Abstract
Recently, a great deal of interest has arisen in resting state fMRI as a measure of tonic brain function in clinical populations. Most studies have focused on the examination of temporal correlation between resting state fMRI low-frequency oscillations (LFOs). Studies on the amplitudes of these low-frequency oscillations are rarely reported. Here, we used amplitude of low-frequency fluctuations (ALFF) and fractional ALFF (fALFF; the relative amplitude that resides in the low frequencies) to examine the amplitude of LFO in schizophrenia. Twenty-six healthy controls and 29 patients with schizophrenia or schizoaffective disorder participated. Our findings show that patients showed reduced low-frequency amplitude in proportion to the total frequency band investigated (i.e., fALFF) in the lingual gyrus, left cuneus, left insula/superior temporal gyrus, and right caudate and increased fALFF in the medial prefrontal cortex and the right parahippocampal gyrus. ALFF was reduced in patients in the lingual gyrus, cuneus, and precuneus and increased in the left parahippocampal gyrus. These results suggest LFO abnormalities in schizophrenia. The implication of these abnormalities for schizophrenic symptomatology is further discussed.
Keywords: Low-frequency oscillation, Schizophrenia, Resting state fMRI
1. Introduction
Recent electrophysiological findings have pointed to the role of abnormal oscillatory processes in schizophrenia (Ford et al., 2007; Uhlhaas et al., 2008). In particular, deficits in gamma band oscillatory processes over frontal electrode sites have been found in unmedicated patients with schizophrenia (Gallinat et al., 2004). The frequency (Spencer et al., 2003; Spencer et al., 2004) and phase locking (Spencer et al., 2008) of gamma oscillations induced by visual Gestalt stimuli is reduced in patients with schizophrenia. In frontal regions, gamma synchrony impairments are associated with cognitive control deficits observed in the disorder (Cho et al., 2006). These oscillations also appear to be important in working memory (Tallon-Baudry et al., 1998). Thus, gamma abnormalities are associated with cognitive (Goldman-Rakic, 1994; Carter, 2006) and perceptual (Butler and Javitt, 2005; Javitt, 2000) domains that are impaired in schizophrenia.
The specificity of these findings to the gamma band is unclear. Abnormalities in other frequency bands have been observed; these abnormalities may be related across bands. For example, Lakatos et al. (2005) have proposed an oscillatory hierarchy that is involved in controlling neuronal excitability and stimulus processing. The key idea is that frequency ranges entrain to each other in a hierarchical manner. Thus, Lakatos et al. (2005) found that in the primary auditory cortex of awake macaque monkeys, delta activity modulated theta activity, which in turn modulated gamma amplitude. This was the case both spontaneously and in stimulus-driven states. These entrainment processes may play a role in attentional (Lakatos et al., 2008) and sensory (Schroeder and Lakatos, 2009) selection. They appear to be, at least partially, under control of glutamatergic systems (Wolf et al., 2005), which are known to be abnormal in schizophrenia (Javitt and Zukin, 1991). In an important recent study, Ehrlichman et al. (2009) found that NMDA receptor antagonist-induced theta and gamma oscillations were consistent with abnormalities in these ranges observed in schizophrenia. In view of the hierarchy of brain oscillatory modulations, the possibility remains that abnormalities in even lower frequency ranges may be observed in schizophrenia.
These abnormalities could be investigated by using the resting state fMRI approaches. Biswal et al. (1995) discovered that spontaneous low-frequency oscillations (LFOs) in the resting state blood oxygen level dependent (BOLD) signal were synchronized across left and right motor cortices (i.e., resting state functional connectivity: RSFC) and gray matter exhibited higher amplitude of LFO than white matter (Biswal et al., 1995). Although several studies demonstrated the altered RSFC in various brain networks of schizophrenia (Garrity et al., 2007; Hoptman, et al., in press; Bluhm et al., 2007; Liang et al., 2006; Zhou et al., 2007), to the best of our knowledge, no resting state fMRI study has examined the amplitude of LFO in schizophrenia. The study of these phenomena may elucidate the understanding of these LFOs.
Several methods have been developed to examine the amplitude of LFOs using resting state fMRI. In the time domain, Biswal et al. (1995) first used the root mean square of LFO to measure the LFO amplitude. More recently, Kannurpatti and Biswal (2008) developed a measure referred to as resting state physiological fluctuation amplitude (RSFA). This measure examines the standard deviation of BOLD signal in the sub-0.1 Hz frequency band. In the frequency domain, Zang et al. have developed two methods to examine these amplitudes (Yang et al., 2007; Zou et al., 2008; Zang et al., 2007). One is referred to as amplitude of low-frequency fluctuations (ALFF) and involves the spectral decomposition of the time series data with a focus on amplitude in these low-frequency ranges. Another measure is fractional ALFF (fALFF), which provides information on the relative amplitude that resides in the low frequencies. Although ALFF is effective at detecting LFO fluctuations, the fluctuations detected can extend over 0.1 Hz, particularly near major vessels (Zou et al., 2008), which are characterized by widespread oscillations across both low and high frequencies. In contrast, as a normalized index of ALFF, fALFF can provide a more specific measure of low-frequency oscillatory phenomena (Zou et al., 2008).
