Abstract
Profound metabolic changes occur in people with type 1 diabetes mellitus during insulin deprivation. These include an increase in basal energy expenditure and reduced mitochondrial function. In addition, protein metabolism is significantly affected during insulin deprivation. A greater increase in whole-body protein breakdown than protein synthesis occurs resulting in a net protein loss. During insulin deprivation the splanchnic bed has a net protein accretion which accounts for the total increase in whole-body protein synthesis while muscle is in a net catabolic state.
Keywords: energy metabolism, insulin, mitochondria, protein synthesis, type 1 diabetes
Introduction
In the absence of insulin replacement type 1 diabetes is a catabolic condition with severe depletion of both energy stores and protein mass. Since most type 1 diabetic individuals are treated with insulin, a short period of insulin withdrawal in these individuals provides a model system to study the role of insulin in energy and protein metabolism. The causes of negative energy balance and protein catabolism have been extensively studied in people with type 1 diabetes and will be reviewed in this article.
Energy Metabolism in Type 1 Diabetes
Profound changes in energy metabolism occur in people with type 1 diabetes mellitus (T1DM) during insulin deprivation in addition to the well known increase in plasma glucose. When glucose levels exceed renal threshold glycosuria and associated water loss occur. Glucose loss in urine will contribute to negative energy balance. Negative energy balance occurs in insulin deficient states in these diabetic people despite a relative increase in energy intake. These clinical observations are the basis of many studies that investigated the potential role of insulin on energy metabolism. The importance of insulin in the regulation of energy metabolism is supported by studies performed in people with type 1 diabetes during insulin deprivation and insulin treatment as well as in non-diabetic people.
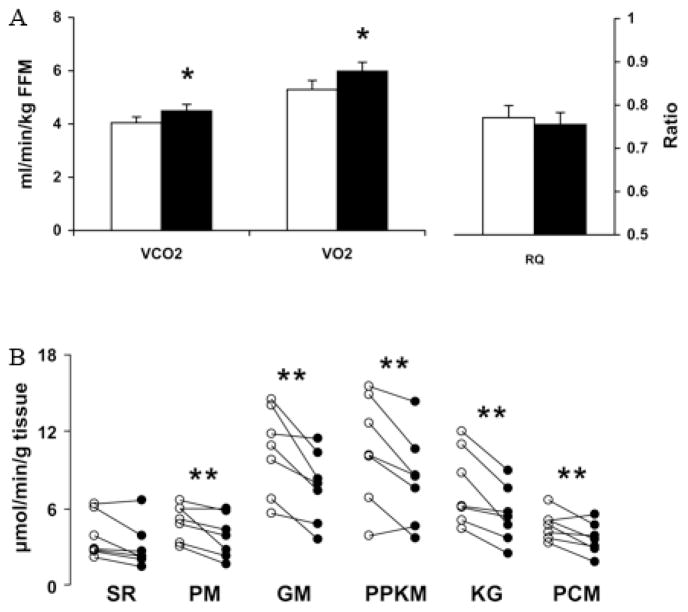
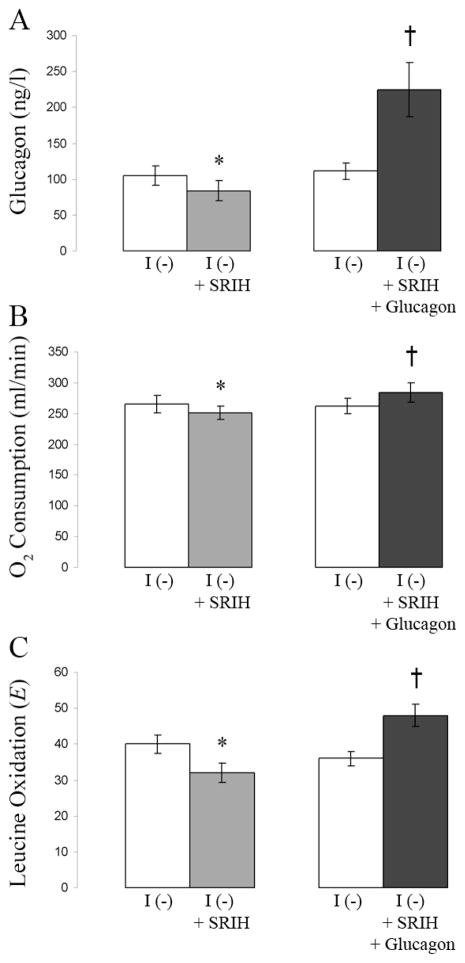
Several studies have shown an increase in basal energy expenditure in type 1 diabetic people following insulin withdrawal (Figure 1A).1–3 In these studies, there is a small but significant increase in whole-body oxygen consumption in type 1 diabetic people during insulin withdrawal when compared with both their own oxygen consumption during insulin treatment and the oxygen consumption of non-diabetic control subjects. One factor involved in causing this increase in energy expenditure during insulin deficiency is the elevation of glucagon levels. Early studies in type 