3,4] by one or more mechanisms: activating intracellular secretory mechanisms (e.g. adenylate cyclase [5]), increasing mucosal permeability [6], and inhibiting apical Cl-/OH- exchange [7]). In addition, bile acids may induce propulsive contractions in human colon (1mM, [8] or 5mM [9]). In humans, there is a relationship between the fecal bile acid excretion and colonic motility; however, this relationship is complicated by associated steatorrhea [9] since the delivery of fatty acids to the colon may also accelerate colonic transit [10]. Bile acid concentration in stool of patients with ileal resection may reach 21mM chenodeoxycholate (CDC) [11]. However, fecal concentrations of bile acids in diarrhea-predominant irritable bowel syndrome (IBS-D) or functional diarrhea are unknown. While earlier studies suggested up-regulation of the ileal active transporter [12] as a result of chronic loss of bile acids (which may reduce the bile acids reaching the colon), other data suggest increased delivery to the colon may occur if the ileal reabsorptive capacity for bile acids is exceeded. In studies of gallstone dissolution with exogenous chenodeoxycholic acid, diarrhea occurred in 40% of patients [13].
The cause of idiopathic BAM is unknown. Walters et al. recently reported reduced plasma levels of fibroblast growth factor (FGF)-19 which is produced by enterocytes [14] in the ileum in response to bile acid absorption and regulates hepatic bile acid synthesis. Thus, it was proposed that reduced FGF-19 (for which the cause is unknown) resulted in increased bile acid synthesis. BAM may contribute to the development of chronic diarrhea or IBS-D, and bile acid binding relieves diarrhea in the setting of BAM (literature systematically analyzed in [2]). Although compliance with cholestyramine (which is prepared as gritty particles) is poor, the bile acid binding agent, colesevelam hydrochloride (prepared in tablet form), is better tolerated, has fewer gastrointestinal side effects and is easily administered [15].
Our aims were: first, to measure the effects of ileocolonic delivery of two doses of sodium CDC on colonic transit and bowel function in healthy subjects; second, to measure fasting serum 7alpha-hydroxy-4-cholesten-3-one (7αC4, an indirect measure of bile acid synthesis), FGF19 which regulates hepatic bile acid synthesis and colonic mucosal permeability in unselected patients with IBS-D; and third, to measure the effects of the bile acid binder, colesevelam hydrochloride, on gastrointestinal and colonic transit, bowel function, and colonic permeability in IBS-D.
METHODS
Study Design, Randomization, Medication, and Measurements
We conducted two double-blind, placebo-controlled, parallel-group, randomized studies evaluating the effects of oral sodium CDC or placebo, once daily for 4 days, in healthy volunteers. In a second trial, oral colesevelam hydrochloride or placebo were administered for 12–14 days in patients with IBS-D. The study was approved by Mayo Clinic Institutional Review Board and all participants signed informed consent. The trial in patients is listed in ClinicTrials.gov (http://www.clinicaltrials.gov/ct2/show/NCT00911612?term=welchol&rank=2). In both studies, allocation was concealed from the study investigators.
In the first trial, participants were randomized to placebo, CDC 500mg, or 1000mg (20 per group). Sodium CDC (unconjugated) was purchased from Calbiochem, EMD Biosciences Inc., San Diego, CA 92121, and the Mayo pharmacy prepared identical placebo and CDC capsules, all of which were coated with the pH sensitive polymer, methacrylate (Eudragit S-100, Rohm Pharmaceuticals, Darmstadt D-64293, Germany). The latter dissolves at the neutral pH found in the distal ileum and was used to ensure ileocolonic delivery of the CDC or placebo. All participants were screened for BAM with fasting serum 7αC4.
In the second trial, we measured fasting serum 7αC4 and FGF-19 and baseline colonic mucosal permeability in patients with IBS-D, who were then randomized to colesevelam or placebo (1:1). The drug was purchased as Welchol ® (Colesevelam HCl) from Daiichi Sankyo Co. Ltd., Parsippany, NJ as 625mg tablets. The tablets were prepared as 312.5mg capsules with matching placebo by Mayo Research Pharmacy.
The selected doses for CDC and colesevelam were respectively based on the observations of diarrhea demonstrated in the gallstone dissolution trials [13] and on the FDA approved doses in the treatment of hypercholesteremia [Welchol® (Colesevelam HCL)] http://www.welchol.com/pdf/Welchol_PI.pdf)
An independent Mayo Clinic statistician generated the randomization codes. Mayo Research Pharmacy maintained the randomization schedule in case of emergency. All clinical and laboratory study personnel were blinded throughout the study until all data were locked and analyzed. Clinical safety monitoring was conducted by the study investigators throughout the study.
Participants
All participants had fasting plasma 7α-C4 (C4) measured to assess for underlying bile acid malabsorption and had serum FGF-19 measured. Participants also completed the following questionnaires: Hospital Anxiety and Depression (HAD) Scale [16], SCL-90 (somatization) [17], and bowel function by validated daily diaries including Bristol Stool Form Scale (BSFS) scores [18].
Effect of Sodium Chenodeoxycholate in Healthy Subjects
We enrolled 60 patients (mean age 38.7 years, 43 female) to the study. Three groups (n=20 each) were randomized to one oral capsule daily containing placebo, 500mg or 1000mg CDC, each for a period of 4 days. Gastrointestinal (GI) and colonic transit was conducted by a scintigraphic method [19] during the last 48 hours of drug ingestion.
Effect of Colesevelam Hydrochloride in Patients with IBS-D
We enrolled 24 patients (all female, mean age 42.7 years) to the study. Participants with a prior cholecystectomy (total 6 enrolled) were eligible to participate if the history of IBS-D anteceded the surgery and the patients reported no aggravation of IBS symptoms by the surgery. Two groups were randomized, balanced on BMI (<25 or ≥25kg/m2), to placebo or colesevelam, 1.875g twice daily, for 12–14days. Gastrointestinal and colonic transit by a scintigraphic method [19] and colonic permeability were conducted at baseline and during the last 48 hours of the study.