Both ALFF and fALFF have been used in various resting state fMRI studies. Under resting conditions, ALFF is higher and dominant in the regions comprising the so-called default mode network (Raichle et al., 2001) than in other regions (Yang et al., 2007; Zou et al., 2008; Zang et al., 2007). Moreover, in healthy individuals, ALFF in visual cortex is higher during scans conducted with eyes open than eyes closed, whereas activity in the posterior cingulate is not (Yang et al., 2007). These measures also have implications for psychiatric populations. For example, in children with attention deficit hyperactivity disorder (ADHD), abnormalities have been shown in ALFF in a number of regions implicated in the disorder (Zang et al., 2007). We are aware of no studies of LFO amplitude in schizophrenia, however.
Here, we examine resting state ALFF and fALFF in patients with schizophrenia and healthy controls in an exploratory voxelwise analysis. Patients with schizophrenia show wellestablished deficits in relatively low level visual (Green et al., 1994; Butler et al., 2005; Butler et al., 2008) and auditory (Javitt et al., 1993; Javitt et al., 2000; Javitt, 2009) sensory functions. These deficits appear to upwardly generalize to other cognitive deficits seen in schizophrenia. That is, deficits in sensory processing appear to account for a significant amount of the variance in higher level cognitive deficits such as prosodic problems (Leitman et al., 2005), reading difficulties (Revheim et al., 2006), and perceptual closure (Doniger et al., 2001). Based on these deficits, we predicted that patients would show reduced ALFF and fALFF in sensory cortical regions. Based on findings of frontal hyperactivity described in medial prefrontal regions in schizophrenia (Whitfield-Gabrieli et al., 2009), we predicted higher levels of ALFF and fALFF in those regions.
2. Materials and methods
2.1. Participants
Participants were 26 healthy controls and 29 patients who met DSM-IV-TR (American Psychiatric Association, 2000) criteria for schizophrenia (n=24) or schizoaffective disorder (n=5) after a Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Patient version (SCID-I/P; First et al., 2002). Controls had no major Axis I disorders as determined with the SCID-I-Nonpatient version (SCID-I/NP; First et al., 2001). Participants with substance dependence diagnoses within the six months prior to assessment were excluded from the study, as were participants with a history of electroconvulsive treatment, head injury with loss of consciousness greater than 15 min, neurological disorders, or HIV seropositivity. Psychiatric symptomatology was evaluated using the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1986). Medication dosages (chlorpromazine equivalents) were computed according to the (American Psychiatric Association (1997) guidelines. The conversion factors for the two patients who were taking risperidone Consta were obtained from the Schizophrenia Patients Outcomes Research Team treatment recommendations (http://www.ahrq.gov/clinic/schzrec.htm). Demographic data are given in Table 1. All participants signed informed consent as approved by the local Institutional Review Boards.
Table 1.
Demographics of study participants
| Variable | Controls (N=26) |
Patients (N=29) |
t | p |
|---|---|---|---|---|
| Age (years) | 41.9±10.9 | 36.5±11.0 | −1.81 | 0.08 |
| Education (years) |
15.2±2.8 | 12.4±2.0 | −4.36 | 0.000006 |
| PANSS totala | - | 76.5±16.6 | - | - |
| PANSS positivea | - | 18.4±6.2 | - | - |
| PANSS negativea | - | 20.2±6.2 | - | - |
| Illness duration (years)b |
- | 13.0±7.2 | - | - |
| CPZ equivalents (mg) |
- | 1118.8±611.2 | - | - |
| χ2 |
p | |||
| Gender | 19/7 | 26/3 | 2.53 | 0.16c |
Note: PANSS=Positive and Negative Syndrome Scale, CPZ=chlorpromazine.
Missing for 1 subject.
Defined as age at first psychiatric hospitalization, missing for 4 subjects.
by Fisher’s exact test.
2.2. MRI
Scanning took place on the 1.5 T Siemens Vision Scanner (Erlangen, Germany) at the Nathan Kline Institute Center for Advanced Brain Imaging. Participants received a magnetization prepared rapidly acquired gradient echo (MPRAGE) T1-weighted scan (TR = 11.6 ms, TE = 4.9 ms, TI = 1122 ms, matrix= 256 × 256, FOV = 256 mm, slice thickness = 1 mm, 190 slices, no gap, 1 acquisition), and a six minute resting state fMRI scan (TR = 2000 ms, TE = 50 ms, matrix = 64 × 64, FOV = 224 mm, 5 mm slice thickness, 22 slices, no gap, 180 acquisitions). For the resting state scan, participants were instructed to close their eyes and remain awake.
