Abstract
Trivalent arsenite (As3+) is a known human carcinogen capable of inducing both cellular transformation and apoptotic cell death by mechanisms involving the production of reactive oxygen species. The tripeptide antioxidant glutathione (GSH) constitutes a vital cellular defense mechanism against oxidative stress. While intracellular levels of GSH are an important determinant of cellular susceptibility to undergo apoptotic cell death, it is not known whether cellular GSH biosynthetic capacity per se regulates As3+-induced apoptosis. The rate-limiting enzyme in GSH biosynthesis is glutamate cysteine ligase (GCL), a heterodimeric holoenzyme composed of a catalytic (GCLC) and a modifier (GCLM) subunit. To determine whether increased GSH biosynthetic capacity enhanced cellular resistance to As3+-induced apoptotic cell death, we utilized a mouse liver hepatoma (Hepa-1c1c7) cell line stably overexpressing both GCLC and GCLM. Overexpression of the GCL subunits increased GCL holoenzyme formation and activity and inhibited As3+-induced apoptosis. This cytoprotective effect was associated with a decrease in As3+-induced caspase activation, cleavage of caspase substrates and translocation of cytochrome c to the cytoplasm. In aggregate, these findings demonstrate that enhanced GSH biosynthetic capacity promotes resistance to As3+-induced apoptosis by preventing mitochondrial dysfunction and cytochrome c release and highlight the role of the GSH antioxidant defense system in dictating hepatocyte sensitivity to As3+-induced apoptotic cell death.
Keywords: arsenite, arsenic, glutathione, apoptosis, glutamate cysteine ligase, GCL
Introduction
Inorganic arsenic is a known human carcinogen associated with the development of cancers of the skin, bladder, lung, kidney and liver (Yoshida et al., 2004). Chronic exposure to low levels of trivalent arsenite (As3+) also promotes cell proliferation and malignant transformation in various cultured cell models in vitro (Zhao et al., 1997; Huang et al., 1999; Achanzar et al., 2002; Chien et al., 2004; Sens et al., 2004). In contrast, acute exposure to high concentrations of As3+ induces apoptotic cell death (Bode and Dong, 2002). Paradoxically, although As3+ is a known carcinogen, As3+ in the form of arsenic trioxide (ATO) is a highly effective chemotherapeutic in the treatment of acute promyelocytic leukemia (Bode and Dong, 2002; Miller et al., 2002). While the molecular mechanisms mediating As3+-induced transformation and apoptosis remain unclear, increased production of reactive oxygen species (ROS) has been implicated in both As3+-induced genotoxicity and cytotoxicity (Kitchin and Ahmad, 2003). Mammalian cells possess a number of antioxidant defense mechanisms to protect against oxidative stress. The tripeptide antioxidant glutathione (GSH) is a highly abundant and particularly effective means of protecting against oxidative injury (Griffith and Mulcahy, 1999). Interestingly, chronic exposure to sub-toxic doses of As3+ induces an adaptive response whereby cells become resistant to acute As3+-induced apoptosis (Romach et al., 2000; Brambila et al., 2002; Chien et al., 2004; Somji et al., 2006). This acquired tolerance to As3+ toxicity occurs in concert with malignant transformation, suggesting that this may provide a selective growth advantage during As3+-induced cellular transformation. The development of this resistant phenotype is associated with elevated intracellular GSH levels and increased expression of various detoxification and antioxidant enzymes, including enzymes involved in GSH biosynthesis, metabolism, and transport (Qu et al., 2001; Brambila et al., 2002; Chien et al., 2004; Coppin et al., 2008). While microarray studies have identified numerous gene products that could potentially mediate this apoptotic resistance (Chen et al., 2001a; Chen et al., 2001b; Hamadeh et al., 2002), inhibition of GSH biosynthesis alone is sufficient to sensitize As3+-transformed rat liver epithelial cells and human prostate cells to As3+-induced apoptosis (Liu et al., 2001a; Brambila et al., 2002). These findings provide compelling evidence that up-regulation of GSH homeostasis contributes to acquired tolerance to As3+ during chronic As3+ exposure.