1 diabetic people were able to correlate high plasma glucagon with increased energy expenditure.3 In one study1 mean fasting glucagon levels in type 1 diabetic individuals during insulin deprivation were reported to be 115 ± 12 ng/L. In these same individuals glucagon levels fell to 67 ± 6 ng/L during insulin treatment, which is similar to fasting glucagon levels (50 ± 9 ng/L) reported in a separate study4 in non-diabetic individuals. Further work using somatostatin (to block endogenous glucagon production) while selectively increasing glucagon to high levels (comparable to those seen during the insulin-deprived state in T1DM) demonstrated the ability of glucagon to increase oxygen consumption in non-diabetic people.5 Moreover, blocking the increase in glucagon levels during insulin deprivation in type 1 diabetic people by infusion of somatostatin prevented the increase in oxygen consumption that normally occurred.1 In this study in type 1 diabetic people, infusion of exogenous glucagon to reach levels that normally occur during insulin deprivation resulted in an increase in energy expenditure that was similar to that observed in these people during insulin deprivation (Figure 2A, 2B). Therefore, the high level of glucagon seen in poorly controlled type 1 diabetic people contributes to their increased energy expenditure and thus to their catabolic state. High glucagon is likely to increase gluconeogenesis,4 which is an energy consuming reaction that may also contribute to a hypermetabolic state in T1DM during insulin deprivation.3 High glucagon levels, unopposed by insulin, enhance hepatic glucose production which is a key contributor to hyperglycemia, glycosuria, and energy wasting. Thus hyperglucagonemia seems to be a pivotal catabolic factor in T1DM during insulin deprivation.
Figure 1.
Indirect calorimetry and muscle mitochondrial ATP production rate (MAPR) in type 1 diabetic people. A, Whole-body Vco2 and Vo2 during rest (n = 8) were significantly higher in type 1 diabetic people during insulin deprivation (I−, ■) compared with insulin treatment (I+, □). There was no difference in respiratory quotient (RQ). *P < 0.05 B, MAPR (n = 7) was significantly lower in type 1 diabetic people during insulin deprivation (I−, ●) compared with insulin treatment (I+, ○) using pyruvate plus malate (PM), glutamate plus malate (GM), pyruvate plus palmitoyl-L-carnitine plus α-ketoglutarate plus malate (PPKM), α-ketoglutarate plus glutamate (KG), and palmitoyl-L-carnitine plus one malate (PCM). There was no significant difference with succinate plus rotenone (SR). **P < 0.02 Taken with permission from Karakelides et al., 2007 Diabetes 56(11):2683–9.
Figure 2.