2.3. Data processing
Resting state data were preprocessed as described elsewhere in detail (Margulies et al., 2007; Castellanos et al., 2008; Kelly et al., 2008). Briefly, the first 10 volumes were discarded to eliminate T1 relaxation effects. Images were then motioncorrected, time shifted, and despiked using AFNI (Cox, 1996). Next, time series were smoothed using a 6 mm FWHM Gaussian kernel and spatially normalized to MNI space (2×2×2 mm3 resolution) using FSL (www.fmrib.ox.ac.uk/fsl). The MPRAGE image was segmented using FSL’s FAST software to obtain the masks used to extract white matter and CSF time series from the BOLD images. The white matter (WM) and cerebrospinal fluid (CSF) time series were then averaged across voxels within each mask. These time series, as well as the global signal intensity and the time series for the six motion parameters were regressed out from each voxel’s time series.
Individual participant analyses were carried out with the GLM implemented in FSL’s FEAT and power spectral density toolbox. The time series for the nuisance covariates (time series regressors for global signal, WM, CSF, and six motion parameters) were entered as predictors. The residual data were then linearly detrended. Then, based upon detrended residual data, the power spectral density of the data was calculated using FSL’s tool (fslpspec). Two measures were obtained: ALFF and fALFF. ALFF represents the amplitude in the low-frequency band, whereas fALFF is the ratio of the amplitude in a low-frequency band to amplitude in the total frequency band. Here, the low-frequency range was the slow-4 band (0.027–0.073 Hz; Buzsaki and Draguhn, 2004; Di Martino et al., 2008). The details of computation can be found in Zang et al. (2007) and Zou et al. (2008). The resulting amplitude measures were converted into Z-scores by subtracting the global mean and dividing the global standard deviation.
Group-level analyses were conducted using FSL’s ordinary least squares (OLS) model implemented in FLAME. These analyses produced thresholded z-statistic maps of ALFF and fALFF based on Gaussian Random Field theory using clusters defined by Z>2.3 and a corrected cluster threshold of P=0.05 (Worsley, 2001). These maps revealed significantly detectable LFO amplitudes for patients and healthy controls, as well as group difference. We first performed group-level analyses on all individual fALFF Z-score maps for patients and controls respectively. This procedure produced significant fALFF z-statistic maps for patients and controls. We then made a mask combining the two fALFF z-statistic maps (patients, healthy controls), and then used this mask for analyzing the differences in ALFF and fALFF between the two groups. As suggested by a previous study, such a masking procedure can remove the influence of large vessels and constrain our analyses within regions generating potentially meaningful LFOs (Zou et al., 2008). Finally, this mask was, in turn, masked by the inclusion mask for all 55 subjects.
Talairach coordinates were derived from the MNI coordinates using the MNI to Talairach Coordinate Converter (http://www.bioimagesuite.org/Mni2Tal/index.html), and brain localizations were derived from the Talairach Daemon (http://www.talairach.org/daemon.html). Surface maps of images in Talairach space were generated using SUMA (Saad et al., 2004), a part of AFNI.
3. Results
3.1. fALFF
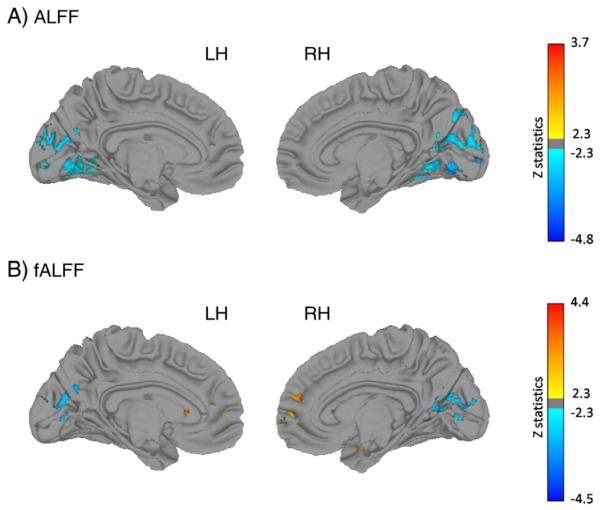
Group differences are shown in Table 2 and Fig. 1B (scatter plots are shown in Supplementary Fig. 1). Compared to controls, patients exhibited reduced fALFF in a cluster in the left insula that extended into the claustrum and transverse temporal gyrus, a cluster in left cuneus, and a region in left middle occipital gyrus that extended into the cuneus. There was also a large area within the right lingual gyrus that extended into the precuneus, cuneus, and middle occipital gyrus, and another in right caudate body and the posterior portion of the caudate head. Patients had higher fALFF in a cluster in the right parahippocampal gyrus extending into the lentiform nucleus, and in the medial prefrontal cortex bilaterally extending into the rostral anterior cingulate and left caudate head.
Table 2.