In these studies, we examined stool diaries [18], gastrointestinal transit [19–23], serum 7α-C4 [22], serum FGF-19, and colonic mucosal permeability [24]. Details of these tests and statistical considerations are in the Appendix.
RESULTS
Participants, Study Conduct and Completion
All medical records were screened for major exclusion criteria (i.e. prior gastrointestinal surgery and concomitant medications). Patients’ responses to bowel disease questionnaire, HAD scores and SCL-90 also excluded the presence of significant gastrointestinal symptoms in the healthy volunteers or anxiety, depression, poor quality of life or psychopathology that could act as confounders in our assessment of the effects of the administered CDC or colesevelam.
Effect of Sodium Chenodeoxycholate
Eighty-five volunteers were recruited for the study through advertisements and mail notifications (Appendix Figure 1). Twenty-five were ineligible based on this initial screen. Of those eligible to participate, all 60 fulfilled the inclusion/exclusion criteria, consented, and were randomized. Demographic data of all participants randomized to CDC or placebo are shown in Figure 1; they had similar age and BMI.
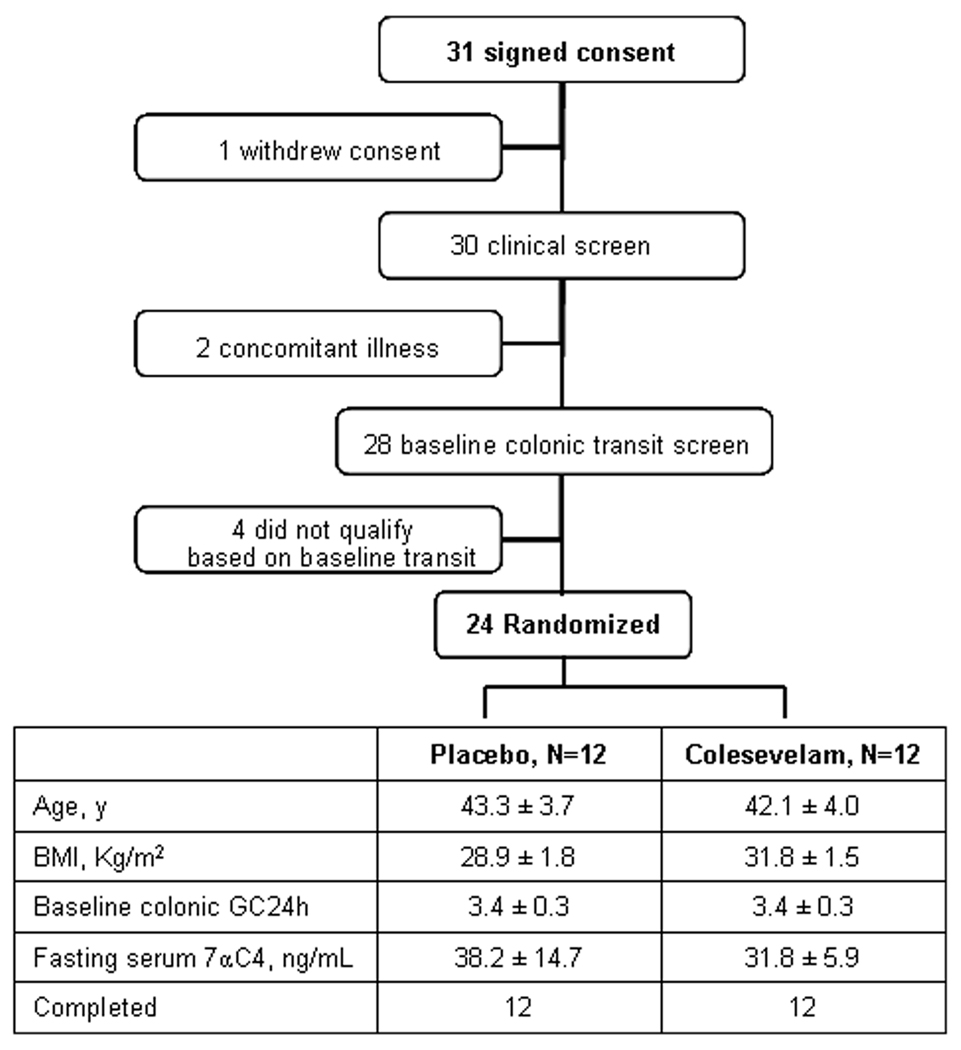
Figure 1.
Study flow chart and demographics of participants in colesevelam study
Effect of Colesevelam Hydrochloride
Thirty-one IBS-D patients were recruited and signed consent, and 24 were randomized and completed the study. One withdrew consent prior to the start of the study; 2 had concomitant illness and were advised not to participate by their primary physicians; and 4 did not qualify based on baseline transit eligibility criteria (GC 24h <2.3) (Figure 1). Demographic data of all randomized patients are shown in Figure 1.
All 60 volunteers and 24 IBS-D patients randomized completed the studies, and there was 100% medication compliance (based on coordinator interview and pill count).
Serum 7αC4 and FGF-19 Measurements in Patients with IBS-D and Healthy Controls
In our laboratory, a value of <61ng/ml is established as the 95th percentile for fasting serum 7αC4 level in healthy volunteers [23]. Fifty-five (92%) out of the 60 healthy volunteers and 20 of the 24 IBS-D patients had normal fasting serum 7αC4 values. There were no clinically important differences in the fasting serum 7αC4 levels in the two treatment groups of IBS-D patients (Figure 1).
The FGF levels in 40 other healthy controls were 161.6 pg/mL (median, IQR 85.9–267.8) and in the 24 patients with IBS-D 105.6 pg/mL (median, IQR 55.8–240.0; p=0.23, Wilcoxon Rank Sum test). Two patients had serum FGF-19 levels below the 5th percentile in health of 36.9 pg/mL. Appendix Figures 1–3 provide plots of serum 7αC4, FGF19, and the correlation between these two measurements (Spearman rank correlation = −0.414, p=0.044).
Effect of Chenodeoxycholate on Gastrointestinal and Colonic Transit
Gastric and Small Bowel Transit
CDC did not significantly affect gastric emptying t1/2 and colonic filling at 6 hours (see Table IA).
