Abstract
Hexavalent chromium is a known inducer of DNA-protein crosslinks (DPC) that contribute to repression of inducible genes and genotoxicity of this metal. Lymphocytic DPC have also shown potential utility as biomarkers of human exposure to Cr(VI). Here, we examined the mechanism of DPC formation by Cr(VI) and the impact of its main cellular reducers. In vitro reactions of Cr(VI) with one-electron reducing thiols (glutathione, cysteine) or two-electron donating ascorbate were all efficient at DPC production, indicating a dispensable role of Cr(V). No Cr(VI) reducer was able to generate DPC in the presence of Cr(III)-chelating EDTA or phosphate. A critical role of Cr(III) in DNA-protein linkages was further confirmed by dissociation of Cr(VI)-induced DPC by phosphate. EDTA was very inefficient in DPC dissociation, indicating its poor suitability for testing of Cr(III)-mediated bridging and reversal of complex DPC. Reactions containing only one Cr-modified component (protein or DNA) showed that Cr(III)-DNA adduction was the initial step in DPC formation. Crosslinking proceeded slowly after the rapid formation of Cr-DNA adducts, indicating that protein conjugation was the rate-limiting step in DPC generation. Experiments with depletion of glutathione and restoration of ascorbate levels in human lung A549 cells showed that high cellular reducing capacity promotes DPC yield. Overall, our data provide evidence for a three-step crosslinking mechanism involving (i) reduction of Cr(VI) to Cr(III), (ii) Cr(III)-DNA binding and (iii) protein capture by DNA-bound Cr(III) generating protein-Cr(III)-DNA crosslinks.
Introduction
Cellular reduction of carcinogenic Cr(VI) generates a wide assortment of small DNA modifications resulting from the crosslinking activity of Cr(III) (1,2) and the oxidizing properties of intermediate Cr species and other reactive byproducts (3,4). Cr(VI) is also known to induce bulky DNA-protein crosslinks (DPC) in various cells in culture and in vivo (5–7). Methodological improvements in DPC measurements and positive results from several human studies pointed to the potential utility of these lesions as biomarkers of exposure to toxic Cr compounds (8). Elevated levels of DPC have been found in peripheral blood lymphocytes among welders (9,10), chrome platers (11,12) and leather tanners (13). Lymphocytic DPC have shown a good correlation with the levels of Cr in red blood cells (11), which is a biomarker of internal dose of Cr(VI) (14,15). Higher levels of lymphocytic DPC have also been found in populations residing in areas with environmental Cr contamination (11,16). The main advantage of DPC measurements over determinations of total Cr in biological samples, including erythrocytes, is that they assess the presence of elevated genetic damage. DPC levels in human lymphocytes are not significantly affected by age, weight or smoking status (17), which permits the use of this biomarker for the detection of Cr-associated genetic damage in relatively small groups of exposed individuals (18). In addition to human biomonitoring, DPC measurements have been used for the assessment of genetic damage in several aquatic species exposed to waterborne Cr(VI) (19,20).
Although the biological significance of DPC in general is poorly understood, these bulky lesions have long been assumed to be genotoxic. A likely impediment of DNA replication by Cr-induced DPC has been suggested to lead to gross genetic rearrangements (9), mutations (8) or S-phase specific DNA double-strand breaks (21). Some chemical forms of DPC were indeed tested mutagenic (22,23) but others were not (24). Puga and co-workers (25) have recently provided experimental evidence that DPC play a major role in the well-established phenomenon of inhibition of inducible gene expression in Cr(VI)-treated cells (26–28). They found that Cr(VI) suppressed the aryl hydrocarbon receptor-mediated gene expression by benzo[α]pyrene via DNA crosslinking of the repressory HDAC1-DNMT1 complexes, which blocked their release from the promoters of the inducible genes and prevented chromatin remodeling and RNA polymerase II recruitment. Thus, in addition to their potential role in genotoxic effects of Cr(VI), DPC can also play an important role in the toxicological effects of mixed exposures, particularly for agents that require gene induction for their activation, efficient detoxification or repair of cell injury.
Broader utilization of DPC as a biomarker, particularly for environmentally exposed populations, is hampered by the inability of current analytical approaches to differentiate between Cr(VI)-induced and other forms of DPC. The basis of this problem largely lies in the poorly understood mechanisms of intermolecular crosslinking by Cr(VI), which makes it difficult to devise more specific methodologies. Better defined crosslinking mechanisms would also make it easier to interpret the effects of altered cellular physiology or coexposure conditions on the DPC formation and the associated toxicological effects. In principle, Cr(VI) can induce DPC either via Cr(III)-mediated crosslinking reactions or oxidative mechanisms. The latter can involve either crosslinking by the initial oxidative lesions on DNA (29,30) and proteins (31) or via reactive products of lipid peroxidation, such as malondialdehyde (32). Formation of advanced products of guanine oxidation by Cr(VI) (4) is one of the potential routes to protein crosslinking via oxidative mechanisms. Studies in CHO cells showed that about 50% of Cr(VI)-induced DPC were sensitive to disruption by EDTA (33), which was indicative of a major role of Cr(III) in DNA-protein crosslinking. However, experimentally very similar work using human MOLT4 lymphoma cells detected only a very small effect of EDTA (7), which has been interpreted as evidence for oxidative linkages in DPC formation. The crosslinking ability of Cr(III) aqua complexes under neutral pH has also been questioned experimentally (34), although results of this and some other similar in vitro studies could have been adversely impacted by the use of Tris buffer (1).
In this work, we conducted detailed mechanistic studies of DPC formation by carcinogenic Cr(VI) and the final product of its reduction, Cr(III). The uncertainty about the role of Cr(III) in DPC production was addressed using a more efficient chelation procedure. The process of DNA-protein crosslinking was dependent on Cr(III), but the sequence of reactions leading to the intermolecular conjugation was different than that established for small reducer-Cr(III)-DNA crosslinks (35,36).
Experimental Procedures
Materials
K2CrO4 (ACS reagent) and [Cr(H2O)4Cl2]Cl•2H2O (99.995% pure) were from Aldrich (Milwaukee, WI). L-ascorbic acid (Asc), bovine serum albumin (BSA), L-buthionine-[S,R]-sulfoximine (BSO), calf thymus DNA type 1, dehydro-L-(+)-ascorbic acid (DHA), 4-morpholinoethanesulfonic acid (MES), 4-morpholinepropanesulfonic acid (MOPS), phenylmethanesulfonyl fluoride (PMSF), SDS, Tris and all salts and electrophoresis materials were from Sigma-Aldrich (St. Louis, MO). L-cysteine, glutathione (GSH) and tissue culture media were from Gibco BRL (Gaithersburg, MD). Fetal bovine serum came from Gemini Bio-Products. PicoGreen and 1,2-diamino-4,5-dimethoxybenzene dihydrochroride dyes were obtained from Molecular Probes (Eugene, OR). Bio-Gel P-30 size-exclusion columns were purchased from Bio-Rad (Hercules, CA). Caution: Cr(VI) compounds are human carcinogens and appropriate precautions should be taken in handling of these materials.
Cells
Human lung A549 cells were from the American Tissue Culture Collection. The cells were grown in F-12K medium with 10% fetal bovine serum at 37°C in atmosphere containing 95% air and 5% CO2.