High glucagon levels increase O2 consumption and leucine oxidation. The same 6 type 1 diabetic people were used in two separate studies. Baseline measurements were taken in each study during insulin deprivation (I (−)). In one study, only somatostatin was infused during insulin deprivation (I (−) + SRIH). In a separate study, somatostatin and high levels of glucagon were infused during insulin deprivation (I (−) + SRIH + Glucagon). Data are means ± SE. Values for glucagon (A), O2 consumption (B), and leucine oxidation (C) are shown. *A value significantly lower than during the baseline period; †a value significantly higher than that obtained during the baseline period. Taken with permission from data in Charlton and Nair, 1998 Diabetes 47(11):1748.
Other factors may also contribute to increased oxygen consumption during insulin deprivation in T1DM. For example, the increased protein turnover that occurs in T1DM during insulin deprivation6, 7 is an energy consuming process.8 Other energy consuming factors including transport of compounds across cell membranes may also contribute to increased energy expenditure. Additionally, the uncoupling of oxidative phosphorylation with proton leak during electron chain transport in the inner mitochondrial compartment should also be investigated as a contributor to increased oxygen consumption.
Increased oxygen consumption suggests an increased rate of oxidative phosphorylation. However, studies in done in type 1 diabetic people demonstrated that despite an increase in whole-body oxygen consumption the skeletal muscle mitochondrial ATP production rate (MAPR) was decreased in type 1 diabetic people during insulin deprivation (Figure 1B).2 The above study did not measure muscle mitochondrial oxygen consumption which may be different from what has been shown at the whole-body level during insulin deprivation. The dissociation between increased energy expenditure and decreased muscle MAPR could be explained by an uncoupling of oxidative phosphorylation during insulin deprivation, although there was no change in mRNA levels of UCP2 or UCP3.2 An alternative explanation has been proposed. With a role for high glucagon levels in increasing energy expenditure already established, it is possible as suggested2 that the increase in whole-body oxygen consumption may be occurring mostly within the liver and not in skeletal muscle since the liver is the major site of glucagon action and skeletal muscle does not contain glucagon receptors. An increase in protein synthesis within the splanchnic region during insulin deprivation in type 1 diabetic people provides further evidence for the aforementioned explanation.6 Since the synthesis of proteins is an energy consuming process8 it is likely that this could also contribute to an increase in whole-body oxygen consumption. These and other changes in protein metabolism that occur in type 1 diabetic people are discussed in greater detail later.
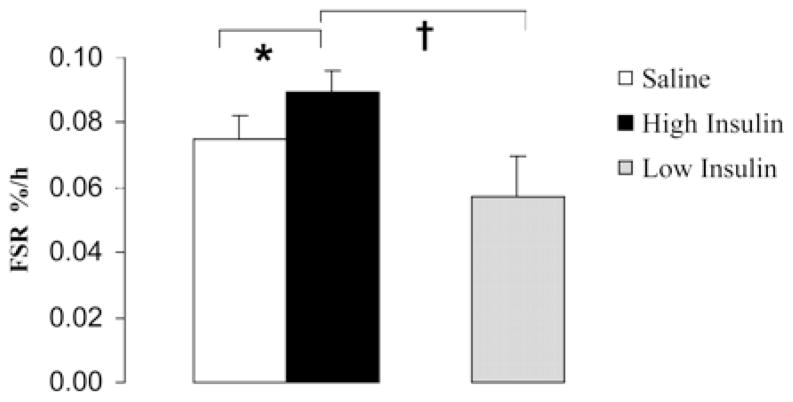
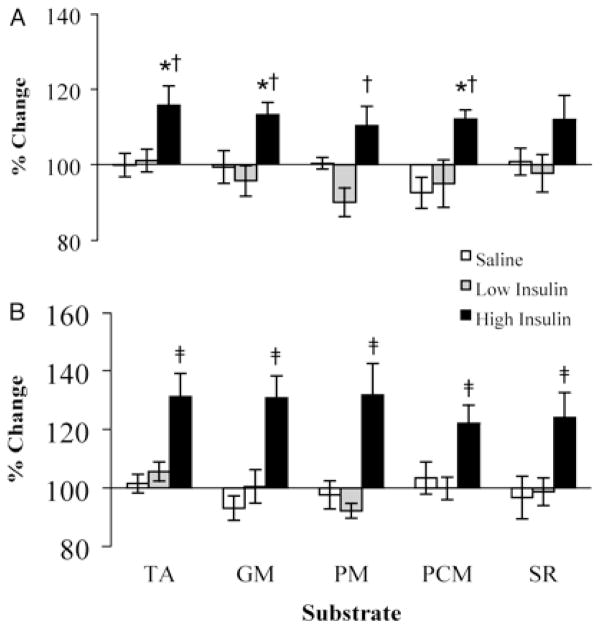