GSH homeostasis is dependent on GSH biosynthesis, utilization and export. The inability of most cells to import GSH highlights the importance of de novo GSH biosynthesis in maintaining GSH homeostasis. GSH biosynthetic capacity is dependent on several factors, including substrate availability and glutamate cysteine ligase (GCL) activity (Griffith and Mulcahy, 1999). GCL mediates the rate-limiting step in GSH biosynthesis and is a heterodimeric holoenzyme composed of a catalytic (GCLC) and a regulatory (GCLM) subunit (Griffith and Mulcahy, 1999). Cellular GCL activity is governed mainly by the relative levels of the GCL subunits which are highly regulated by transcriptional control mechanisms (Wild and Mulcahy, 2000; Franklin et al., 2009; Lu, 2009). We and others have demonstrated that both acute and chronic exposure to sub-toxic concentrations of As3+ coordinately induce GCL subunit expression resulting in increased cellular GCL activity and GSH (Li et al., 2002; Schuliga et al., 2002; Pi et al., 2003; Coppin et al., 2008; Pi et al., 2008; Thompson et al., 2009). There is also strong evidence that alterations in GSH levels play an important role in dictating cellular sensitivity to As3+-induced apoptotic cell death (Bode and Dong, 2002; Miller et al., 2002). This is based on both comparative analyses and acute manipulation of cellular GSH levels. In this regard, increasing cellular GSH levels with the GSH precursor N-acetylcysteine (NAC) promotes cellular resistance to As3+-induced apoptosis, while depletion of cellular GSH levels with the GCLC inhibitor buthionine sulfoximine (BSO) dramatically potentiates As3+-induced apoptosis (Bode and Dong, 2002; Miller et al., 2002). Indeed, sufficiently elevating cellular GSH levels can abolish As3+-induced apoptosis, while depletion of cellular GSH is capable of enhancing As3+ toxicity in rat liver epithelial cells by an order of magnitude (Shimizu et al., 1998; Bode and Dong, 2002; Miller et al., 2002). While these findings clearly demonstrate that cellular sensitivity to As3+-induced apoptosis is inversely related to cellular GSH levels, they provide no information on the functional effects of altered GSH biosynthetic capacity per se.
GSH homeostasis is disrupted in most models of apoptotic cell death (Circu and Aw, 2008). This is mainly the result of the rapid depletion of cellular GSH levels due to extrusion of reduced GSH (Circu and Aw, 2008). However, altered GSH biosynthesis may also be a contributing factor as we have demonstrated that GCLC is a direct target for caspase-mediated cleavage during apoptosis (Siitonen et al., 1999; Pierce et al., 2000; Franklin et al., 2002; Franklin et al., 2003). While it is still unclear how altered GSH homeostasis affects apoptotic cell death, GSH is thought to function at the level of the mitochondrion to prevent the loss of mitochondrial membrane potential and release of pro-apoptotic factors into the cytoplasm (Circu and Aw, 2008). Consistent with this working model, we have found that increased GCL activity resulting from GCL overexpression inhibits TNF-induced apoptosis by maintaining mitochondrial integrity and preventing the release of cytochrome c (Botta et al., 2004). While TNF induces by cytochrome c release indirectly via Bid-mediated mitochondrial dysfunction, As3+ initiates cytochrome c release and activation of the apoptotic machinery by a direct effect on the mitochondrion (Larochette et al., 1999; Costantini et al., 2000). Based on the functional similarities of these cell death pathways, we believe that increased GSH biosynthetic capacity should also promote resistance to As3+-induced apoptosis.
In this study, we directly examined whether increased expression of the GCL subunits and enhanced GSH biosynthetic capacity promotes cellular resistance to apoptotic cell death utilizing an established mouse liver hepatoma (Hepa-1c1c7) cell line overexpressing GCLC and GCLM (Botta et al., 2004). Hepa-1c1c7 cells are an excellent model to selectively examine the effects of increased GCL subunit expression and GCL activity as the relative levels of the GCL subunits, and not substrate availability, are limiting for GSH biosynthesis (Shertzer et al., 1995). Furthermore, Hepa-1c1c7 cells overexpressing the GCL subunits exhibit increased GSH biosynthetic capacity, but only a modest increase in cellular GSH content (Botta et al., 2004). GCL overexpression was found to suppress As3+-induced translocation of cytochrome c to the cytoplasm and caspase activation, and inhibit As3+-induced apoptosis. These findings provide proof-of-principle that up-regulation of GSH biosynthesis could mediate apoptotic resistance, promote cell survival and provide a selective growth advantage during chronic As3+ exposure.