DNA-protein crosslinking in vitro
All in vitro DNA-protein crosslinking reaction mixtures had a final volume of 50 μL. With the exception of the preliminary optimization experiments in which the BSA concentration was varied, the crosslinking reactions contained 25 mM MOPS buffer (pH 7.0) or 25 mM MES buffer (pH 6.0), 5 μg calf thymus DNA, 60 μg BSA, and 0–20 μM of freshly prepared chromic chloride (CrIII) in distilled H2O. Crosslinking reactions with Cr(VI) (K2CrO4) additionally contained one of the three reducers (Asc, Cys or GSH). Unless indicated otherwise, the samples were incubated at 37°C for 3 hr. Crosslinking reactions were stopped by the addition of 1% SDS (final volume of 500 μL). Samples were stored at −80°C prior to DPC measurements.
To purify A549 nuclei, cells were lysed in a homogenization buffer (250 mM sucrose, 5 mM CaCl2, 30 mM KCl, 1 mM PMSF, 0.5% Triton X-100, 20 mM Tris-HCl, pH 8.0,) using a Dounce homogenizer. The lysates were spun down (900g for 5 min, 4 °C) and the nuclear pellets were washed twice in the homogenization buffer. The nuclei were stored at −80°C in 25% glycerol, 5 mM CaCl2, 20 mM Tris-HCl (pH 8.0). Standard crosslinking reactions with purified A549 nuclei contained 25 mM MOPS (pH 7.0), 150 mM NaCl, Cr(VI) and a mixture of three main biological reducers (1 mM Asc, 2 mM GSH and 0.5 mM Cys). Some crosslinking reactions additionally contained 5 mM EDTA or MOPS was replaced by 25 mM Na-phosphate buffer. All samples were incubated for 3 hr at 37°C with continuous gentle rotation, nuclei were washed with MOPS-NaCl and lysed in 1% SDS for DPC measurements.
Reversibility of DPC by Cr(III) chelators
The BSA-DNA mixtures contained 25 μM MOPS buffer (pH 7.0), 5 μg calf thymus DNA, 60 μg BSA, 0–100 μM K2CrO4 and 1 mM Asc. Samples were reacted at 37°C for 0.5–3 hr and then received 50 mM Na-phosphate (pH 7.0) or 10 mM EDTA followed by additional incubation for 24 hr at 37°C. The crosslinking reactions were stopped by the addition of 1% SDS. For assessment of reversibility of nuclear DPC, nuclei were treated as above, washed with the buffer, resuspended in either 25 mM MOPS or 25 mM Na-phosphate (both pH 7.0) and incubated for additional 24 hr at 37°C.
Determination of the order of conjugation reactions
Cr-DNA or Cr-BSA complexes were formed by incubating 5 μg DNA or 60 μg BSA with 0–20 μM Cr(III) or with 0–100 μM K2CrO4 plus 1 mM Asc at 37°C for 30 min. Cr-modified DNA and Cr(III)-BSA were then purified by Bio-Gel P-30 size-exclusion chromatography. The missing DPC component, either 60 μg BSA or 5 μg DNA, was then added and the samples were incubated at 37°C for 3 hr. The crosslinking reactions were stopped by addition of 1% SDS.
Treatments of A549 cells with Cr(VI)
Cells were seeded on 12-well plates, allowed to attach overnight and exposed to K2CrO4 in serum-free medium for 3 hr. Cells were washed with PBS, collected by trypsinization, and spun down at 4°C for 5 min at 1100 g. The cell pellets were washed once with PBS and resuspended in 50 μL PBS. The resuspended cells were added to a lysis solution containing 1% SDS, 0.4 mg/mL BSA, 20 mM Tris-HCI (pH 7.5), and 1 mM PMSF. The lysed samples were vortexed and frozen at −80°C. Restoration of physiological concentrations of Asc was carried out by preincubation of the cells with 2 mM DHA at 37°C for 90 min in Krebs buffer supplemented with 0.5 mM D-glucose. The depletion of intracellular GSH was done by incubating the cells at 37°C for 24 hr in complete medium supplemented with 0.1 mM BSO.
Determination of cellular Asc
Intracellular Asc was measured by HPLC detection of a specific conjugate with 1,2-diamino-4,5-dimethoxybenzene dihydrochroride (37). Cells were collected by trypsinization, washed three times with cold PBS (1100 × g for 5 min at 4°C) and resuspended in equal volumes of water and 100 mM methane sulfonic acid, 10 mM diethylenetriaminepentaacetic acid. Samples were subjected to two cycles of freezing (−80°C) and thawing (37°C), and Asc was recovered in the supernatant after centrifugation at 12,000g for 10 min at 4°C. Pellets were resuspended in 1% SDS, 50 mM NaOH for determination of protein concentration. Derivatization reactions contained 10 μl of Asc-containing samples and 90 μl of a dye solution containing 0.2 units/μl ascorbate oxidase, 50 mM Na-acetate (pH 6.2), and 0.5 mM 1,2-diamino-4,5-dimethoxybenzene and were incubated for 4 hr at room temperature in the dark. Fluorescent Asc conjugates were separated using isocratic elution with 75% 50 mM phosphoric acid (pH 2.0) and 25% acetonitrile.
Measurements of cellular GSH
Total cellular GSH was determined by HPLC as described previously (38). In brief, cells were collected by trypsinization, washed three times with cold PBS and suspended in a 40 mM methanesulfonic acid-10 mM diethylenetriaminepentaacetic acid solution, lysed by two cycles of freezing/thawing and GSH-containing supernatants were recovered by centrifugation at 12,000g for 10 min at 4°C. The cell extracts were reacted with 2 mM monobromobimane in the presence of DTT and the amount of the fluorescent GSH conjugate was measured by HPLC.
Measurements of DPC
Both in vitro and cellular DPC were measured by the K+/SDS precipitation assay (39), as previously modified (40). This frequently used assay is based on the selective precipitation of protein-crosslinked DNA by K+/SDS. The anionic detergent SDS binds proteins but not DNA. The addition of K+ ions produces a K+/SDS precipitate, which can be recovered by low speed centrifugation. Protein-free DNA remains in the supernatant whereas protein-crosslinked DNA is found in the SDS pellet. Repeated washes and high temperature heating steps ensure the dissociation of DNA from noncovalently bound proteins. The percentage of the SDS-precipitable DNA represents a quantitative measure of the number of DPC. Frozen samples were thawed at 37°C and mixed with 0.5 ml DPC wash buffer (200 mM KCl, 20 mM Tris-HCI pH 7.5). The samples were vortexed and heated at 50°C for 10 min (initially) and 5 min (between washes). The samples were placed on ice for 10 min to reform the K+/SDS-protein precipitate. The SDS pellet was recovered by centrifugation at 4°C, 3000g for 5 min. The supernatant was saved and the pellet was resuspended in 1 ml of DPC wash buffer. The heating, cooling, and centrifugation steps were repeated for a total of three washes. The final pellet was resuspended in 500 μL DPC wash buffer containing 10 mM EDTA (pH 8.0) and 0.5 mg/mL proteinase K and incubated at 50°C overnight. The DNA from the proteinase K digested pellet was then recovered by placing the samples on ice for 30 min and spinning them down at 4°C for 20 min at 3,000g. The DNA content of the supernatant from the washes and the proteinase K-digested K+/SDS pellet were measured in a multi-well fluorescence reader using the PicoGreen dye (emission 535 nm, excitation 485 nm).