The withdrawal of insulin in type 1 diabetic people affects mitochondrial function, protein synthesis, and enzyme activity. Insulin stimulates the transcription of genes involved in oxidative phosphorylation2, 9 in addition to mitochondrial protein synthesis rates in muscle (Figure 3).9, 10 There is evidence that the amount of mitochondrial protein present is closely related to the rate of ATP production.11 In fact, treatment of non-diabetic people with insulin (provided there is an exogenous infusion of glucose and amino acids to prevent their insulin induced decline) increases muscle MAPR (Figure 4).9 Futhermore muscle MAPR is decreased in type 1 diabetic people during periods of insulin deprivation (Figure 1B).2 Additionally, insulin is able to increase the activity of the mitochondrial oxidative enzymes, citrate synthase and COX.9 Therefore insulin, either directly or through it’s modulation of other signaling pathways,2 is a major regulator of mitochondrial function.
Figure 3.
Skeletal muscle mitochondrial protein fractional synthesis rates (FSR). Mitochondrial FSR in healthy people during saline and high-dose insulin treatment. FSR during low-dose insulin infusion in a separate group of healthy people is also shown. Low-dose or high-dose insulin was infused while clamping glucose, amino acids, glucagon, and growth hormone. Values are means ± SEM. *, Significantly different from the saline value (P < 0.05); †, significantly different from the low insulin value (P < 0.05). Taken with permission from Stump et al., 2003 PNAS 100(13):7996–8001. Copyright (2003) National Academy of Sciences, U.S.A.
Figure 4.
Muscle MAPR in healthy people during insulin infusion. Vastus lateralis muscle mitochondrial ATP production rates after 4 h (A) and 8 h (B) of saline, low-dose insulin, and high-dose insulin infusion in healthy people. Low-dose or high-dose insulin was infused while clamping glucose, amino acids, glucagon, and growth hormone. Values are expressed as a percentage of preinfusion baseline (means ± SEM). Measurements were made in the presence of five different substrate combinations: N, N, N′, N′ – tetramethyl-p-phenylenediamine plus ascorbate (TA), glutamate plus malate (GM), pyruvate plus malate (PM), palmitoyl-L-carnitine plus malate (PCM), or succinate plus rotenone (SR). *, Significantly different (P < 0.05) from the saline values; †, significantly different from the low insulin values at 4 h (P < 0.05); ‡, significant difference from the saline values and low insulin values at 8 h (P < 0.01). Taken with permission from Stump et al., 2003 PNAS 100(13):7996–8001. Copyright (2003) National Academy of Sciences, U.S.A.
Protein Metabolism in Type 1 Diabetes
The regulation of protein synthesis and breakdown is a central component of metabolic and physiological homeostasis.12 Type 1 diabetic people are in a profound protein catabolic state without insulin replacement. The role of insulin in protein metabolism has been investigated in whole-body, regional and individual protein studies.