Table I.
Effects of Bile Salt and Bile Salt Sequestrant on Gastrointestinal Transit and Bowel Function
| A. Effect of NaCDC in Health (mean ± SEM) | ||||
|---|---|---|---|---|
| Placebo N=20 |
CDC 500mg N=20 |
CDC 1000mg N=20 |
P | |
| GE t1/2 (min) | 122.8 ± 6.1 | 126.9 ± 5.3 | 143.0 ± 14.1 | |
| CF 6 (%) | 54.6 ± 6.8 | 49.6 ± 7.0 | 46.0 ± 7.1 | |
| GC 4 | 0.97 ± 0.24 | 0.87 ± 0.18 | 1.27 ± 0.33 | |
| GC 24 | 2.69 ± 0.24 | 2.80 ± 0.27 | 3.76 ± 0.30 | 0.01 |
| GC 48 | 3.76 ± 0.20 | 4.10 ± 0.21 | 4.92 ± 0.05 | <0.0001 |
| AC t1/2 (h) | 14.5 ± 1.7 | 12.1 ± 2.1 | 10.7 ± 1.9 | |
| Stool frequency per day | 1.09 ± 0.13 | 1.50 ± 0.18 | 2.01 ± 0.15 | <0.001 |
| Stool consistency by Bristol Stool Form Scale |
3.51 ± 0.16 | 4.29 ± 0.19 | 4.80 ± 0.15 | <0.0001 |
| Ease of passage (scale1–7) | 3.9 ± 0.03 | 4.1 ± 0.06 | 4.3 ± 0.06 | <0.0001 |
| B. Effects of Colesevelam on Transit, Bowel Function and Mucosal Permeability in Diarrhea-Predominant Irritable Bowel Syndrome (mean ± SEM) | |||
|---|---|---|---|
| Placebo N=12 |
Colesevelam N=12 |
P | |
| GE t1/2 (min) | 119.6 ± 7.69 | 156.1 ± 17.36 | 0.14 |
| CF 6 (%) | 64.5 ± 8.17 | 58.5 ± 8.72 | |
| GC 4 | 0.81 ± 0.19 | 0.42 ± 0.16 | |
| GC 24 | 3.30 ± 0.33 | 2.68 ± 0.32 | 0.18 |
| GC 48 | 4.47 ± 0.20 | 4.65 ± 0.13 | |
| AC t1/2 (h) | 14.9 ± 3.58 | 18.85 ± 2.88 | |
| Stool frequency per day | 2.25 ± 0.34 | 2.14 ± 0.31 | |
| Stool consistency by Bristol Stool Form Scale | 4.57 ± 0.35 | 3.78 ± 0.27 | 0.12 |
| Ease of passage (scale1–7) | 4.39 ± 0.11 | 4.18 ± 0.14 | 0.047 |
| Urinary excretion of mannitol 8–24 hr, mg | 45.8±8.8 | 64.3±13.3 | 0.28 |
| Urinary excretion of lactulose 8–24 hr, mg | 19.9±3.12 | 28.5±5.9 | 0.30 |
| L:M ratio 8–24 hr | 0.49±0.05 | 0.59±0.19 | 0.62 |
Overall Colonic Transit Time Assessed by Geometric Center
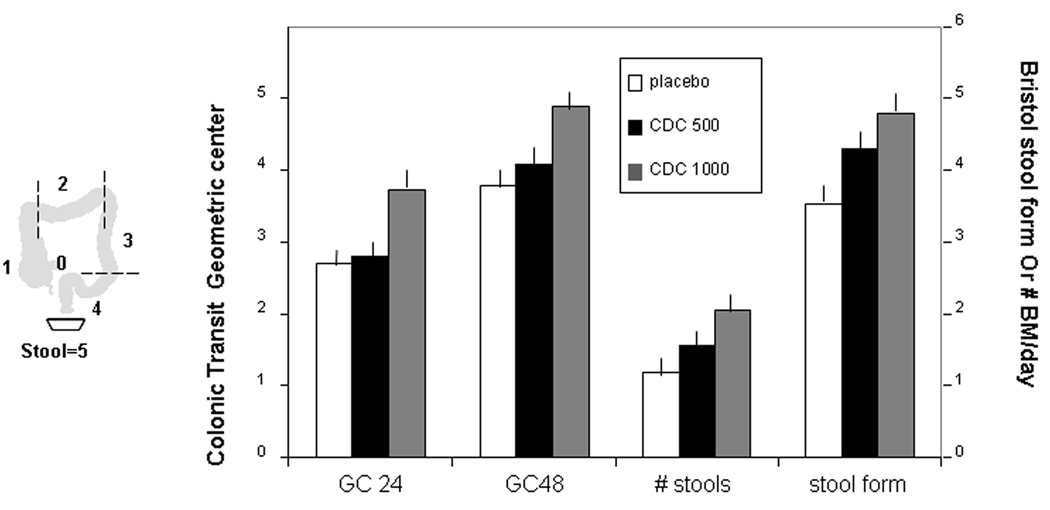
CDC accelerated overall colonic transit at 24 and 48 hours (ANCOVA p=0.01 and <0.0001 respectively), as detailed in the Appendix and illustrated in Figure 2. The effect of the 1000mg dose was significantly greater than the 500mg dose on the study primary endpoint, colonic GC at 24 hours (p<0.05).
Figure 2.
Effects of NaCDC on colonic transit (GC) at 24 and 48 hours, and stool form and frequency. Data show least square means ± SEM. Note that there is an overall acceleration of colonic transit, and this is significantly greater with the 1000 mg dose of Na CDC (p<0.0001 vs. placebo) relative to the 500 mg dose (p<0.01 vs. placebo), particularly evident with the transit data at 24 hours (overall p<0.05, 1000 mg vs. 500 mg). Note also that the acceleration of colonic transit is associated with an increase in stool frequency (p<0.001) and a more liquid consistency stool (p<0.0001).
Effect of Chenodeoxycholate on Bowel Function in Healthy Volunteers
There were also significant overall treatment effects of CDC (Table IA) on stool frequency, consistency, ease of passage (all p<0.001), and on sense of complete evacuation (p=0.02).