Cr(VI) uptake
Cellular Cr was extracted by hot nitric acid and measured by graphite furnace atomic absorption spectroscopy (Perkin-Elmer GF-AAS, model 41002L) (41). Cells were collected by trypsinization, washed twice with cold PBS and resuspended in 50 μl of cold water and 50 μl of 10% nitric acid. Samples were frozen at −80°C and then heated to 50°C for 60 min, followed by incubation on ice for 30 min and centrifugation at 10,000 g for 10 min at 4°C. Supernatants were diluted with water 2.5 times and analyzed for chromium content. Pellets were washed twice with cold 5% nitric acid and dissolved in 200 μl of 0.5 M NaOH by heating at 37°C for 30 min. Lysates were used for protein determination.
Results and Discussion
In vitro DPC formation by Cr(III) and Cr(VI)
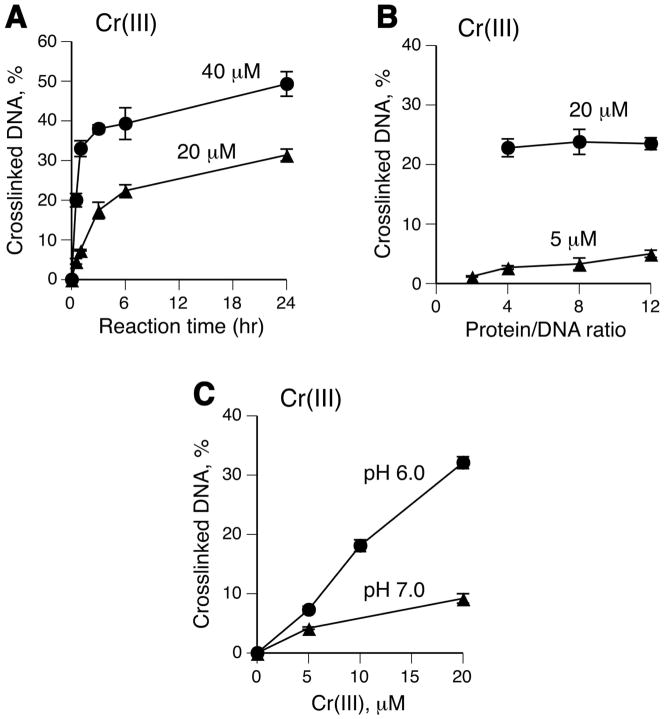
We have chosen to conduct a detailed study of DPC formation in vitro using BSA, which has been previously found to be capable of crosslinking to DNA by Cr(VI) with higher efficiency than actin (42), the main constituent of cellular DPC (43). Neither protein binds DNA but the use of BSA avoids precipitation and other common problems associated with the polymerization properties of actin. BSA readily forms DPC through decomposition of protein hydroperoxides (44) and it was found to be as efficient in DPC generation by ionizing radiation as were histones (45). Because BSA is also rich in metal-binding amino acids, it should be able to participate in both oxidative and Cr(III)-mediated reactions leading to DNA-protein crosslinking. In the first series of experiments, we examined the conditions for optimal DPC production by Cr(III). To prevent the formation of poorly soluble, unreactive Cr(III) hydroxides, our reactions contained either MES or MOPS buffers, which weakly bind Cr(III) via their sulfonate groups retaining its solubility and reactivity towards DNA (46). We found that the majority of DPC were formed by Cr(III) within the first 3 hr with almost half of crosslinking occurring already during 5–30 min (Fig. 1A). The DPC production appeared to increase with higher BSA/DNA ratios at low Cr(III) concentrations (Fig. 1B). Similar to the increased reactivity of Cr(III) toward DNA binding at lower pH (36), the DPC yield was about 3-times greater in MES, pH 6.0, than in MOPS, pH 7.0 (Fig. 1C). These results showed that Cr(III) was clearly capable of generating DPC at neutral and near neutral pH at as low as 5 μM concentration. We attribute the need for millimolar Cr(III) concentrations and low pH to cause DNA-BSA crosslinks in two previous studies to their use of Tris buffer (34,47), which has low buffering capacity at neutral pH and is unable to bind Cr(III) and thereby prevent its hydrolysis to unreactive hydroxides and concomitant acidification of the reaction mixtures.
Figure 1. DPC formation by Cr(III) under different reaction conditions.
Standard reaction mixtures contained 5 μg DNA, 60 μg BSA, 25 mM MES (pH 6.0) and indicated Cr(III) concentrations. Data are means ±SD (n=3). Where not seen, error bars were smaller than symbols. (A) Time-course of DNA-BSA crosslinking by Cr(III). (B) Yield of DPC as a function of BSA concentration (reaction time =3 hr). (C) Effect of pH on the formation of DPC (pH 6.0 – 25 mM MES, pH 7.0 – 25 mM MOPS).
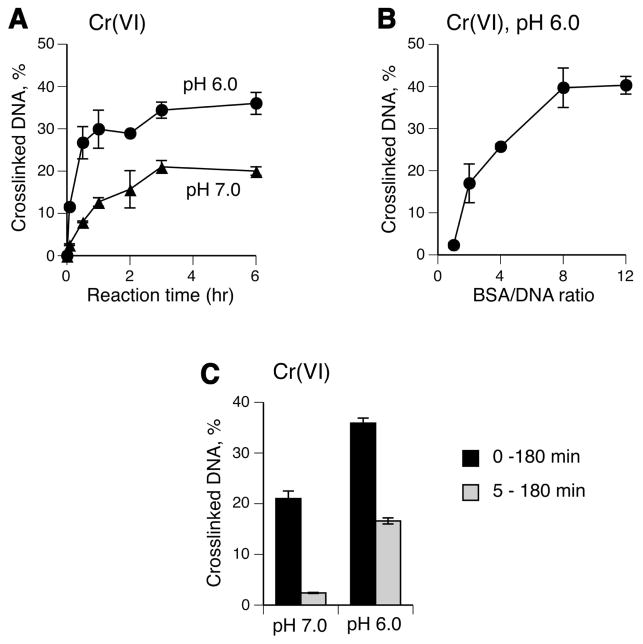
Examination of the crosslinking activity of Cr(VI) in the presence of 1 mM Asc, which is a principal reducer of Cr(VI) in vivo (48,49), showed that DPC formation was about 2-times higher at pH 6.0 than at pH 7.0 and reached plateau after 3 hr incubation under both conditions (Fig. 2A). In addition to the overall greater yield of crosslinks, pH 6.0 reactions generated DPC much faster as evidenced by nearly completed crosslinking already at 30 min while the yield of crosslinks at this time in pH 7.0 samples reached only about 30% of its maximal levels. Increasing ratio of BSA to DNA ratio up to 8:1 strongly enhanced the production of DPC (Fig. 2B). Reduction of Cr(VI) by 1 mM Asc is fast (t1/2 =1 min) and essentially complete after 5 min (49). Addition of DNA 5 min after the start of Cr(VI) reduction inhibited the DPC production with a particularly severe suppression in pH 7.0 reactions (Fig. 2C). This result suggests that DNA modifications generated during or very shortly after Cr(VI) reduction were important for the subsequent formation of crosslinks. Slow crosslinking following the initial rapid formation of DPC-producing damage in 0–5 min reactions indicates that protein conjugation is the rate-limiting step in DPC generation. The observed pH dependence for DPC formation in complete and 5–180 min reactions was consistent with the pattern of Cr(III) reactivity (Fig. 1C).