Resting state activity in controls and patients with schizophrenia or schizoaffective disorder (fractional amplitude of low-frequency fluctuations [fALFF]).
| Region | Voxels | BA | X | Y | Z | Z value |
|---|---|---|---|---|---|---|
| Decreased in schizophrenia | ||||||
| Left insula | 353 | 13 | −48 | −36 | 18 | −4.26 |
| Claustrum | −38 | −14 | 6 | |||
| Transverse temporal gyrus | 41 | −34 | −28 | 8 | ||
| Left cuneus | 78 | 17 | −16 | −92 | 4 | −3.59 |
| Left middle occipital gyrus | 48 | 18 | −24 | −92 | 20 | −3.17 |
| Cuneus | 18 | −22 | −78 | 22 | ||
| Right lingual gyrus | 606 | 19 | 20 | −60 | 2 | −4.22 |
| Precuneus | 31 | 2 | −68 | 22 | ||
| Cuneus | 17 | 4 | −80 | 12 | ||
| Lingual gyrus | 18 | 10 | −82 | −8 | ||
| Posterior cingulate | 31 | 26 | −60 | 16 | ||
| Middle occipital gyrus | 18 | 12 | −92 | 18 | ||
| Right caudate | 226 | 20 | 22 | 14 | −4.52 | |
| Caudate | 20 | −14 | 26 | |||
| Caudate | 16 | 2 | 22 | |||
| Increased in schizophrenia | ||||||
| Right parahippocampal gyrus | 97 | 30 | −6 | −14 | 3.90 | |
| Lentiform nucleus | 14 | 8 | −14 | |||
| Medial prefrontal cortex | 244 | 9 | 6 | 50 | 18 | 4.35 |
| Medial frontal gyrus | 10 | 8 | 58 | 2 | ||
| Medial frontal gyrus | 10 | −6 | 54 | −4 | ||
| Anterior Cingulate | 24 | 0 | 36 | 6 | ||
| Caudate | −2 | 20 | 8 | |||
| Caudate | −12 | 18 | −8 |
Note: SCZ=schizophrenia, CON=Controls, BA=Brodmann Area, [X, Y, Z] = Talairach coordinate.
Fig. 1.
Group differences in ALFF (top) and fALFF (bottom), LH = left hemisphere, RH = right hemisphere, blue = higher in controls. Z value scale is to the right of each pair.
To examine whether there was a relationship between areas in which fALFF was reduced and increased in patients, we extracted the fALFF for all subjects for areas in which patients showed higher and lower fALFF. LFO in these areas were significantly and negatively correlated with each other in the patient group, r=−0.47, p=0.011, n=29. The correlation was nonsignificant but in the same direction within the control group, r=−0.26, p=0.20, n= 26. These two correlations did not differ in magnitude; Fisher’s r-to-z for the difference=0.85, p=0.30.
3.2. ALFF
Talairach coordinates and cluster information are given in Table 3 and Fig. 1A (scatter plots are shown in Supplementary Fig. 2). ALFF was higher in controls than patients in the cuneus/precuneus, precentral gyrus and lingual gyrus. The cuneus/precuneus cluster extended to the middle occipital gyrus. The precentral area extended to the postcentral gyrus, and the right lingual gyrus cluster extended to the parahippocampal gyrus, middle occipital gyrus, and dorsal lingual gyrus, as well as then culmen and declive of the cerebellum as well as to the left lingual gurus and culmen. ALFF was significantly higher in patients than controls in left hippocampus/parahippocampus.
Table 3.
Resting state activity in controls and patients with schizophrenia or schizoaffective disorder (amplitude of low-frequency fluctuations [ALFF]).
| Region | Voxels | BA | X | Y | Z | Z value |
|---|---|---|---|---|---|---|
| Decreased in schizophrenia | ||||||
| Right lingual gyrus/culmen | 2182 | 18 | 6 | −70 | −8 | −4.10 |
| Lingual gyrus | 18 | 14 | −62 | 6 | ||
| Lingual gyrus | 18 | 16 | −86 | −10 | ||
| Lingual gyrus | 19 | 22 | −70 | −4 | ||
| Middle occipital gyrus | 18 | 20 | −98 | 14 | ||
| Cuneus | 12 | −96 | −2 | |||
| Cuneus | 17 | 8 | −90 | 20 | ||
| Parahippocampal gyrus | 19 | 24 | −56 | −2 | ||
| Declive | 8 | −74 | −10 | |||
| Culmen | 10 | −58 | −8 | |||
| Culmen | 20 | −44 | −18 | |||
| Left lingual gyrus | 18 | −4 | −72 | 2 | ||
| Left lingual gyrus | 18 | −14 | −98 | −4 | ||
| Left lingual gyrus | 18 | −10 | −82 | −10 | ||
| Left lingual gyrus | 18 | −22 | −56 | 2 | ||
| Left cuneus | 18 | −6 | −92 | 16 | ||
| Left cuneus | 19 | −18 | −88 | 26 | ||
| Left middle occipital gyrus | −28 | −84 | 16 | |||
| Left culmen | −12 | −58 | −8 | |||
| Left culmen | −22 | −52 | −20 | |||
| Right precuneus | 321 | 19 | 12 | −80 | 42 | −3.84 |
| Cuneus | 18 | 28 | −82 | 36 | ||
| Precuneus | 31 | 28 | −72 | 26 | ||
| Left precuneus | 19 | −2 | −82 | 40 | ||
| Right precentral gyrus | 300 | 4 | 48 | −8 | 46 | −4.77 |
| Precentral gyrus | 6 | 54 | −2 | 28 | ||
| Precentral gyrus | 6 | 46 | −14 | 26 | ||
| Postcentral gyrus | 2 | 46 | −26 | 52 | ||
| Postcentral gyrus | 3 | 32 | −32 | 46 | ||
| Postcentral gyrus | 3 | 40 | −22 | 36 | ||
| Increased in schizophrenia | ||||||
| Left hippocampus | 183 | 30 | −28 | −24 | −8 | 3.70 |
| Parahippocampal gyrus | −24 | −36 | 2 |
Note: SCZ=schizophrenia, CON=Controls, BA=Brodmann Area, [X, Y, Z]=Talairach coordinate.