Effect of Colesevelam on Gastrointestinal and Colonic Transit in IBS-D
These data are summarized in Table IB and Figure 3.
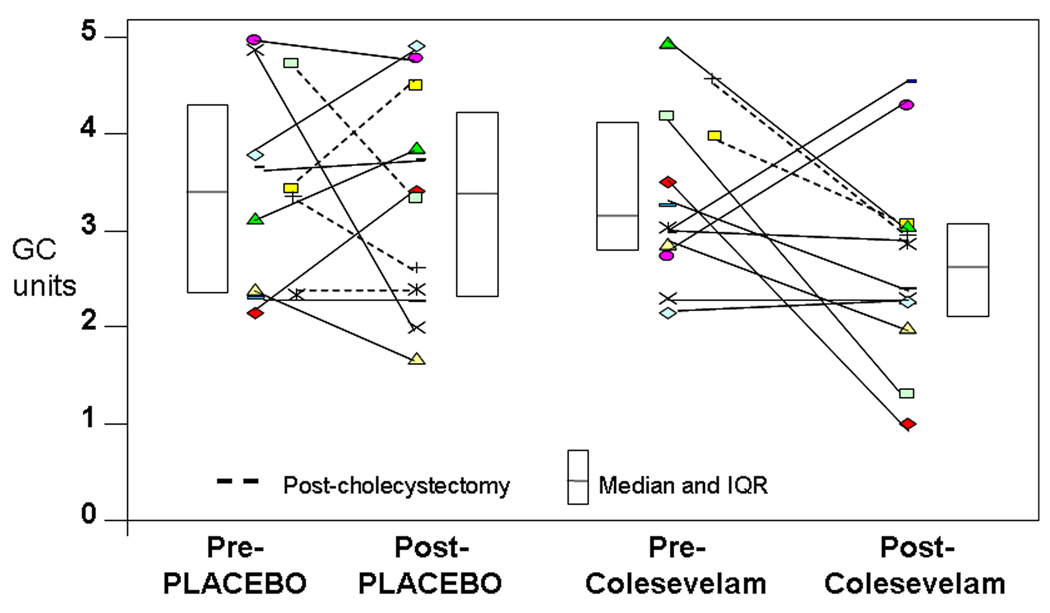
Figure 3.
Effects of placebo or colesevelam on overall colonic transit at 24 hours in individual patients. Note that 7/12 participants in the colesevelam arm had reduced colonic transit by >0.7. The lines link the transit results at baseline and post-treatment.
Gastric, Small Bowel and Overall Colonic Transit
Colesevelam moderately delayed gastric emptying (proportion emptied at 4h: p = 0.06; GE t1/2 p = 0.14) and did not have a significant effect on small bowel transit. There was a tendency for colesevelam to slow colonic transit at 24 hours compared with placebo treatment (ANCOVA using baseline GC24, BMI and serum 7αC4 as covariates; p=0.22). Seven of 12 in the colesevelam group and 4 of 12 in the placebo group demonstrated a decrease of GC24 of at least 0.7 GC units. Appreciable retardation of colonic transit was observed at 6 and 8 hours (data not shown).
There were 4 patients in the placebo group and 2 in the colesevelam group who had prior cholecystectomy. Two of the 4 patients with prior cholecystectomy in the placebo group and the 2 in the colesevelam group had >0.7 GC unit slowing of colonic transit at 24 hours. Thus, cholecystectomy state did not appear to have a significant effect on the results (Figure 3).
Ascending Colonic Emptying
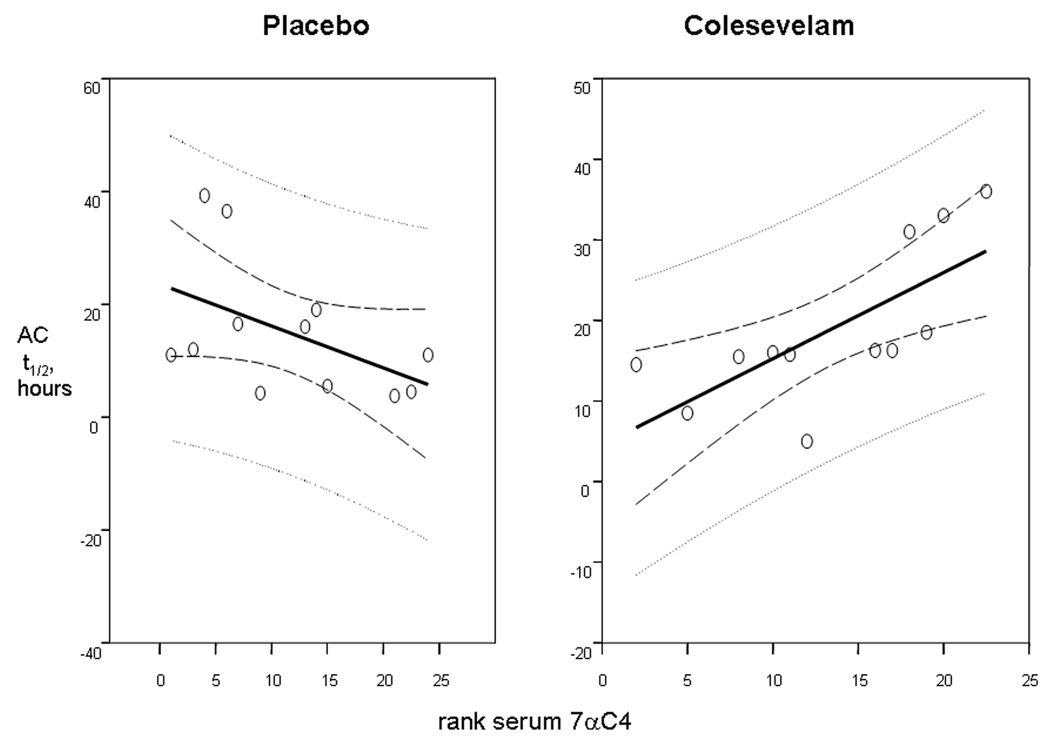
On average, AC t1/2 was >4 hours slower in the colesevelam group compared to the placebo group. There was a statistically significant interaction between treatment and baseline serum 7αC4 (p=0.0025). Figure 4 shows the relationship between (the rank transformation of) baseline serum 7αC4 and observed AC t1/2 values, separately for the two treatment groups. In the colesevelam group, the Spearman correlation coefficient was 0.87 (p<0.001), and in the placebo group the coefficient was −0.49 (p=0.11). Thus, the higher the baseline serum 7αC4, the higher was the observed AC t1/2 after colesevelam treatment.