Figure 2. Influence of reaction conditions on DPC formation by Cr(VI).
Standard reaction mixtures contained 5 μg DNA, 60 μg BSA, 25 mM MES (pH 6.0) or 25 mM MOPS (pH 7.0), 1 mM Asc and 0 or 100 μM Cr(VI). Data are means ±SD (n=3). Where not seen, error bars were smaller than symbols. (A) Time-course of DNA-BSA crosslinking. (B) DPC formation as a function of BSA concentration (reaction time =3 hr, MES buffer). (C) Importance of the initial reaction period in the DPC production (0 – 180 min, DNA was added before the start of Cr(VI) reduction; 5 – 180 min, DNA was added 5 min after the start of Cr(VI) reduction).
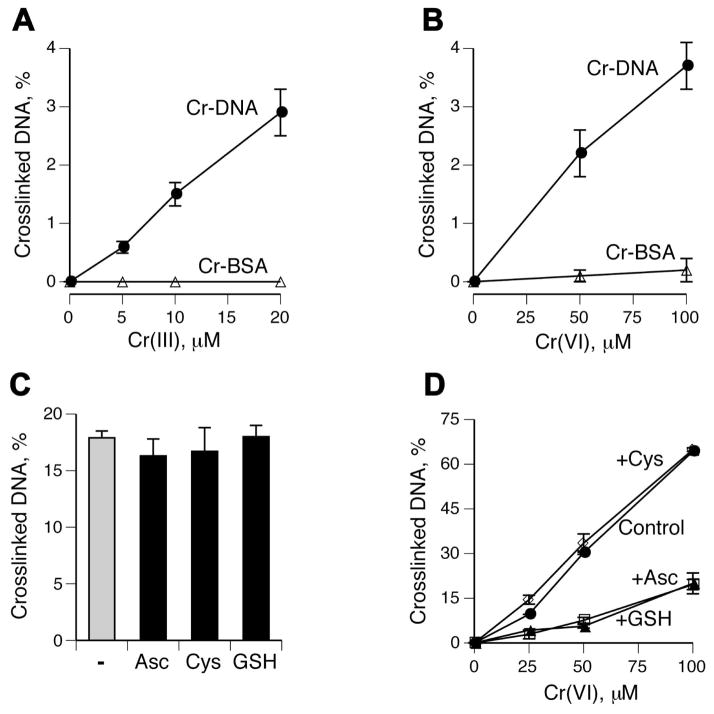
Cr(III) is responsible for DPC formation in Cr(VI) reactions
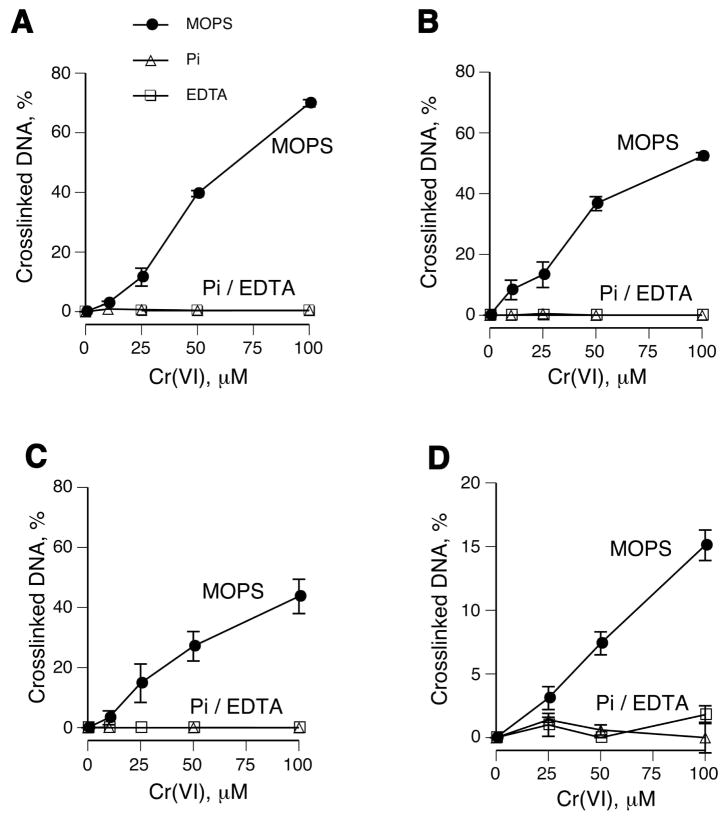
To test a role of Cr(III) more directly, we examined DPC yield in the presence of two chelating agents, EDTA and inorganic phosphate. Both chelators completely abrogated DNA-BSA crosslinking in reactions containing Asc, GSH or Cys as Cr(VI) reducers (Fig. 3A-C). These three reducing agents account for more than 95% of Cr(VI) reducing activity in tissues (1,48,49). The overall yield of DNA-BSA crosslinks was modestly higher with Asc followed by GSH and Cys reactions. Because there is no detectable Cr(V) formation at the 10:1 to 100:1 Asc to Cr(VI) ratios used here (50,51), this intermediate appears to be dispensable for DPC production. To analyze a more complex set of DPC, we examined crosslinking in purified A549 nuclei using Cr(VI) and a mixture of its three main reducers: Asc, GSH and Cys. We found that chelation of Cr(III) by EDTA or phosphate also completely blocked DPC formation in nuclei (Fig. 3D).
Figure 3. DPC formation by Cr(VI) in the presence of the different reducers and Cr(III) chelators.
Standard reaction mixtures in panels A-C contained 5 μg DNA, 60 μg BSA, 25 mM MOPS (pH 7.0) or 25 mM Na-phosphate buffer (pH 7.0), Cr(VI) and indicated reducers. Data are means ±SD (n=3). Where not seen, error bars were smaller than symbols. (A) BSA-DNA crosslinking in reactions containing 1 mM Asc (Pi - phosphate buffer, EDTA – MOPS buffer containing 5 mM EDTA). (B) BSA-DNA crosslinking in the presence of 10 mM GSH or (C) 2 mM Cys. (D) DPC formation in purified A549 nuclei by Cr(VI) activated by a mixture of its main reducers (1 mM Asc, 2 mM GSH and 0.5 mM Cys).