Materials and Methods
Reagents
NaAsO2, 4′,6-diamidino-2-phenylindole (DAPI), and digitonin were purchased from Sigma Chemical Co. (St. Louis, MO). All reagents were prepared in either H2O, sterile phosphate-buffered saline (PBS) or DMSO. z-VAD-fmk was from Bachem Bioscience (Torrance, CA) and Ac-DEVDAMC, Ac-IETD-AMC, and Ac-LEHD-AMC were from Alexis Biochemicals (San Diego, CA).
Cell culture and treatments
Murine Hepa-1c1c7 cells (American Type Culture Collection, Manassas, VA) were maintained in DMEM/F12 media (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Cells were cultured at 37C in a humidified atmosphere of 95% air/5% CO2. Hepa-1c1c7 cell lines expressing murine GCLC and GCLM expression vectors (Hepa-CR17) or a pMC1-neo vector (Hepa-V3) (Stratagene, La Jolla, CA) were established as previously described (Botta et al., 2004). Cells were seeded at 1.0-1.5 × 106 cells per 6-cm dish 18–24 h before treatment and all experiments were performed at 90-100% confluency.
Immunoblot analysis
Cells were harvested and lysed by a brief sonication on ice in TES/SB buffer (20 mM Tris, pH 7.4, 1 mM EDTA, 250 mM sucrose, 20 mM boric acid, 1 mM L-serine) containing 1× Complete protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN). Lysates were clarified by centrifugation at 13,000 × g for 10 min at 4C and protein quantified by the Bradford assay (Bio-Rad, Hercules, CA). Equal amounts of soluble protein (20 ug) were resolved on 10% SDSpolyacrylamide gels and transferred to PVDF membranes (Millipore, Bedford, MA). For analysis of GCL holoenzyme formation whole cell extracts were prepared in the absence of reducing equivalents and were resolved on native 10% PAGE gels in the absence of SDS. Samples for these studies were not boiled prior to gel loading and the gels were resolved in Tris/Glycine buffer lacking SDS at 4C prior to transfer to PVDF membranes. Membranes were blocked in Tris-buffered saline/0.1%Tween-20 (TBST) containing 5% non-fat milk prior to incubation with primary antibody in TBST containing 0.5% milk. Membranes were probed for GCLC, GCLM (Thompson et al., 1999; Franklin et al., 2002; Franklin et al., 2003; Thompson et al., 2009), βActin (Sigma), pro-caspase-3, cytochrome c (BD Biosciences, San Diego, CA), Bid (R&D Systems, Minneapolis, MN), GADD153 and ATF-3 (Santa Cruz Biotechnology, Santa Cruz, CA). Anti-mouse-HRP and anti-rabbit HRP secondary antibodies (Amersham Biosciences) were used at 1:5,000 in TBST containing 0.5% milk. Antigen-antibody complexes were detected with Western Lightning Chemoluminescent Reagent (PerkinElmer, Boston, MA).
To detect translocation of cytochrome c from the mitochondria to cytosol, cells were fractionated by a rapid digitonin lysis procedure (Single et al., 1998). Cells were incubated on ice for 5 min in digitonin lysis buffer (DLB; 75 mM NaCl, 1 mM NaH2PO4, 8 mM Na2HPO4, 250 mM sucrose) containing 20 ug digitonin per 1×106 cells. Lysates were centrifuged at 13,000 × g for 5 min at 4C. Supernatants were recovered and the pellets lysed by sonication in DLB and centrifuged as described above. Soluble protein (10 ug) from the fractions was resolved on 15% SDS-polyacrylamide gels and analyzed for cytochrome c by immunoblotting as described above.
GSH and GCL activity assays
Total GSH content (GSH + GSSG) was determined by a modification of the Tietze assay (Baker et al., 1990). Cell extracts were prepared by sonication in TES/SB as described above (Thompson et al., 1999; Franklin et al., 2002; Franklin et al., 2003) and GSH levels were determined against a standard curve of GSSG and levels calculated per μg of soluble protein in the original cell extract. This value was utilized to determine the relative change in intracellular GSH levels compared to untreated samples. GCL activity was measured by a fluorescence-based NDA assay as described previously (White et al., 2003).