An increase in both whole-body protein breakdown and synthesis occurs in poorly controlled type 1 diabetic people. This increase in protein breakdown is greater than the increase in protein synthesis resulting in a net protein loss.7 Protein breakdown during insulin deprivation occurs mostly in skeletal muscle, while protein synthesis occurs in the splanchnic region.6 Glucagon, the hormone determined to be largely responsible for the increased energy expenditure during insulin deprivation in type 1 diabetic individuals, increases leucine (Figure 2C) and phenylalanine oxidation and protein breakdown.1, 13, 14 Moreover, following an amino acid load in fasted individuals, hyperglucagonemia reduces glucogenic plasma amino acids by more than 50 percent.15 By reducing the availability of glucogenic amino acids, glucagon inhibits protein synthesis.15 Although the major role of glucagon appears to be catabolic, it is may also be involved in the increase in splanchnic protein synthesis that occurs in the insulin deprived state. For example, Tessari et al.16 has shown that the increase in fibrinogen synthesis reported during insulin deprivation17 is likely related to high glucagon levels. Increased energy expenditure as a result of increased protein turnover may also be caused by factors other than glucagon. During insulin deprivation in type 1 diabetic people there is an increase in circulating amino acids (particularly branched chain amino acids) and ketones.18 Branched chain amino acids increase leucine oxidation and whole-body protein synthesis and inhibit protein breakdown.19, 20 Amino acids have also been shown to be a key regulator of protein synthesis in the splanchnic bed.21 It has been proposed that amino acids are released from the breakdown of skeletal muscle protein during insulin deprivation. These amino acids are then taken up by the splanchnic bed which stimulates splanchnic protein synthesis.18 Ketoacids decrease nitrogen loss and leucine oxidation and increase whole-body and skeletal muscle protein synthesis.22 Together these data provide evidence that the increased protein turnover that occurs during insulin deprivation in type 1 diabetic people may be related to secondary events such as hyperaminoacidemia, hyperglucagonemia, and high ketone levels.
Insulin has a differential effect on protein metabolism dependent on the tissue, subcellular fraction, or individual protein studied. While insulin inhibits whole-body protein synthesis, the main action of insulin treatment on protein metabolism in type 1 diabetic people in the whole-body is to decrease protein breakdown.6, 23 Insulin treatment inhibits protein breakdown in splanchnic and skeletal muscle tissues and selectively inhibits protein synthesis in the splanchnic region.6, 24 When skeletal muscle is subdivided into mitochondrial and sarcoplasmic fractions, the ability of insulin to increase mitochondrial protein synthesis is seen.10 Furthermore, insulin has differential effects on the synthesis rates of individual proteins. Fractional synthesis rates of albumin are decreased in type I diabetic people during insulin deficiency while fibrinogen fractional synthesis rates are increased.17 Additionally, the synthesis rates of several plasma proteins are altered during short-term insulin deprivation. The effects of insulin on protein synthesis rates are not seen in whole-body or even regional tissue studies since these studies provide a summation of the effects on individual proteins.
The inability to fully demonstrate whether insulin can stimulate protein synthesis based on whole-body or regional isotopic tracer studies in type 1 diabetic individuals indicate the importance of studying insulin action on individual protein synthesis. A new method was developed in our lab to simultaneously measure the fractional synthesis rates of multiple plasma proteins25 or multiple skeletal muscle proteins.26 This method enables us to investigate the effects of insulin deprivation on the synthesis rates of individual mitochondrial proteins in type 1 diabetic people. Using this method to determine synthesis rates of proteins involved in signaling pathways might be of interest since a decline in amino acid levels upon insulin replacement may down-regulate anabolic signaling pathways such as mTOR.