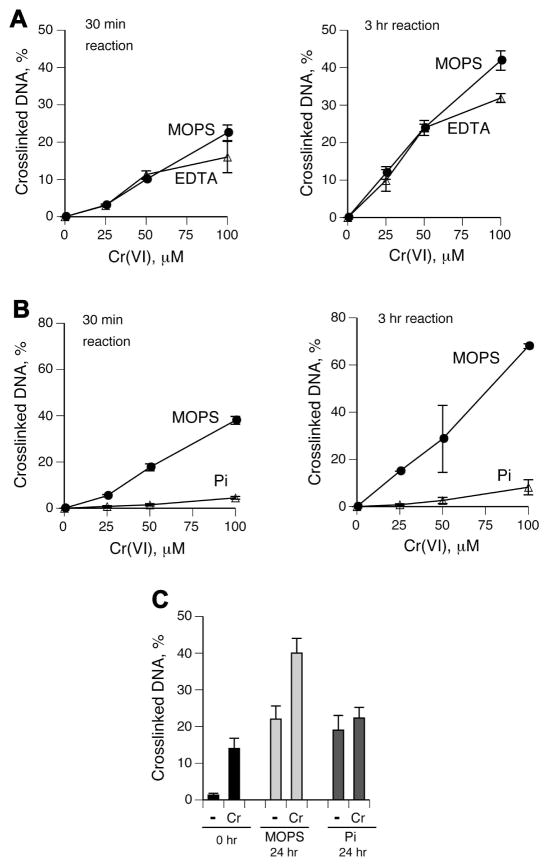
To further investigate the importance of Cr(III) in crosslinking reactions, we tested the susceptibility of the already formed DPC to dissociation by EDTA or phosphate. This approach excludes the possibility of potential alterations in the reduction process and/or the spectrum of intermediate Cr species and other products. We found that DNA-BSA crosslinks produced in 0.5 and 3 hr reactions were resistant to disruption by 10 mM EDTA during the subsequent 24 hr incubations (Fig. 4A). In contrast, a post-crosslinking addition of phosphate nearly completely dissociated Cr(VI)-induced DNA-BSA DPC (Fig. 4B). Post-incubation in phosphate but not in control MOPS buffer also dissociated nuclear DPC induced by Cr(VI) in the presence of Asc, GSH and Cys (Fig. 4C), further confirming a crucial role of Cr(III) in crosslinking. These findings indicate that one approach for the assessment of Cr-specific DPC in biomonitoring studies could be based on the measurements of the fraction of DPC that is susceptible to rupture by phosphate ions. However, a practical application of this strategy would benefit from further modifications to the described phosphate reversal procedure with the aim of suppressing observed increases in background DPC. The use of deoxygenated solutions could potentially be helpful in ameliorating this problem.
Figure 4. Reversibility of Cr(VI)-induced DPC by Cr(III) chelators.
Reaction conditions and definitions were as in Fig. 3. Stability of DPC was assessed during 24 hr incubations with 5 mM EDTA or 50 mM phosphate (pH 7.0) at 37°C. (A) Resistance of DNA-BSA crosslinks formed in Cr(VI)/1mM Asc reactions to dissociation by EDTA. EDTA was added 0.5 (left panel) or 3 hr (right panel) after the start of Cr(VI) reduction. (B) As panel A except that phosphate buffer (Pi) was tested for the DPC reversibility. (C) Reversibility of Cr(VI)-induced DPC in A549 nuclei. DPC were formed by incubating purified nuclei in 25 mM MOPS (pH 7.0) in the presence of 100 μM Cr(VI) and a mixture of its main biological reducers (1 mM Asc, 2 mM GSH and 0.5 mM Cys). Nuclei were processed for DPC measurements immediately after 3 hr initial reactions (−) and following additional 24 hr incubations at 37°C in 50 mM MOPS, pH 7.0 (MOPS) or 50 mM phosphate, pH 7.0 (Pi).
Order of the first- and second-arm reactions in DPC formation
To examine the sequence of reactions leading to DPC, we separately modified DNA and BSA with Cr(III) or Cr(VI)+Asc and then assessed DPC yield in mixtures with the unmodified component added subsequently. Both Cr(III)- and Cr(VI)-modified DNA generated crosslinks after incubations with BSA but neither type of Cr-BSA modifications produced any DPC after mixing with unmodified DNA (Fig. 5A,B). Thus, DPC formation proceeds through initial Cr(III)-DNA binding followed by conjugation of protein. This mechanism is different from DNA crosslinking of small molecules, such as His, Cys or Asc, which involves attack of DNA by binary Cr(III)-ligand complexes (35,36). Consistent with the observed order of reactions, the presence of 2 mM Asc, Cys or GSH had no effect on DPC yield in reactions with unmodified BSA and preformed Cr-DNA adducts (Fig. 5C). However, the presence of 2 mM Asc or GSH but not Cys during Cr(VI) reduction with 1 mM Asc (during Cr-DNA binding) inhibited DPC formation (Fig. 5D), reflecting decreased yields of crosslink-forming binary Cr-DNA adducts at high concentrations of Asc and GSH (36,52).
Figure 5. DPC formation using separately produced Cr-DNA and Cr-BSA adducts.
DNA and BSA were separately modified in 30-min long reactions with Cr(III) or mixtures of Cr(VI)-1 mM Asc, purified using BioGel P-30 columns and then incubated for 3 hr with the unmodified component to produce DPC. Data are means ± SD (n=3). If not seen, error bars were smaller than symbols. (A) DPC formation using BSA or DNA modified with Cr(III) or (B) Cr(VI). (C) The presence of small ligands has no effect on DPC formation in reactions of BSA with pre-formed Cr-DNA adducts. DNA was modified with 100 μM Cr(VI)/1 mM Asc for 30 min, purified using BioGel P-30 columns and incubated for 3 hr with BSA in the presence of 2 mM Asc, Cys or GSH. (D) Influence of small ligands on DPC production during Cr(VI) reduction with 1 mM Asc. Ctrl – standard reaction, +Asc – additional 2 mM Asc was added, +Cys – 2 mM Cys was added, +GSH – 2 mM GSH was added.
Role of the main cellular reducers in DPC formation
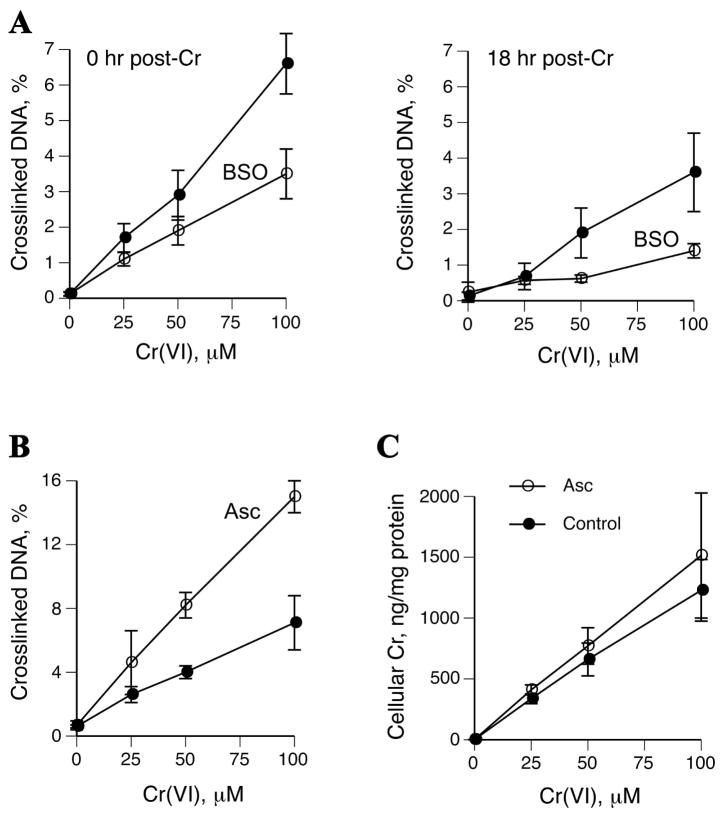
Similarly to cultured primary human lung cells (53), A549 cells contain barely detectable Asc concentrations under standard culture conditions (36,54). Asc plays a dominant role in reductive activation of Cr(VI) in vivo (48) and it has been found to strongly enhance apoptotic, genotoxic and mutagenic effects of Cr(VI) in human and rodent cells (37,53). However, the role of this key reducer in the production of DPC by Cr(VI) remains poorly understood as previous studies attempted to deliver Asc into cells through inefficient approaches (55,56) that are associated with significant side effects (57). Under Asc-deficient culture conditions, human cells largely rely on GSH for Cr(VI) reduction (1). Therefore, we examined the role of both Asc and GSH in DPC formation in A549 cells. These cells were depleted of GSH by 24 hr preincubation with the inhibitor of glutathione synthase, BSO, which decreased GSH levels from 4.2±0.6 to 0.15±0.2 mM (n=3). This severe drop in cellular GSH resulted in lower DPC levels immediately and 18 hr after Cr(VI) exposure (Fig. 6A). On average, GSH-depleted cells contained 1.9- and 3.2-times fewer DPC at 0 and 18 hr post-exposure, respectively. BSO treatment had only a marginal impact on Cr(VI) uptake, which averaged 84.5% of the control cells. Increasing the cellular reducing capacity by restoration of physiological Asc levels (0.9±0.1 mM, n=3) prior to Cr(VI) treatment led to 2.2-fold higher DPC yield (Fig. 6B). This elevated crosslinking activity did not result from significant changes in Cr(VI) uptake following Asc loading (Fig. 6C). We have previously determined that normalization of Asc levels in human lung H460 cells led to about 2-fold decrease in Cr(VI) uptake (37). This difference between A549 and H460 cells was further confirmed by parallel uptake measurements, detecting on average 2.2-times lower accumulation of Cr in Asc-preloaded H460 cells. These analyses also found that A549 cells had approximately 5-times lower Cr(VI) accumulation than H460 cells, which explains the strong resistance of A549 cells to Cr(VI) toxicity (54) and the need for the relatively high Cr(VI) concentrations for the induction of genotoxic effects under short exposure conditions.