To examine whether there was a relationship between areas in which ALFF was reduced and increased in patients, we extracted the ALFF for all subjects for areas in which patients showed higher and lower ALFF. LFO in these areas were significantly and negatively correlated with each other in the patient group, r=−0.40, p=0.033, n=29. The correlation was significant and in the same direction within the control group, r=−0.48, p=0.012, n=26. These two correlations did not differ in magnitude; Fisher’s r-to-z for the difference=−0.38, p=0.70.
3.3. Correlations with clinical and demographic variables
We examined the relationship between both ALFF and fALFF to demographic variables in regions with significant group differences. In patients, neither measure was correlated with PANSS scores, with only the PANSS Negative and Total scores trending toward significant correlations with regions in which ALFF was reduced in patients relative to controls (r<−0.34, p=0.07, n=28). The other correlations with PANSS scores were all less than ±0.26. Medication dosage (chlorpromazine equivalents) was not correlated with either ALFF or fALFF in any of the group difference regions, r<±0.03, p>0.88. Illness duration was negatively correlated with ALFF in the region in which ALFF was reduced in patients, r=−0.41, p=0.04, n=25. In controls, age was negatively correlated with fALFF in the region in which patients had higher fALFF, r=−0.68, p=0.00015, n=26, however, the group difference in this region persisted even after controlling for age.
4. Discussion
In the current study, we report that LFO amplitudes (i.e., ALFF and fALFF) were abnormal in patients with schizophrenia and schizoaffective disorder compared to healthy controls. fALFF measures were higher in controls than patients in the left cuneus and insula, right lingual gyrus and right caudate, and ALFF was higher in controls than patients in the right precuneus/cuneus and lingual gyrus, as well as right precentral gyrus. Patients had higher ALFF than controls in left hippocampus/parahippocampus, and higher fALFF than controls in the right parahippocampal and medial frontal gyri. Thus, our data suggest that there are abnormalities in LFOs in patients with schizophrenia. The current findings add to a literature suggesting abnormalities of neural synchrony in schizophrenia (Uhlhaas et al., 2008) and extend these findings to the LFO domain. Whether these findings are related to the oscillatory hierarchies observed in higher frequency ranges (i.e., between delta, theta, and gamma ranges) remains an important question for future work.
Many of the areas in which reduced LFO amplitude in patients with schizophrenia was observed are sensory and motor regions, although deficits also were found in right precuneus, cerebellum, caudate, and paralimbic regions. These are consistent with deficits in low level visual and auditory sensory functions in schizophrenia (Javitt et al., 2000; Rabinowicz et al., 2000; Butler and Javitt, 2005; Butler et al., 2005), as well as with deficits in motor control seen in patients with schizophrenia (Bilder et al., 2000; Bilder et al., 2002). It may be speculated that the right striatal findings might relate to deficits in reward processing and/or stimulus saliency observed in schizophrenia (Murray et al., 2008), and possibly to dulling of emotional expression in patients (Tremeau, 2006), although this would have to be addressed in a study in which both behavioral measures and resting state measures are obtained. Cerebellar abnormalities have also been commonly observed in schizophrenia (Rusch et al., 2007; Andreasen et al., 1999).
The areas of increased amplitude in schizophrenia are in some of the same frontal and temporal regions previously associated with abnormal function in the disorder. The finding of increased fALFF in frontal regions is consistent with data showing that frontal regions may be dysregulated in the context of working memory task performance (Callicott et al., 2003). Moreover, they may also be consistent with the notion that dysregulation of medial frontal regions is associated with self-directed thoughts, with the consequence that the source of internal and external stimuli could become confused, which may provide a neurophysiological basis for hallucinations (Whitfield-Gabrieli et al., 2009). This would have to be verified in future work. These frontal increases were not detected by the ALFF measure. Taking into account the total power over the entire spectrum present in the BOLD signal, fALFF is the normalized ALFF, which can provide a more specific measure of low-frequency oscillatory phenomena.