Figure 4.
Effects of colesevelam and placebo on ascending colon emptying t1/2 in relation to the rank of baseline fasting serum 7αC4. Note that the relationship between baseline fasting serum 7αC4 and ascending colon emptying times was positive in patients on colesevelam treatment, but negative in patients on placebo, implying that treatment effects were enhanced by 7αC4 on active drug, but not on placebo. Solid lines represent the regression lines (predicted value for a given X-axis value), dashed lines are the 95% confidence limits for the regression lines, and the dotted lines represent the prediction or tolerance limits for the regression lines.
Effect of Colesevelam on Bowel Function and Colonic Mucosal Permeability in IBS-D
Colesevelam had no effect on the number of bowel movements per day, but there was a tendency to improve stool consistency (p=0.12) and ease stool passage (p=0.048).
Urinary excretion of mannitol 8–24 hours after ingestion was not significantly associated with fasting serum 7αC4 (Spearman rank correlation −0.18, p=0.41). There were no clinically important differences in permeability measurements in the two groups at baseline (data not shown). There was also no significant treatment effect on colonic mucosal permeability (Table IB).
Adverse Events with Sodium Chenodeoxycholate and Colesevelam
The most common adverse events for CDC (see Table IIA) were loose stools, diarrhea and lower abdominal cramps, each being more frequently observed in the CDC 1000mg group (15–75%). Headache was observed at similar rates (20–25%) between the placebo and two treatment groups. Gas, bloating and nausea were experienced by 5–20% of the participants.
Table II.
| A. Percent of Participants with Each Adverse Effect Recorded in the Entire Group of Healthy Participants Receiving Each Dose | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Loose stools |
Diarrhea* | Lower abdominal pain/cramps |
Nausea | Gas | Lower abdominal bloating |
Headache | Muscle aches |
URI | Dizzy, Light-headed |
Sensation of warmth |
Sweating | |
|
Placebo, N=20 |
0 | 0 | 0 | 0 | 5 | 5 | 20 | 5 | 5 | 0 | 0 | 0 |
|
CDC 500mg, N=20 |
15 | 40 | 15 | 5 | 5 | 5 | 25 | 5 | 0 | 10 | 5 | 5 |
|
CDC 1000mg, N=20 |
20 | 75 | 75 | 20 | 5 | 0 | 25 | 0 | 10 | 5 | 5 | 5 |
| B. Percent of Participants with Each Adverse Effect Recorded in the Entire Group of IBS Participants Receiving Each Dose | |||||||
|---|---|---|---|---|---|---|---|
| Uterine cramps |
Headache | URI | Lower abdominal cramps |
Flatulence | Green colored stools |
Nausea | |
| Placebo, n=12 | 17 | 33 | 33 | 0 | 8 | 17 | 24 |
| Colesevelam, n=12 | 8 | 40 | 8 | 17 | 24 | 17 | 17 |
p<0.05 by Chi-square vs. placebo. Feeling of fullness, nasal congestion, anxiety, sore throat, abnormal low body temperature, feeling of weakness and rectal pain were each experienced by one participant. URI= upper respiratory infection
(backache, constipation, lack of appetite, sinusitis, rectal bleeding, borborygmi, vomiting, chills/fever, musculoskeletal sprain, canker sore and lower abdominal bloating occurred in less than 10 percent of participants)
In the colesevelam study, the most common adverse events (see Table IIB) were headache, nausea, flatulence and green colored stools, which each occurred at similar rates in the placebo and treatment groups (10–45%). Smaller numbers experienced uterine and abdominal cramps and urinary tract infection. There were no serious adverse events and no participant had to stop the study due to an adverse event in either of the two studies.
DISCUSSION
We used two approaches to assess the effect of bile acids on colonic transit and of binding bile acids in the treatment of bowel dysfunction in unselected patients in IBS-D in whom indirect serum markers of bile acid synthesis were measured.
Effects of Chenodeoxycholate in Health
In healthy volunteers, sodium CDC administered at lower doses previously used for gallstone dissolution (500mg and 1000mg) accelerated whole colonic transit, but the higher dose was more effective. The acceleration in colonic transit was accompanied by looser stool form and increased stool frequency and ease of passage. Only a small minority of healthy participants had increased serum 7αC4 at baseline and, therefore, the findings cannot be attributed to undiagnosed BAM in these participants. Idiopathic BAM was previously reported to be associated with accelerated small bowel and distal colonic transit [25].
Our study increases understanding of the action of unabsorbed bile salts on colonic transit. Chenodeoxycholate undergoes metabolism to lithocholate in the colon; however, the latter does not cause colonic secretion [26]. Therefore, the effects on colonic transit and on fecal parameters likely result from effects of chenodeoxycholate rather than lithocholate.
It is generally perceived that bile salts accelerate colonic transit by causing secretion. This perception is reinforced by observations of diarrhea post-cholecystectomy and the assumption that this results from bile salt loss. However, <20% patients develop diarrhea postcholecystectomy [27]. Bile acid loss [28] occurs in some, but not all, patients. Evidence of loss is not correlated with change in bowel function [28], and population-based studies show that, as a group, post-cholecystectomy patients do not have increased frequency or altered consistency of bowel movements [29]. Accelerated colonic transit may result from stimulation of motility; concentrations of >5mM bile acids infused directly into the human sigmoid and rectum stimulated colonic phasic contractions [9]. Such concentrations are seldom achieved in the colon in the absence of ileal resection [9], and effects of 5mM bile salts delivered to the proximal colon (as might occur in humans with BAM) were unknown. Studies of effects of bile acids on colon motility are contradictory: Taylor et al. did not observe stimulation with 5 and 15 mM CDCA in the sigmoid colon of IBS patients [30]; whereas, Bampton et al. [8] noted propulsive contractions in the colon of healthy volunteers with rapid rectal delivery of 1 mM CDCA. Therefore, our detailed transit measurements clarified the dose of bile salt required to accelerate colonic transit and suggest that ileocolonic delivery of CDC may enhance its efficacy, relative to the effects on fecal parameters in patients with constipation observed by Bazzoli et al. [31] with oral administration of CDC.