Figure 6. Impact of Asc and GSH on DPC formation in A549 cells.
All Cr(VI) exposures were for 3 hr in serum-free medium. Data are means±SD. (A) DPC levels in cells with and without pretreatment with 0.1 mM BSO for 24 hr. DPC were measured either immediately (left panel) or 18 hr after Cr(VI) treatments (right panel). (B) Formation of DPC in control and 1 mM Asc-preloaded cells. (C) Cr accumulation by A549 cells with and without Asc-preloading.
Conclusions
Our results showed that DPC formation involved reduction of Cr(VI) to Cr(III), formation of DNA-Cr(III) adducts and subsequent capture of proteins by DNA-bound Cr(III). The second-arm reaction of protein conjugation was the rate-limiting step in the crosslinking process. The inability of Cr-protein adducts to produce crosslinks probably resulted from the blocked reactivity of Cr(III) due to its multidentate coordination to several readily available groups (peptide bonds, SH, COOH, imidazole nitrogen). Unlike aldehydes (32,40), Cr(VI)-induced crosslinking does not require stable protein-DNA binding, which explains the presence of large amounts of the nonbinding protein actin but a complete absence of histones in Cr(VI)-induced cellular DPC (6,7,33,43). Stable association of proteins with DNA typically involves ionic interactions between negatively charged phosphate groups and positively charged Lys or Arg side chain groups. Cr(III) preferentially binds Cys, His and negatively charged side chain COOH groups in Glu and Asp (1), all of which are rarely found in close contact with the duplex in DNA-binding proteins. While some promoter-bound repressor complexes were crosslinked in Cr(VI)-treated cells (25), this could reflect a somewhat unusual proximity of Cr(III)-coordinating amino acids to DNA either in the bound state or transiently during binding. The observed slow conjugation of the protein component at neutral pH helps explain a prolonged DPC accumulation in primary human lymphocytes (8), which are inefficient in repair of these lesions (40). Poor ability of EDTA to dissociate Cr(III)-containing linkages could have been be responsible for the variable results obtained with this chelator in assessing the role of Cr(III) in chromate-induced crosslinking in cells (7,33). In contrast, phosphate was very efficient in DPC dissociation and this property could be exploited for a more specific determination of Cr-induced DPC in human biomonitoring studies. The inability of protein-bound Cr(III) to react with DNA indicates that, in contrast to DPC-forming bifunctional aldehydes targeting duplex-proximal lysine groups (32), Cr(III) binding by chromatin proteins diminishes the overall DNA damage by Cr(VI).
Acknowledgments
This work was supported by grants R01 ES008786 and P42 ES013660 from the National Institute of Environmental Health Sciences (NIEHS).
Abbreviations
- Asc
ascorbate
- BSA
bovine serum albumin
- DPC
DNA-protein crosslink
- GSH
glutathione
- MES
4-morpholinoethanesulfonic acid
- MOPS
4-morpholinepropanesulfonic acid
- PMSF
phenylmethanesulfonyl fluoride
References
- 1.Zhitkovich A. Importance of chromium-DNA adducts in mutagenicity and toxicity of chromium(VI) Chem Res Toxicol. 2005;18:3–11. doi: 10.1021/tx049774+. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien T, Mandel GH, Pritchard DE, Patierno SR. Critical role of chromium(Cr)-DNA interactions in the formation of Cr-induced polymerase arresting lesions. Biochemistry. 2002;41:12529–12537. doi: 10.1021/bi020452j. [DOI] [PubMed] [Google Scholar]
- 3.Lay PA, Levina A. Activation of molecular oxygen during the reactions of chromium(VI/V/IV) with biological reductants: implications for chromium-induced genotoxicities. J Am Chem Soc. 1998;120:6704–6714. [Google Scholar]
- 4.Slade PG, Hailer MK, Martin BD, Sugden KD. Guanine-specific oxidation of double-stranded DNA by Cr(VI) and ascorbic acid forms spiroiminodihydantoin and 8-oxo-2′-deoxyguanosine. Chem Res Toxicol. 2005;18:1140–1149. doi: 10.1021/tx050033y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton JW, Wetterhahn KE. Chromium (VI)-induced DNA damage in chick embryo liver and blood cells in vivo. Carcinogenesis. 1986;7:2085–2088. doi: 10.1093/carcin/7.12.2085. [DOI] [PubMed] [Google Scholar]
- 6.Costa M. DNA-protein complexes induced by chromate and other carcinogens. Environ Health Perspect. 1991;92:45–52. doi: 10.1289/ehp.919245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattagajasingh SN, Misra HP. Analysis of EDTA-chelatable proteins from DNA-protein crosslinks induced by a carcinogenic chromium(VI) in cultured intact human cells. Mol Cell Biochem. 1999;199:149–162. doi: 10.1023/a:1006910732307. [DOI] [PubMed] [Google Scholar]
- 8.Zhitkovich A, Voitkun V, Kluz T, Costa M. Utilization of DNA-protein crosslinks as a biomarker of chromium exposure. Environ Health Persp. 1998;106:969–974. doi: 10.1289/ehp.98106s4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa M, Zhitkovich A, Toniolo P. DNA-protein crosslinks in welders: molecular implications. Cancer Res. 1993;53:460–463. [PubMed] [Google Scholar]
- 10.Werfel U, Langen V, Eickhoff I, Schoonbrood J, Vahrenholtz C, Brauksiepe A, Popp W, Norpoth K. Elevated DNA single-strand breakage frequencies in lymphocytes of welders exposed to chromium and nickel. Carcinogenesis. 1998;19:413–418. doi: 10.1093/carcin/19.3.413. [DOI] [PubMed] [Google Scholar]
- 11.Zhitkovich A, Lukanova A, Popov T, Taioli E, Cohen H, Costa M, Toniolo P. DNA-protein crosslinks in peripheral lymphocytes of individuals exposed to hexavalent chromium compounds. Biomarkers. 1996;1:86–93. doi: 10.3109/13547509609088675. [DOI] [PubMed] [Google Scholar]
- 12.Budhwar R, Das M, Bihari V, Kumar S. Exposure estimates of chromeplaters in India: an exploratory study. Biomarkers. 2005;10:252–257. doi: 10.1080/13547500500218625. [DOI] [PubMed] [Google Scholar]