Apoptosis and caspase assays
Apoptosis was quantified by assessing apoptotic nuclear morphology of DAPI-stained cells (Pierce et al., 2000). Cells were harvested, fixed in 70% ethanol and either analyzed immediately or stored at −20C. After a brief centrifugation, cells were resuspended in a solution containing 0.5% Nonidet P-40 and 10 ug/ml DAPI and analyzed by fluorescence microscopy. Caspase activities were measured using fluorogenic substrates as previously described (Franklin et al., 1998; Franklin et al., 2002; Franklin et al., 2003). Soluble protein extract (5–25 μg) was added to an equal volume of 2× caspase cleavage buffer (40 mM PIPES, pH 7.2, 200 mM NaCl, 20% sucrose, 0.2% CHAPS, 20 mM DTT) containing 40 μM fluorogenic substrate (Ac-DEVD-AMC for caspase-3, AcIETD-AMC for caspase-8, or Ac-LEHD-AMC for caspase-9). Reactions were carried out at 37C, and fluorescence was monitored on a SpectraMax Gemini EM (Molecular Devices, Sunnyvale, CA) with an excitation wavelength of 360 nm and an emission wavelength of 460 nm. Substrate autofluorescence was subtracted from each point, and specific activity was calculated against a standard curve of AMC (Sigma, St. Louis, MO).
Statistical analysis
Data are presented as averages +/− SEM of at least three experiments. Statistical analysis was performed using GraphPad Prism 4 (GraphPad Software, San Diego, CA). Results were compared by one-way or two-way ANOVA with Tukey's post test and mean differences were considered significant when p < 0.05.
Results
As3+-induced apoptosis in parental Hepa-1c1c7 cells
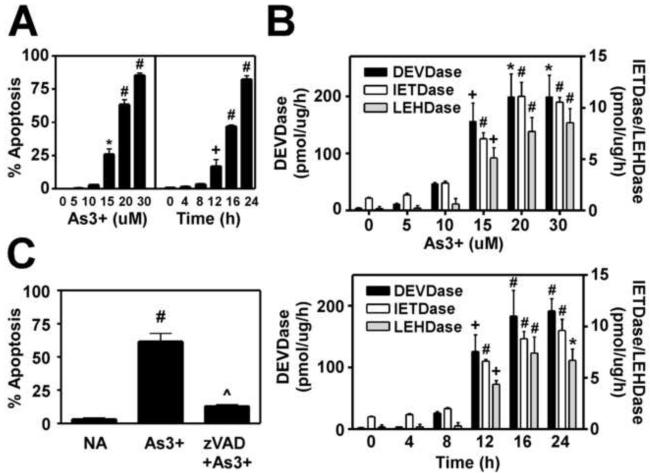
As3+ exhibits clear dose-dependent effects in most cultured cell systems, with low levels promoting cell proliferation and transformation and higher concentrations inducing apoptosis (Bode and Dong, 2002). To determine whether Hepa-1c1c7 cells were sensitive to As3+-induced apoptosis, cells were treated with varying concentrations of As3+ for 16 h and apoptosis was quantified by assessing apoptotic nuclear morphology of DAPI-stained cells (Pierce et al., 2000). In contrast to previous studies (Elbekai and El-Kadi, 2004), we found that As3+ was highly cytotoxic in the Hepa-1c1c7 cell line. As3+-induced apoptotic cell death was dose-dependent with near maximal apoptosis occurring at 20 uM As3+ (Fig. 1A, left panel), while evidence of necrotic cell death was observed at higher concentrations (data not shown). For this reason, 20 uM As3+ was employed for all future studies. As3+-induced apoptosis in Hepa-1c1c7 was also time-dependent, with significant apoptotic cell death (~20%) occurring within 12 h of treatment and ~80% of cells appearing apoptotic within 24 h (Fig. 1A, right panel). As3+-induced apoptosis was accompanied by a coordinate increase in the activities of caspases-3/8/9 as measured utilizing fluorogenic caspase substrates (Fig. 1B). The time- and dose-dependent activation of these caspases correlated well with the relative level of apoptotic cell death. Importantly, As3+-induced apoptosis was found to be caspase-dependent as pretreatment with the pan-caspase inhibitor z-VAD-fmk nearly abolished As3+-induced apoptotic cell death (Fig. 1C). These findings demonstrate that Hepa-1c1c7 cells are sensitive to As3+-induced apoptotic cell death, which occurs in a caspase-dependent manner.
Figure 1. Time- and dose-dependent As3+-induced apoptosis in Hepa-1c1c7 cells.