Effect of Colesevelam in IBS-D
Our second treatment trial showed tendencies for overall colonic transit to be retarded in patients with IBS-D, of whom only 4/24 had evidence of BAM at baseline. The most intriguing finding, however, is that the baseline serum 7αC4 is significantly associated with the slowing of AC t 1/2 emptying; the latter is a determinant of stool consistency. In a pooled analysis of data from 193 patients or healthy controls from previous studies and the current study (Camilleri, Deiteren, Zinsmeister unpublished observation), we estimated increase in stool consistency scores (Bristol Stool Form Scale [18]) of 0.56 ± 0.06 units per unit change in overall colonic transit at 24 hours. The difference in colonic transit in the colesevelam and placebo groups of 0.7 GC units at 24 hours would be expected to correspond to a 0.4 unit change in stool form scale.
The prevalence of BAM in patients with IBS or chronic diarrhea may be as high as 33% [2], and up to 76% of patients with functional diarrhea or IBS-D with reduced bile acid retention on 75SeHCAT respond to bile acid binders [2,32]. In addition to benefit in full blown BAM [35], our data suggest that bile acid binding might be beneficial in patients with IBS-D without overt BAM, given the significant treatment by fasting serum 7αC4 interaction in the AC emptying in response to colesevelam.
Treatment with colesevelam did not significantly affect colonic mucosal permeability in IBS-D patients. However, only 4/24 had evidence of BAM at baseline, and pre-treatment colonic mucosal permeability does not appear to be significantly associated with subject status. Specifically, based on our measurements [24] in healthy volunteers without GI symptoms (n=12, median [range] mannitol excretion over 8–24 hours of 64.8[20.2, 429.3]), the 24 IBS-D patients did not have increased baseline permeability (53.9 [17.1, 182.9], p=0.13, Wilcoxon rank sum test).
It is interesting to note that colesevelam treatment was associated with slower gastric emptying. Colesevelam improves glycemic and lipid parameters in patients with type 2 diabetes mellitus inadequately controlled with metformin-based therapy [33], and this effect may conceivably result from increased release of incretins such as glucagon-like peptide-1.
In summary, the significant colesevelam treatment by serum 7αC4 interaction in determining ascending colon emptying time suggests that effects of bile acid binding on the secretory and motor effects of malabsorbed bile acids and clinical endpoints in IBS-D deserve further study.
Potential of Therapies Modulating Bile Salts
Defective sulfation of CDC may cause functional constipation in children [34]. Conversely, 2.25g per day CDC may improve stool frequency and consistency in some, but not all, constipated patients [31]. Methacrylate-coated capsule delivery of CDC to the ileocolonic region is associated with demonstrable colonic transit effects in healthy participants. Follow-up studies of the effects in patients with IBS-C are currently underway using 500mg and 1000mg doses of CDC in methacrylate-coated capsules.
The effects of colesevelam on colonic transit in IBS-D suggest that larger studies should explore the clinical outcomes over a longer period of treatment. The study also suggests fasting serum 7αC4 might enhance selection of IBS-D patients for bile acid binding treatment and initiate a paradigm shift in the management of patients with IBS-D.
Safety
The literature on gallstone dissolution shows that 37% of patients treated with typical CDC doses of 14–15 mg per kg per day had effectively dissolved gallstones [35] and had hypertransaminasemia (within 3 times upper limit of normal) in the first 6 months of treatment [36]. On the other hand, doses of 7–20 mg CDC kg−1 day−1 were not hepatotoxic in humans [37]. Among patients with gallstones treated with CDC (over 1000mg day−1 for several months), and patients who underwent partial ileal bypass for hyperlipidemia, there are no reports of colon cancer [38]. With ileocolonic delivery of CDC, the bile salt is probably absorbed passively from the colonic mucosa (as observed in the human colon [39]), rather than the active transport mechanism of the ileum, potentially reducing hepatic levels of CDC and reducing the risk of transaminasemia.
In these studies, colesevelam was used at the highest approved dose; the extensive use of the drug for hyperlipidemia suggests that it could be used safely in patients with IBS-D or BAM.
CONCLUSIONS
In summary, our studies show that the acceleration of overall colonic transit at 24 and 48 hours induced by sodium CDC is significant and comparable with the effects observed with a variety of pharmacological agents (e.g. prucalopride, lubiprostone, linaclotide) using the same colonic transit measurement. In addition, a subgroup of patients with IBS-D has amelioration of transit in response to colesevelam, fasting serum 7αC4 may predict responsiveness to bile acid binding in patients with IBS-D, and the current studies are consistent with the hypothesis that BAM contributes to the phenotype of IBS-D.
Supplementary Material
Acknowledgments
The excellent secretarial support of Mrs. Cindy Stanislav is gratefully acknowledged. Dr. Camilleri is supported by NIH grant DK-54681.
Abbreviations
- 7αC4
7α-hydroxy-4-cholesten-3-one
- BAM
bile acid malabsorption
- CDC
chenodeoxycholic acid or chenodeoxycholate
- FGF-19
fibroblast growth factor 19
- IBS
irritable bowel syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Mayo Clinic has filed a provisional patent application (inventors: M. Camilleri, D. Burton) related to this technology: No. 61/143,727.
Authors’ contributions: Dr. Odunsi-Shiyanbade: fellow co-investigator, writing; Dr. Camilleri: PI, concept, writing; S. McKinzie: study coordinator, participant recruitment; D. Burton: technologist, transit measurements and assays; P. Carlson: technologist, biochemical assays; I. Busciglio: technologist, transit measurements and assays; J. Lamsam: technologist, biochemical assays; R. Singh: Director of Immunochemistry Lab, biochemical assays; Dr. Zinsmeister: biostatistician co-investigator, concept, writing.