- 13.Medeiros MG, Rodrigues AS, Batoreu MC, Laires A, Rueff J, Zhitkovich A. Elevated levels of DNA-protein crosslinks and micronuclei in peripheral lymphocytes of tannery workers exposed to trivalent chromium. Mutagenesis. 2003;18:19–24. doi: 10.1093/mutage/18.1.19. [DOI] [PubMed] [Google Scholar]
- 14.Lukanova A, Toniolo P, Zhitkovich A, Nikolova V, Panev T, Popov T, Taioli E, Costa M. Occupational exposure to Cr(VI): comparison between chromium levels in lymphocytes, erythrocytes and urine. Int Arch Occup Environ Health. 1996;69:39–44. doi: 10.1007/BF02630737. [DOI] [PubMed] [Google Scholar]
- 15.Kerger BD, Finley BL, Corbett GE, Dodge DG, Paustenbach DJ. Ingestion of chromium(VI) in drinking water by human volunteers: Absorption, distribution, and excretion of single and repeated doses. J Tox Environ Health. 1997;50:67–95. doi: 10.1080/009841097160618. [DOI] [PubMed] [Google Scholar]
- 16.Taioli E, Zhitkovich A, Kinney P, Udasin I, Toniolo P, Costa M. Increased DNA-protein crosslinks in lymphocytes of residents living in chromium contaminated areas. Biol Trace Element Res. 1995;50:175–180. doi: 10.1007/BF02785408. [DOI] [PubMed] [Google Scholar]
- 17.Taioli E, Zhitkovich A, Toniolo P, Costa M. Normal values of DNA-protein crosslinks in mononuclear cells of a population of healthy controls. Cancer J. 1995;8:76–78. [Google Scholar]
- 18.Taioli E, Kinney P, Zhitkovich A, Fulton H, Voitkun V, Cosma G, Frenkel K, Toniolo P, Garte S, Costa M. Application of reliability models to studies of biomarker validation. Environ Health Persp. 1994;102:306–309. doi: 10.1289/ehp.94102306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuykendall JR, Miller KL, Mellinger KN, Cain AV. Waterborne and dietary hexavalent chromium exposure causes DNA-protein crosslink (DPX) formation in erythrocytes of largemouth bass (Micropterus salmoides) Aquat Toxicol. 2006;78:27–31. doi: 10.1016/j.aquatox.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Kuykendall JR, Miller KL, Mellinger KM, Cain AJ, Perry MW, Bradley M, Jarvi EJ, Paustenbach DJ. DNA-protein cross-links in erythrocytes of freshwater fish exposed to hexavalent chromium or divalent nickel. Arch Environ Contam Toxicol. 2009;56:260–267. doi: 10.1007/s00244-008-9175-9. [DOI] [PubMed] [Google Scholar]
- 21.Ha L, Ceryak S, Patierno SR. Generation of S phase-dependent DNA double-strand breaks by Cr(VI) exposure: involvement of ATM in Cr(VI) induction of gamma-H2AX. Carcinogenesis. 2004;25:2265–2274. doi: 10.1093/carcin/bgh242. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Hachey DL, Valadez G, Williams KM, Guengerich FP, Loktionova NA, Kanugula S, Pegg AE. Characterization of a mutagenic DNA adduct formed from 1,2-dibromoethane by O6-alkylguanine-DNA alkyltransferase. J Biol Chem. 2004;279:4250–4259. doi: 10.1074/jbc.M311105200. [DOI] [PubMed] [Google Scholar]
- 23.Minko IG, Kozekov ID, Kozekova A, Harris TM, Rizzo CJ, Lloyd RS. Mutagenic potential of DNA-peptide crosslinks mediated by acrolein-derived DNA adducts. Mutat Res. 2008;637:161–172. doi: 10.1016/j.mrfmmm.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loecken EM, Dasari S, Hill S, Tabb DL, Guengerich FP. The bis-electrophile diepoxybutane cross-links DNA to human histones but does not result in enhanced mutagenesis in recombinant systems. Chem Res Toxicol. 2009;22:1069–1076. doi: 10.1021/tx900037u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnekenburger M, Talaska G, Puga A. Chromium cross-links histone deacetylase 1-DNA methyltransferase 1 complexes to chromatin, inhibiting histone-remodeling marks critical for transcriptional activation. Mol Cell Biol. 2007;27:7089–7101. doi: 10.1128/MCB.00838-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCaffrey J, Wolf CM, Hamilton JW. Effects of the genotoxic carcinogen chromium(VI) on basal and hormone-inducible phosphoenolpyruvate carboxykinase gene expression in vivo: correlation with glucocorticoid- and developmentally regulated expression. Mol Carcinog. 1994;10:189–198. doi: 10.1002/mc.2940100403. [DOI] [PubMed] [Google Scholar]
- 27.Wei YD, Tepperman K, Huang MY, Sartor MA, Puga A. Chromium inhibits transcription from polycyclic aromatic hydrocarbon-inducible promoters by blocking the release of histone deacetylase and preventing the binding of p300 to chromatin. J Biol Chem. 2004;279:4110–4119. doi: 10.1074/jbc.M310800200. [DOI] [PubMed] [Google Scholar]
- 28.O’Hara KA, Nemec AA, Alam J, Klei LR, Mossman BT, Barchowsky A. Chromium (VI) inhibits heme oxygenase-1 expression in vivo and in arsenic-exposed human airway epithelial cells. J Cell Physiol. 2006;209:113–121. doi: 10.1002/jcp.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu X, Muller JG, Ye Y, Burrows CJ. DNA-protein cross-links between guanine and lysine depend on the mechanism of oxidation for formation of C5 vs C8 guanosine adducts. J Am Chem Soc. 2008;130:703–709. doi: 10.1021/ja077102a. [DOI] [PubMed] [Google Scholar]
- 30.Bjorklund CC, Davis WB. Stable DNA-protein cross-links are products of DNA charge transport in a nucleosome core particle. Biochemistry. 2007;46:10745–10755. doi: 10.1021/bi700475b. [DOI] [PubMed] [Google Scholar]
- 31.Luxford C, Dean RT, Davies MJ. Induction of DNA damage by oxidised amino acids and proteins. Biogerontology. 2002;3:95–102. doi: 10.1023/a:1015228001561. [DOI] [PubMed] [Google Scholar]
- 32.Voitkun V, Zhitkovich A. Analysis of DNA-protein crosslinking activity of malondialdehyde in vitro. Mutat Res. 1999;424:97–106. doi: 10.1016/s0027-5107(99)00011-1. [DOI] [PubMed] [Google Scholar]
- 33.Miller CA, 3rd, Costa M. Analysis of proteins cross-linked to DNA after treatment of cells with formaldehyde, chromate, and cis-diamminedichloroplatinum(II) Mol Toxicol. 1989;2:11–26. [PubMed] [Google Scholar]