Protein and Energy Metabolism in Asian Indians and Type 2 Diabetes
While most studies indicate that insulin positively regulates mitochondrial function, Asian Indians show a dissociation between mitochondrial ATP production and insulin sensitivity.27 Similarly, caloric restriction improves insulin sensitivity and reduces intramuscular triglycerides, but does not increase energy metabolism or mitochondrial functions.28 The interaction between insulin resistance in specific organs and energy metabolism remains to be fully understood. Asian Indians have enhanced mitochondrial function and mitochondrial DNA copy number despite having a substantial reduction in insulin sensitivity when compared with Northern European Americans.27 Further investigation needs to be conducted to determine whether this is a result of an adaptive process developed by Asian Indians or if this is modulated through genetic factors. Additionally, the site of insulin resistance (liver versus skeletal muscle) may prove to be important since Asian Indians have a high prevalence of nonalcoholic fatty liver disease.29 An association between intramyocellular triglyceride levels and insulin resistance has been demonstrated.30 Asian Indian diabetic people may have a tendency to accumulate greater amounts of intramyocellular triglyceride although no difference between non-diabetic Asian Indians and non-diabetic Northern European Americans has been noted.27
Changes in protein and energy metabolism have also been studied in type 2 diabetic individuals. Studies done while maintaining identical blood glucose and insulin concentrations demonstrated that skeletal muscle ATP production and mitochondrial DNA copy number were similar between type 2 diabetic and non-diabetic individuals during periods of low insulin (similar to postabsorptive levels). The transcript levels of most genes encoding mitochondrial proteins were lower in skeletal muscle of type 2 diabetic individuals; however, the transcript and protein levels of genes encoding subunits of the electron transport chain complexes were higher in type 2 diabetic individuals compared to non-diabetic individuals indicating a compensatory response to maintain MAPR.31 Increasing insulin to postprandial levels did not change mitochondrial DNA copy number in type 2 diabetic or non-diabetic individuals. Treatment with high levels of insulin stimulated muscle MAPR and mitochondrial protein synthesis in non-diabetic individuals but not in type 2 diabetic individuals.9, 31, 32 Additionally, the glucose infusion rate necessary to maintain similar glucose levels was lower in type 2 diabetic individuals during periods of high insulin indicating reduced insulin sensitivity compared to nondiabetic individuals.31 These data indicate that reduced insulin sensitivity in type 2 diabetic people explains the inability of high dose insulin to increase their muscle MAPR and mitochondrial protein synthesis.
Summary
The changes in both energy and protein metabolism in type 1 diabetic people without insulin replacement are extensive. Insulin treatment returns their increased energy expenditure to normal levels and retains mitochondrial function. In addition, insulin treatment reduces whole-body and muscle protein breakdown and stimulates the synthesis of select individual proteins including muscle mitochondrial proteins. The increase in splanchnic protein synthesis that occurs with insulin deprivation needs to be studied further to understand the specific proteins affected. The new methodology developed to measure individual protein synthesis will substantially help to accomplish the above goal.
Acknowledgments
The studies are supported by grants from the National Institute of Health R01 DK41973 and UL1 RR02415-01.
Non-standard abbreviations
- COX
cyclooxygenase
- FSR
fractional synthesis rate
- MAPR
mitochondrial ATP production rate
- T1DM
type 1 diabetes mellitus
- UCP
uncoupling protein
Footnotes
Conference presentation: 2008 Protein Symposium, Padua, Italy
Conflict of Interest
The authors have no conflicts of interest to report.
Statement of authorship: SLH and KSN drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Sadie L. Hebert, Email: hebert.sadie@mayo.edu, Mayo Clinic, 200 First St. SW, Joseph 5-194, Rochester, MN 55905