We also observed hyperactivity in hippocampus/parahippocampus. These results are consistent with findings from other work showing hippocampal hyperactivity in patients with schizophrenia (Heckers, 2001; Krieckhaus et al., 1992; Lodge and Grace, 2007). Some papers have suggested a left lateralization to this hyperactivity (Gur, 1978). It is unclear why this abnormality was left sided for the ALFF measure and right sided for the fALFF measure. This difference will be the topic of future work.
The reduced LFO in the precuneus is consistent with abnormalities in the DMN in schizophrenia (see references in Introduction). The decrease there is in contrast with increased fALFF in medial prefrontal cortex, which is also a part of the DMN. It is possible that these differential effects on amplitude in patients are related to the disruption in coordination among elements of the DMN in schizophrenia (e.g., Whitfield-Gabrieli et al., 2009; Garrity et al., 2007).
Left caudate head fALFF was increased in patients whereas right caudate body fALFF was lower in patients than controls. This dissociation might be attributable to segregation of neural circuitry in the head vs. body of the caudate (Alexander et al., 1986). Subsequent work has shown that activation in the head of the caudate is associated with feedback of performance and that activation in the body of the caudate is more involved in learning stimulus-category associations (Seger and Cincotta, 2005; Cincotta and Seger, 2007).
It is potentially interesting that the LFOs in the areas in which patients showed increases and decreases in ALFF and fALFF were negatively correlated in the patient group. This may suggest mutual inhibition among these sets of regions. Further work using diffusion tensor imaging might be particularly fruitful to examine this hypothesis.
Our findings for fALFF are similar to those in children with ADHD (Zang et al., 2007) in showing increases in the right anterior cingulate cortex. It may be that some of the disinhibitory behaviors seen in both of these disorders might be shared by a common neural substrate. This raises interesting questions from a neurodevelopmental perspective, as well as from a nosological perspective that can be examined in future studies.
These current results were obtained under resting conditions. It would be important to determine whether similar results would be obtained under task conditions, or indeed whether differences in resting fALFF or ALFF would predict task induced signal changes. This issue can and should be examined in studies in which both resting and task-based fMRI are collected. It is possible, for example, that resting LFOs represent a neuronal activity baseline that can be entrained for the purposes of sensory selection of task-relevant stimuli (Schroeder and Lakatos, 2009).
Several demographic variables were correlated with abnormalities in LFO. Illness duration was negatively correlated with ALFF in the patient deficit region. It is not known whether this might be a marker for possible progression of the illness. This could be addressed in longitudinal studies. In controls, age was negatively correlated with fALFF in the region in which patients showed heightened fALFF. It is unclear what the effects of age are in ALFF and fALFF. This should be addressed in future work.
The current study has several limitations. One of these is the unconstrained nature of the resting state. We should point out in this regard that Fransson (2006) examined so-called task-unrelated thoughts (concerning inner speech, imagery, planning for the future, episodic memory, and task-unrelated attention) and found that these were correlated with neither activation during a working memory task nor with resting state activation in the default mode network. Moreover, we have shown short- and long-term test–retest reliability in the moderate to high range for resting state functional connectivity (Shehzad et al., 2009). We also have shown that both fALFF and ALFF are reliable over time (Zuo et al., in press). Secondly, patient participants all had chronic schizophrenia or schizoaffective disorders and were all on antipsychotic medication. However, neither fALFF nor ALFF differences were related to medication dosages. Subjects were instructed to keep eyes closed during the resting scan, but it is possible that there were group differences in the extent to which individual subjects might have failed to comply with this instruction. Future studies would benefit from the use of eye tracking or visual monitoring equipment during the resting state session. Finally, we examined only one frequency range. It would be important to examine other frequency ranges and their interrelationships, especially because these frequencies may exhibit a hierarchical structure (Lakatos et al., 2008; Sirota et al., 2008).
The current findings show that ALFF and fALFF are abnormal in schizophrenia, with multiple areas showing either increases or decreases in these measures. Although the relationship between these LFO measures and resting state functional connectivity (RSFC) remains to be determined, these group differences may form the basis for some of the RSFC deficits seen in schizophrenia (Garrity et al., 2007; Bluhm et al., 2007; Jafri et al., 2008; Hoptman, et al., in press). Moreover, our findings may have implications for abnormalities in the oscillatory hierarchy in schizophrenia that has been proposed by a number of different investigators, as noted above. The areas in which decreases were found are consistent with other data showing deficits in motor and low level sensory processing in this disorder, as well as with deficits in reward sensitivity in patients with schizophrenia. The areas in which increases were found are relevant to internally directed thought, which might lead to an inappropriate emphasis on internally generated stimuli, as well as confusion as to their source. For this reason, it has been postulated that this dysregulation might be associated with hallucinations. Further work will be needed to examine the functional significance of these findings.