Contributor Information
Suwebatu T. Odunsi-Shiyanbade, Clinical Enteric Neuroscience Translational and Epidemiological Research (C.E.N.T.E.R.), College of Medicine, Mayo Clinic, Rochester, Minnesota
Michael Camilleri, Clinical Enteric Neuroscience Translational and Epidemiological Research (C.E.N.T.E.R.), College of Medicine, Mayo Clinic, Rochester, Minnesota
Sanna McKinzie, Clinical Enteric Neuroscience Translational and Epidemiological Research (C.E.N.T.E.R.), College of Medicine, Mayo Clinic, Rochester, Minnesota
Duane Burton, Clinical Enteric Neuroscience Translational and Epidemiological Research (C.E.N.T.E.R.), College of Medicine, Mayo Clinic, Rochester, Minnesota.
Paula Carlson, Clinical Enteric Neuroscience Translational and Epidemiological Research (C.E.N.T.E.R.), College of Medicine, Mayo Clinic, Rochester, Minnesota
Irene A. Busciglio, Clinical Enteric Neuroscience Translational and Epidemiological Research (C.E.N.T.E.R.), College of Medicine, Mayo Clinic, Rochester, Minnesota
Jesse Lamsam, Immunochemistry Core Laboratory, College of Medicine, Mayo Clinic, Rochester, Minnesota.
Ravinder Singh, Immunochemistry Core Laboratory, College of Medicine, Mayo Clinic, Rochester, Minnesota
Alan R. Zinsmeister, Department of Health Sciences Research, Division of Biostatistics, College of Medicine, Mayo Clinic, Rochester, Minnesota
REFERENCES
- 1.Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159:2647–2658. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- 2.Wedlake L, A'hern R, Russell D, Thomas K, Walters J, Andreyev H. Systematic review: the prevalence of idiopathic bile acid malabsorption (I-BAM) as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome (IBS) Aliment Pharmacol Ther. 2009 Jun 30; doi: 10.1111/j.1365-2036.2009.04081.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Mekhjian HS, Phillips SF, Hofmann AF. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J Clin Invest. 1971;50:1569–1577. doi: 10.1172/JCI106644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wingate DL, Phillips SF, Hofmann AF. Effect of glycine-conjugated bile acids with and without lecithin on water and glucose absorption in perfused human jejunum. J Clin Invest. 1973;52:1230–1236. doi: 10.1172/JCI107290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conley DR, Coyne MJ, Bonorris GG, Chung A, Schoenfield LJ. Bile acid stimulation of colonic adenylate cyclase and secretion in the rabbit. Am J Dig Dis. 1976;21:453–458. doi: 10.1007/BF01072128. [DOI] [PubMed] [Google Scholar]
- 6.Chadwick VS, Gaginella TS, Carlson GL, Debongnie JC, Phillips SF, Hofmann AF. Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. J Lab Clin Med. 1979;94:661–674. [PubMed] [Google Scholar]
- 7.Alrefai WA, Saksena S, Tyagi S, Gill RK, Ramaswamy K, Dudeja PK. Taurodeoxycholate modulates apical Cl-/OH- exchange activity in Caco2 cells. Dig Dis Sci. 2007;52:1270–1278. doi: 10.1007/s10620-006-9090-8. [DOI] [PubMed] [Google Scholar]
- 8.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol. 2002;282:G443–G449. doi: 10.1152/ajpgi.00194.2001. [DOI] [PubMed] [Google Scholar]
- 9.Kirwan WO, Smith AN, Mitchell WD, Falconer JD, Eastwood MA. Bile acids and colonic motility in the rabbit and the human. Gut. 1975;16:894–902. doi: 10.1136/gut.16.11.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiller RC, Brown ML, Phillips SF. Decreased fluid tolerance, accelerated transit, and abnormal motility of the human colon induced by oleic acid. Gastroenterology. 1986;91:100–107. doi: 10.1016/0016-5085(86)90445-2. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell WD, Findlay JM, Prescott RJ, Eastwood MA, Horn DB. Bile acids in the diarrhoea of ileal resection. Gut. 1973;14:348–353. doi: 10.1136/gut.14.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Tilburg AJ, de Rooij FW, van Blankenstein M, van den Berg JW, Bosman-Jacobs EP. Na+dependent bile acid transport in the ileum: the balance between diarrhea and constipation. Gastroenterology. 1990;98:25–32. doi: 10.1016/0016-5085(90)91286-f. [DOI] [PubMed] [Google Scholar]
- 13.Mok HY, Bell GD, Dowling RH. Effect of different doses of chenodeoxycholic acid on bile-lipid composition and on frequency of side-effects in patients with gallstones. Lancet. 1974;304:253–257. doi: 10.1016/s0140-6736(74)91415-9. [DOI] [PubMed] [Google Scholar]
- 14.Walters JR, Tasleem AM, Omer OS, Brydon WG, Dew T, le Roux CW. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009 May 6; doi: 10.1016/j.cgh.2009.04.024. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Davidson MH, Dillon MA, Gordon B, et al. Colesevelam hydrochloride: a new potent bile acid sequestrant associated with a low incidence of gastrointestinal side effects. Arch Intern Med. 1999;159:1893–1900. doi: 10.1001/archinte.159.16.1893. [DOI] [PubMed] [Google Scholar]
- 16.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 17.Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale preliminary report. Psychopharmacol Bull. 1973;9:13–28. [PubMed] [Google Scholar]
- 18.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 19.Cremonini F, Mullan BP, Camilleri M, Burton DD, Rank MR. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther. 2002;16:1781–1790. doi: 10.1046/j.1365-2036.2002.01344.x. [DOI] [PubMed] [Google Scholar]