K. Sreekumaran Nair, Email: nair.sree@mayo.edu, Mayo Clinic, 200 First St. SW, Joseph 5-194, Rochester, MN 55905.
References
- 1.Charlton MR, Nair KS. Role of hyperglucagonemia in catabolism associated with type 1 diabetes: effects on leucine metabolism and the resting metabolic rate. Diabetes. 1998;47(11):1748–56. doi: 10.2337/diabetes.47.11.1748. [DOI] [PubMed] [Google Scholar]
- 2.Karakelides H, Asmann YW, Bigelow ML, Short KR, Dhatariya K, Coenen-Schimke J, Kahl J, Mukhopadhyay D, Nair KS. Effect of insulin deprivation on muscle mitochondrial ATP production and gene transcript levels in type 1 diabetic subjects. Diabetes. 2007;56(11):2683–9. doi: 10.2337/db07-0378. [DOI] [PubMed] [Google Scholar]
- 3.Nair KS, Halliday D, Garrow JS. Increased energy expenditure in poorly controlled Type 1 (insulin-dependent) diabetic patients. Diabetologia. 1984;27(1):13–6. doi: 10.1007/BF00253494. [DOI] [PubMed] [Google Scholar]
- 4.Chhibber VL, Soriano C, Tayek JA. Effects of low-dose and high-dose glucagon on glucose production and gluconeogenesis in humans. Metabolism. 2000;49(1):39–46. doi: 10.1016/s0026-0495(00)90638-3. [DOI] [PubMed] [Google Scholar]
- 5.Nair KS. Hyperglucagonemia increases resting metabolic rate in man during insulin deficiency. J Clin Endocrinol Metab. 1987;64(5):896–901. doi: 10.1210/jcem-64-5-896. [DOI] [PubMed] [Google Scholar]
- 6.Nair KS, Ford GC, Ekberg K, Fernqvist-Forbes E, Wahren J. Protein dynamics in whole body and in splanchnic and leg tissues in type I diabetic patients. J Clin Invest. 1995;95(6):2926–37. doi: 10.1172/JCI118000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair KS, Garrow JS, Ford C, Mahler RF, Halliday D. Effect of poor diabetic control and obesity on whole body protein metabolism in man. Diabetologia. 1983;25(5):400–3. doi: 10.1007/BF00282518. [DOI] [PubMed] [Google Scholar]
- 8.Welle S, Nair KS. Relationship of resting metabolic rate to body composition and protein turnover. Am J Physiol. 1990;258(6 Pt 1):E990–8. doi: 10.1152/ajpendo.1990.258.6.E990. [DOI] [PubMed] [Google Scholar]
- 9.Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci U S A. 2003;100(13):7996–8001. doi: 10.1073/pnas.1332551100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boirie Y, Short KR, Ahlman B, Charlton M, Nair KS. Tissue-specific regulation of mitochondrial and cytoplasmic protein synthesis rates by insulin. Diabetes. 2001;50(12):2652–8. doi: 10.2337/diabetes.50.12.2652. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi M, Hood DA. Protein import into subsarcolemmal and intermyofibrillar skeletal muscle mitochondria. Differential import regulation in distinct subcellular regions. J Biol Chem. 1996;271(44):27285–91. doi: 10.1074/jbc.271.44.27285. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. John Wiley & Sons; Hoboken, New Jersey: 2005. Whole Body Protein Synthesis and Breakdown. [Google Scholar]
- 13.Nair KS, Halliday D, Matthews DE, Welle SL. Hyperglucagonemia during insulin deficiency accelerates protein catabolism. Am J Physiol. 1987;253(2 Pt 1):E208–13. doi: 10.1152/ajpendo.1987.253.2.E208. [DOI] [PubMed] [Google Scholar]
- 14.Tessari P, Inchiostro S, Barazzoni R, Zanetti M, Vettore M, Biolo G, Iori E, Kiwanuka E, Tiengo A. Hyperglucagonemia stimulates phenylalanine oxidation in humans. Diabetes. 1996;45(4):463–70. doi: 10.2337/diab.45.4.463. [DOI] [PubMed] [Google Scholar]
- 15.Charlton MR, Adey DB, Nair KS. Evidence for a catabolic role of glucagon during an amino acid load. J Clin Invest. 1996;98(1):90–9. doi: 10.1172/JCI118782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tessari P, Iori E, Vettore M, Zanetti M, Kiwanuka E, Davanzo G, Barazzoni R. Evidence for acute stimulation of fibrinogen production by glucagon in humans. Diabetes. 1997;46(8):1368–71. doi: 10.2337/diab.46.8.1368. [DOI] [PubMed] [Google Scholar]