Supplementary Material
Acknowledgements
We thank Raj Sangoi RT(R) (MR) for his assistance in scanning the participants.
Role of funding source Funding for this study was provided by NIMH grants RO1 MH064783 and R21 MH084031 (MJH), R37 MH049334 (DJC), R01 MH066374 (PDB), and by grants provided by the National Alliance for Research on Schizophrenia and Depression and gifts from Linda and Richard Schaps, Jill and Bob Smith, and the Taubman Foundation to Francisco Xavier Castellanos. These funding sources had no further role in study design; in the collection, analysis and interpretation of data; in writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of interest Drs. Hoptman, Zuo, Milham, Butler, Javitt, Ms. D’Angelo, and Ms. Mauro reported no biomedical financial interests or potential conflicts of interest.
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.schres.2009.09.030.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association Practice guideline for the treatment of patients with schizophrenia. Am. J. Psychiat. 1997;154(Suppl):1–63. doi: 10.1176/ajp.154.4.1. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) American Psychiatric Association; Washington D.C.: 2000. [Google Scholar]
- Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol. Psychiatry. 1999;46:908–920. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Willson DF, Alvir J, Ma J, Woerner MG, Geisler S, Kane JM, Lieberman JA. Neuropsychology of first-episode schizophrenia: Initial characterization and clinical correlates. Am. J. Psychiatr. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Kunz M, Chakos M, Cooper TB, Horowitz TL, Lieberman JA. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. Am. J. Psychiatry. 2002;159:1018–1028. doi: 10.1176/appi.ajp.159.6.1018. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld R, Theberge J, Schaefer B, Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr. Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Curr. Opin. Psychiatr. 2005;18:151–157. doi: 10.1097/00001504-200503000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, Revheim N, Silipo G, Javitt DC. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch. Gen. Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biol. Psychiatry. 2008;64:40–47. doi: 10.1016/j.biopsych.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am. J. Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Carter CS. Re-conceptualizing schizophrenia as a disorder of cognitive and emotional processing: a shot in the arm for translational research. Biol. Psychiatry. 2006;60:1169–1170. doi: 10.1016/j.biopsych.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJ, Rotrosen J, Adler LA, Milham MP. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cincotta CM, Seger CA. Dissociation between striatal regions while learning to categorize via feedback and via observation. J. Cogn. Neurosci. 2007;19:249–265. doi: 10.1162/jocn.2007.19.2.249. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Ghaffari M, Curchack J, Reiss P, Hyde C, Vannucci M, Petkova E, Klein DF, Castellanos FX. Decomposing intra-subject variability in children with attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2008;64:607–614. doi: 10.1016/j.biopsych.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniger GM, Silipo G, Rabinowicz EF, Snodgrass JG, Javitt DC. Impaired sensory processing as a basis for object-recognition deficits in schizophrenia. Am. J. Psychiatry. 2001;158:1818–1826. doi: 10.1176/appi.ajp.158.11.1818. [DOI] [PubMed] [Google Scholar]
- Ehrlichman RS, Gandal MJ, Maxwell CR, Lazarewicz MT, Finkel LH, Contreras D, Turetsky BI, Siegel SJ. N-methyl-d-aspartic acid receptor antagonist-induced frequency oscillations in mice recreate pattern of electrophysiological deficits in schizophrenia. Neuroscience. 2009;158:705–712. doi: 10.1016/j.neuroscience.2008.10.031. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) Biometrics Division. New York State Psychiatric Institute; New York: 2001. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders—Patient Edition (SCID-I/P, 11/2002 revision) Biometrics Research Department. New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Ford JM, Krystal JH, Mathalon DH. Neural synchrony in schizophrenia: from networks to new treatments. Schizophr. Bull. 2007;33:848–852. doi: 10.1093/schbul/sbm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Winterer G, Herrmann CS, Senkowski D. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin. Neurophysiol. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am. J. Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. I. Specifying a mechanism. Arch. Gen. Psychiatry. 1994;51:939–944. doi: 10.1001/archpsyc.1994.03950120011003. [DOI] [PubMed] [Google Scholar]
- Gur RE. Left hemisphere dysfunction and left hemisphere over-activation in schizophrenia. J. Abnorm. Psychology. 1978;87:226–238. doi: 10.1037//0021-843x.87.2.226. [DOI] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, D’Angelo D, Catalano D, Mauro MJ, Shehzad ZE, Kelly AMC, Castellanos FX, Javitt DC, Milham MP. Amygdalofrontal functional disconnectivity and aggression in schizophrenia. Schizophrenia Bulletin. doi: 10.1093/schbul/sbp012. in press. doi:10.1093/schbul/sbp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent restingstate components in schizophrenia. NeuroImage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Intracortical mechanisms of mismatch negativity dysfunction in schizophrenia. Audiol. Neuro-Otol. 2000;5:207–215. doi: 10.1159/000013882. [DOI] [PubMed] [Google Scholar]