- 34.Kortenkamp A, Curran B, O’Brien P. Defining conditions for the efficient in vitro cross-linking of proteins to DNA by chromium(III) compounds. Carcinogenesis. 1992;13:307–308. doi: 10.1093/carcin/13.2.307. [DOI] [PubMed] [Google Scholar]
- 35.Zhitkovich A, Voitkun V, Costa M. Formation of the amino acid-DNA complexes by hexavalent and trivalent chromium in vitro: importance of trivalent chromium and the phosphate group. Biochemistry. 1996;35:7275–7282. doi: 10.1021/bi960147w. [DOI] [PubMed] [Google Scholar]
- 36.Quievryn G, Messer J, Zhitkovich A. Carcinogenic chromium(VI) induces cross-linking of vitamin C to DNA in vitro and in human lung A549 cells. Biochemistry. 2002;41:3156–3167. doi: 10.1021/bi011942z. [DOI] [PubMed] [Google Scholar]
- 37.Reynolds M, Zhitkovich A. Cellular vitamin C increases chromate toxicity via a death program requiring mismatch repair but not p53. Carcinogenesis. 2007;28:1613–1620. doi: 10.1093/carcin/bgm031. [DOI] [PubMed] [Google Scholar]
- 38.Quievryn G, Goulart M, Messer J, Zhitkovich A. Reduction of Cr(VI) by cysteine: significance in human lymphocytes and formation of DNA damage in reactions with variable reduction rates. Mol Cell Biochem. 2001;222:107–118. [PubMed] [Google Scholar]
- 39.Zhitkovich A, Costa M. A simple, sensitive assay to detect DNA-protein-crosslinks in intact cells and in vivo. Carcinogenesis. 1992;13:1485–1489. doi: 10.1093/carcin/13.8.1485. [DOI] [PubMed] [Google Scholar]
- 40.Quievryn G, Zhitkovich A. Loss of DNA-protein crosslinks from formaldehyde-exposed cells occurs through spontaneous hydrolysis and an active repair process linked to proteasome function. Carcinogenesis. 2000;21:1573–1580. [PubMed] [Google Scholar]
- 41.Messer J, Reynolds M, Stoddard L, Zhitkovich A. Causes of DNA single-strand breaks during reduction of chromate by glutathione in vitro and in cells. Free Radic Biol Med. 2006;40:1981–1992. doi: 10.1016/j.freeradbiomed.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 42.Salnikow K, Zhitkovich A, Costa M. Analysis of the binding sites of chromium to DNA and protein in vitro and in intact cells. Carcinogenesis. 1992;13:2341–2346. doi: 10.1093/carcin/13.12.2341. [DOI] [PubMed] [Google Scholar]
- 43.Miller CA, 3rd, Cohen MD, Costa M. Complexing of actin and other nuclear proteins to DNA by cis-diamminedichloroplatinum(II) and chromium compounds. Carcinogenesis. 1991;12:269–276. doi: 10.1093/carcin/12.2.269. [DOI] [PubMed] [Google Scholar]
- 44.Gebicki S, Gebicki JM. Crosslinking of DNA and proteins induced by protein hydroperoxides. Biochem J. 1999;338:629–636. [PMC free article] [PubMed] [Google Scholar]
- 45.Distel L, Distel B, Schuessler H. DNA doeble-strand breaks and DNA-protein crosslinks: a comparison of the influence of histones and non-histones. Radiat Res. 1997;148:517–518. [Google Scholar]
- 46.Zhitkovich A, Messer J, Shrager S. Reductive metabolism of Cr(VI) by cysteine leads to the formation of binary and ternary Cr-DNA adducts in the absence of oxidative DNA damage. Chem Res Toxicol. 2000;13:1114–1124. doi: 10.1021/tx0001169. [DOI] [PubMed] [Google Scholar]
- 47.Cohen MD, Miller CA, Xu LS, Snow ET, Costa M. A blotting method for monitoring the formation of chemically induced DNA-protein complexes. Anal Biochem. 1990;186:1–7. doi: 10.1016/0003-2697(90)90562-n. [DOI] [PubMed] [Google Scholar]
- 48.Standeven AM, Wetterhahn KE. Ascorbate is the principal reductant of chromium(VI) in rat lung ultrafiltrates and cytosols, and mediates chromium-DNA binding in vitro. Carcinogenesis. 1992;13:1319–1324. doi: 10.1093/carcin/13.8.1319. [DOI] [PubMed] [Google Scholar]
- 49.Quievryn G, Peterson E, Messer J, Zhitkovich A. Genotoxicity and mutagenicity of chromium(VI)/ascorbate-generated DNA adducts in human and bacterial cells. Biochemistry. 2003;42:1062–1070. doi: 10.1021/bi0271547. [DOI] [PubMed] [Google Scholar]
- 50.Stearns DM, Wetterhahn KE. Reaction of Cr(VI) with ascorbate produces chromium(V), chromium(IV), and carbon-based radicals. Chem Res Toxicol. 1994;7:219–230. doi: 10.1021/tx00038a016. [DOI] [PubMed] [Google Scholar]
- 51.Lay PA, Levina A. Activation of molecular oxygen during the reactions of chromium(VI/V/IV) with biological reductants: implications for chromium-induced genotoxicities. J Am Chem Soc. 1998;120:6704–6714. [Google Scholar]
- 52.Guttmann D, Poage G, Johnston T, Zhitkovich A. Reduction with glutathione is a weakly mutagenic pathway in chromium(VI) metabolism. Chem Res Toxicol. 2008;21:2188–2194. doi: 10.1021/tx800265g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reynolds M, Stoddard L, Bespalov I, Zhitkovich A. Ascorbate acts as a highly potent inducer of chromate mutagenesis and clastogenesis: linkage to DNA breaks in G2 phase by mismatch repair. Nucleic Acids Res. 2007;35:465–476. doi: 10.1093/nar/gkl1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin BD, Schoenhard JA, Hwang JM, Sugden KD. Ascorbate is a pro-oxidant in chromium-treated human lung cells. Mutat Res. 2006;610:74–84. doi: 10.1016/j.mrgentox.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 55.Sugiyama M, Tsuzuki K, Ogura R. Effect of ascorbic acid on DNA damage, cytotoxicity, glutathione reductase, and formation of paramagnetic chromium in Chinese hamster V-79 cells treated with sodium chromate(VI) J Biol Chem. 1991;266:3383–3386. [PubMed] [Google Scholar]
- 56.Capellmann M, Mikalsen A, Hindrum M, Alexander J. Influence of reducing compounds on the formation of DNA-protein cross-links in HL-60 cells induced by hexavalent chromium. Carcinogenesis. 1995;16:1135–1139. doi: 10.1093/carcin/16.5.1135. [DOI] [PubMed] [Google Scholar]
- 57.Koh WS, Lee SJ, Lee H, Park C, Park MH, Kim WS, Yoon SS, Park K, Hong SI, Chung MH, Park CH. Differential effects and transport kinetics of ascorbate derivatives in leukemic cell lines. Anticancer Res. 1998;18:2487–2493. [PubMed] [Google Scholar]