- 17.De Feo P, Gaisano MG, Haymond MW. Differential effects of insulin deficiency on albumin and fibrinogen synthesis in humans. J Clin Invest. 1991;88(3):833–40. doi: 10.1172/JCI115384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlton M, Nair KS. Protein metabolism in insulin-dependent diabetes mellitus. J Nutr. 1998;128(2 Suppl):323S–327S. doi: 10.1093/jn/128.2.323S. [DOI] [PubMed] [Google Scholar]
- 19.Louard RJ, Barrett EJ, Gelfand RA. Effect of infused branched-chain amino acids on muscle and whole-body amino acid metabolism in man. Clin Sci (Lond) 1990;79(5):457–66. doi: 10.1042/cs0790457. [DOI] [PubMed] [Google Scholar]
- 20.Nair KS, Schwartz RG, Welle S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol. 1992;263(5 Pt 1):E928–34. doi: 10.1152/ajpendo.1992.263.5.E928. [DOI] [PubMed] [Google Scholar]
- 21.Nygren J, Nair KS. Differential regulation of protein dynamics in splanchnic and skeletal muscle beds by insulin and amino acids in healthy human subjects. Diabetes. 2003;52(6):1377–85. doi: 10.2337/diabetes.52.6.1377. [DOI] [PubMed] [Google Scholar]
- 22.Nair KS, Welle SL, Halliday D, Campbell RG. Effect of beta-hydroxybutyrate on whole-body leucine kinetics and fractional mixed skeletal muscle protein synthesis in humans. J Clin Invest. 1988;82(1):198–205. doi: 10.1172/JCI113570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nair KS, Ford GC, Halliday D. Effect of intravenous insulin treatment on in vivo whole body leucine kinetics and oxygen consumption in insulin-deprived type I diabetic patients. Metabolism. 1987;36(5):491–5. doi: 10.1016/0026-0495(87)90049-7. [DOI] [PubMed] [Google Scholar]
- 24.Meek SE, Persson M, Ford GC, Nair KS. Differential regulation of amino acid exchange and protein dynamics across splanchnic and skeletal muscle beds by insulin in healthy human subjects. Diabetes. 1998;47(12):1824–35. doi: 10.2337/diabetes.47.12.1824. [DOI] [PubMed] [Google Scholar]
- 25.Jaleel A, Nehra V, Persson XM, Boirie Y, Bigelow M, Nair KS. In vivo measurement of synthesis rate of multiple plasma proteins in humans. Am J Physiol Endocrinol Metab. 2006;291(1):E190–7. doi: 10.1152/ajpendo.00390.2005. [DOI] [PubMed] [Google Scholar]
- 26.Jaleel A, Short KR, Asmann YW, Klaus KA, Morse DM, Ford GC, Nair KS. In vivo measurement of synthesis rate of individual skeletal muscle mitochondrial proteins. Am J Physiol Endocrinol Metab. 2008;295(5):E1255–68. doi: 10.1152/ajpendo.90586.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nair KS, Bigelow ML, Asmann YW, Chow LS, Coenen-Schimke JM, Klaus KA, Guo ZK, Sreekumar R, Irving BA. Asian Indians have enhanced skeletal muscle mitochondrial capacity to produce ATP in association with severe insulin resistance. Diabetes. 2008;57(5):1166–75. doi: 10.2337/db07-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toledo FG, Menshikova EV, Azuma K, Radikova Z, Kelley CA, Ritov VB, Kelley DE. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes. 2008;57(4):987–94. doi: 10.2337/db07-1429. [DOI] [PubMed] [Google Scholar]
- 29.Petersen KF, Dufour S, Feng J, Befroy D, Dziura J, Dalla Man C, Cobelli C, Shulman GI. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci U S A. 2006;103(48):18273–7. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119(5 Suppl 1):S10–6. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML, Nair KS. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes. 2006;55(12):3309–19. doi: 10.2337/db05-1230. [DOI] [PubMed] [Google Scholar]
- 32.Halvatsiotis P, Short KR, Bigelow M, Nair KS. Synthesis rate of muscle proteins, muscle functions, and amino acid kinetics in type 2 diabetes. Diabetes. 2002;51(8):2395–404. doi: 10.2337/diabetes.51.8.2395. [DOI] [PubMed] [Google Scholar]