- Javitt DC. When doors of perception close: bottom–up models of disrupted cognition in schizophrenia. Annu. Rev. Clin. Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Doneshka P, Zylberman I, Ritter W, Vaughan HG., Jr. Impairment of early cortical processing in schizophrenia: an eventrelated potential confirmation study. Biol. Psychiatry. 1993;33:513–519. doi: 10.1016/0006-3223(93)90005-x. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia: defining the pattern. Arch. Gen. Psychiatry. 2000;57:1131–1137. doi: 10.1001/archpsyc.57.12.1131. [DOI] [PubMed] [Google Scholar]
- Kannurpatti SS, Biswal BB. Detection and scaling of task-induced fMRI-BOLD response using resting state fluctuations. NeuroImage. 2008;40:1567–1574. doi: 10.1016/j.neuroimage.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Bronx Psychiatric Center; N.Y.: 1986. [Google Scholar]
- Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Krieckhaus EE, Donahoe JW, Morgan MA. Paranoid schizophrenia may be caused by dopamine hyperactivity of CA1 hippocampus. Biol. Psychiatry. 1992;31:560–570. doi: 10.1016/0006-3223(92)90242-r. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J. Neurophysiol. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Foxe JJ, Butler PD, Saperstein A, Revheim N, Javitt DC. Sensory contributions to impaired prosodic processing in schizophrenia. Biol. Psychiatry. 2005;58:56–61. doi: 10.1016/j.biopsych.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. NeuroReport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J. Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. NeuroImage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Murray GK, Corlett PR, Clark L, Pessiglione M, Blackwell AD, Honey G, Jones PB, Bullmore ET, Robbins TW, Fletcher PC. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol. Psychiatry. 2008;13:239, 267–239, 276. doi: 10.1038/sj.mp.4002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowicz EF, Silipo G, Goldman R, Javitt DC. Auditory sensory dysfunction in schizophrenia: imprecision or distractibility? Arch. Gen. Psychiatry. 2000;57:1149–1155. doi: 10.1001/archpsyc.57.12.1149. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revheim N, Butler PD, Schechter I, Jalbrzikowski M, Silipo G, Javitt DC. Reading impairment and visual processing deficits in schizophrenia. Schizophr. Res. 2006;87:238–245. doi: 10.1016/j.schres.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch N, Spoletini I, Wilke M, Bria P, Di PM, Di IF, Martinotti G, Caltagirone C, Spalletta G. Prefrontal–thalamic–cerebellar gray matter networks and executive functioning in schizophrenia. Schizophr. Res. 2007;93:79–89. doi: 10.1016/j.schres.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Reynolds RC, Argall B, Japee S, Cox RW. SUMA: an interface for surface-based intra- and inter-subject analysis with AFNI. 2nd IEEE International Symposium on Biomedical Imaging: Macro to Nano.2004. pp. 1510–1513. [Google Scholar]
- Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger CA, Cincotta CM. The roles of the caudate nucleus in human classification learning. J. Neurosci. 2005;25:2941–2951. doi: 10.1523/JNEUROSCI.3401-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, Milham MP. The resting brain: unconstrained yet reliable. Cereb. Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsaki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J. Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, Shenton ME, McCarley RW. Sensoryevoked gamma oscillations in chronic schizophrenia. Biol. Psychiatry. 2008;63:744–747. doi: 10.1016/j.biopsych.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. J. Neurosci. 1998;18:4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremeau F. A review of emotion deficits in schizophrenia. Dial. Clin. Neurosci. 2006;8:59–70. doi: 10.31887/DCNS.2006.8.1/ftremeau. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr. Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, Laviolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JA, Moyer JT, Lazarewicz MT, Contreras D, Benoit-Marand M, O’Donnell P, Finkel LH. NMDA/AMPA ratio impacts state transitions and entrainment to oscillations in a computational model of the nucleus accumbens medium spiny projection neuron. J. Neurosci. 2005;25:9080–9095. doi: 10.1523/JNEUROSCI.2220-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. Oxford University Press; Oxford, UK: 2001. [Google Scholar]
- Yang H, Long XY, Yang Y, Yan H, Zhu CZ, Zhou XP, Zang YF, Gong QY. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage. 2007;36:144–152. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]
- Zang YF, Yang H, Zhu CZ, Qing-Ju C, Man-Qiu S, Meng L, Li-Xia T, Tian-Zi J, Yu-Feng W. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;92:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, Liu Z, Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr. Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J. Neurosci. Methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee D, Klein DF, Castellanos FX, Biswal BB, Milham MP. The oscillating brain: complex and reliable. Neuroimage. doi: 10.1016/j.neuroimage.2009.09.037. in press. doi:10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.