- 20.Camilleri M, Talley NJ. Pathophysiology as a basis for understanding symptom complexes and therapeutic targets. Neurogastroenterol Motil. 2004;16:135–142. doi: 10.1111/j.1365-2982.2004.00516.x. [DOI] [PubMed] [Google Scholar]
- 21.Viramontes BE, Camilleri M, McKinzie S, Pardi DS, Burton D, Thomforde GM. Gender related differences in slowing colonic transit by a 5-HT3 antagonist in subjects with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2001;92:2671–2676. doi: 10.1111/j.1572-0241.2001.04138.x. [DOI] [PubMed] [Google Scholar]
- 22.Sauter GH, Münzing W, von Ritter C, Paumgartner G. Bile acid malabsorption as a cause of chronic diarrhea: diagnostic value of 7alpha-hydroxy-4-cholesten-3-one in serum. Dig Dis Sci. 1999;44:14–19. doi: 10.1023/a:1026681512303. [DOI] [PubMed] [Google Scholar]
- 23.Camilleri M, Nadeau A, Tremaine WJ, Lamsam J, Burton D, Odunsi S, Sweetser S, Singh R. Measurement of serum 7α-hydroxy-4-cholesten-3-one (or 7αC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil. 2009 Jul 10; doi: 10.1111/j.1365-2982.2009.01288.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camilleri M, Nadeau A, Lamsam J, Linker-Nord S, Ryks M, Burton D, Sweetser S, Zinsmeister AR, Singh R. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol Motil. doi: 10.1111/j.1365-2982.2009.01361.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadik R, Abrahamsson H, Ung KA, Stotzer PO. Accelerated regional bowel transit and overweight shown in idiopathic bile acid malabsorption. Am J Gastroenterol. 2004;99:711–718. doi: 10.1111/j.1572-0241.2004.04139.x. [DOI] [PubMed] [Google Scholar]
- 26.Keely SJ, Scharl MM, Bertelsen LS, Hagey LR, Barrett KE, Hofmann AF. Bile acid-induced secretion in polarized monolayers of T84 colonic epithelial cells: structure-activity relationships. Am J Physiol. 2007;292:G290–G297. doi: 10.1152/ajpgi.00076.2006. [DOI] [PubMed] [Google Scholar]
- 27.Ros E, Zambo´n D. Postcholecystectomy symptoms. A prospective study of gallstone patients before and two years after surgery. Gut. 1987;28:1500–1504. doi: 10.1136/gut.28.11.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauter GH, Moussavian AC, Meyer G, Steitz HO, Parhofer KG, Jüngst D. Bowel habits and bile acid malabsorption in the months after cholecystectomy. Am J Gastroenterol. 2002;97:1732–1735. doi: 10.1111/j.1572-0241.2002.05779.x. [DOI] [PubMed] [Google Scholar]
- 29.Heaton KW, Parker D, Cripps H. Bowel function and irritable bowel symptoms after hysterectomy and cholecystectomy — a population based study. Gut. 1993;34:1108–1111. doi: 10.1136/gut.34.8.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor I, Basu P, Hammond P, Darby C, Flynn M. Effect of bile acid perfusion on colonic motor function in patients with the irritable colon syndrome. Gut. 1980;21:843–847. doi: 10.1136/gut.21.10.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bazzoli F, Malavolti M, Petronelli A, Barbara L, Roda E. Treatment of constipation with chenodeoxycholic acid. J Int Med Res. 1983;11:120–123. doi: 10.1177/030006058301100211. [DOI] [PubMed] [Google Scholar]
- 32.Wildt S, Nørby Rasmussen S, Lysgård Madsen J, Rumessen JJ. Bile acid malabsorption in patients with chronic diarrhoea: clinical value of SeHCAT test. Scand J Gastroenterol. 2003;38:826–830. doi: 10.1080/00365520310004461. [DOI] [PubMed] [Google Scholar]
- 33.Bays HE, Goldberg RB, Truitt KE, Jones MR. Colesevelam hydrochloride therapy in patients with type 2 diabetes mellitus treated with metformin: glucose and lipid effects. Arch Intern Med. 2008;168:1975–1983. doi: 10.1001/archinte.168.18.1975. [DOI] [PubMed] [Google Scholar]
- 34.Hofmann AF, Loening-Baucke V, Lavine JE, Hagey LR, Steinbach JH, Packard CA, Griffin TL, Chatfield DA. Altered bile acid metabolism in childhood functional constipation: inactivation of secretory bile acids by sulfation in a subset of patients. J Pediatr Gastroenterol Nutr. 2008;47:598–606. doi: 10.1097/MPG.0b013e31816920a6. [DOI] [PubMed] [Google Scholar]
- 35.Iser JH, Dowling H, Mok HY, Bell GD. Chenodeoxycholic acid treatment of gallstones. A follow-up report and analysis of factors influencing response to therapy. N Engl J Med. 1975;293:378–383. doi: 10.1056/NEJM197508212930804. [DOI] [PubMed] [Google Scholar]
- 36.Meredith TJ, Williams GV, Maton PN, Murphy GM, Saxton HM, Dowling RH. Retrospective comparison of 'Cheno' and 'Urso' in the medical treatment of gallstones. Gut. 1982;23:382–389. doi: 10.1136/gut.23.5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell GD, Mok HY, Thwe M, Murphy GM, Henry K, Dowling RH. Liver structure and function in cholelithiasis: effect of chenodeoxycholic acid. Gut. 1974;15:165–172. doi: 10.1136/gut.15.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buchwald H, Varco RL, Bowen JR, Williams SE, Hansen BJ, Campos CT, Campbell GS, Pearce MB, Yellin AE, Edmiston WA, Smink RD, Jr, Sawin HS., Jr Effective lipid modification by partial ileal bypass reduced long-term coronary heart disease mortality and morbidity: five-year post-trial follow-up report from the POSCH, Program on the Surgical Control of the Hyperlipidemias. Arch Intern Med. 1998;158:1253–1261. doi: 10.1001/archinte.158.11.1253. [DOI] [PubMed] [Google Scholar]
- 39.Mekhjian HS, Phillips SF, Hofmann AF. Colonic absorption of unconjugated bile acids: perfusion studies in man. Dig Dis Sci. 1979;24:545–550. doi: 10.1007/BF01489324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.