(A and B) Hepa-1c1c7 cells were treated for 16 h with the indicated concentrations of As3+ or with 20 uM As3+ for the time periods indicated. (A) As3+-induced apoptosis was quantified by assessing apoptotic nuclear morphology of DAPI-stained cells (Pierce et al., 2000). (B) Caspase activities were determined as described in the Methods section utilizing the fluorogenic substrates Ac-DEVD-AMC (caspase-3), AcIETD-AMC (caspase-8), and Ac-LEHD-AMC (caspase-9). (C) Cells were pretreated with z-VAD-fmk (50 uM) for 1 h prior to treatment with As3+ (20 uM) for 16 h and apoptosis quantified as described above. Data presented are averages +/- SEM of at least three experiments. + p < 0.05, * p < 0.01, # p < 0.001, indicates a significant difference compared to untreated control, and ^ p < 0.001 indicates a significant difference from As3+-only treated cells.
Hepa-1c1c7 cells overexpressing murine GCLC and GCLM exhibit enhanced GSH biosynthetic capacity
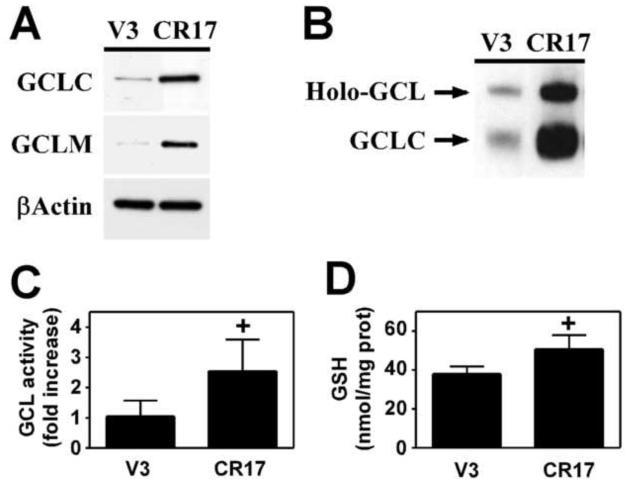
While As3+-induced apoptosis is highly sensitive to intracellular GSH levels (Bode and Dong, 2002), the goal of this study was to examine whether enhanced GSH biosynthetic capacity per se promotes resistance to As3+-induced apoptosis. To directly examine this hypothesis, we employed a clonally derived Hepa-1c1c7 cell line overexpressing both murine GCLC and GCLM (Hepa-CR17) (Botta et al., 2004). The Hepa-1c1c7 cell line was chosen for these studies as GCL subunit expression, and not cysteine availability, is limiting for GSH biosynthesis in these cells (Shertzer et al., 1995). While GCL subunit expression is under the control of a heavy metal-inducible metallothionein promoter in the Hepa-CR17 cell line, basal GCL subunit expression and GCL activity are constitutively elevated in CR17 cells and all studies were performed in the absence of heavy metals (Botta et al., 2004). As previously reported (Botta et al., 2004), Hepa-CR17 cells expressed 2-3 fold more GCLC and GCLM protein than the vector control cell line (Hepa-V3) (Fig. 2A). Overexpression of the GCL subunits also led to increased GCL holoenzyme formation as detected by resolving whole cell extracts by native gel electrophoresis and immunoblotting for GCLC (Fig. 2B). Importantly, increased GCL subunit expression and GCL holoenzyme formation was associated with a significant ~2.5-fold increase in cellular GCL activity (Fig. 2C), but only a modest increase in GSH content (Fig. 2D).
Figure 2. Hepa-1c1c7 cells overexpressing GCLC and GCLM exhibit enhanced GCL activity.
Hepa-1c1c7 cells were transfected with pMC1-neo vector alone (V3) or together with GCLC and GCLM expression vectors (CR17) and stable cell lines established as previously described (Botta et al., 2004). (A) GCLC, GCLM, and βActin protein expression were analyzed by immunoblotting. (B) GCL holoenzyme formation was assessed by native gel electrophoresis and immunoblotting for GCLC. (C) GCL activity was measured by a fluorescence-based NDA assay as previously described (White et al., 2003). (D) Total GSH levels (GSH + GSSG) were measured by a modified Tietze assay (Baker et al., 1990). Data presented are averages +/− SEM of at least three experiments. + p < 0.05, indicates a significant difference compared to the Hepa-V3 vector control cell line.
Enhanced GSH biosynthetic capacity promotes resistance to As3+-induced apoptosis
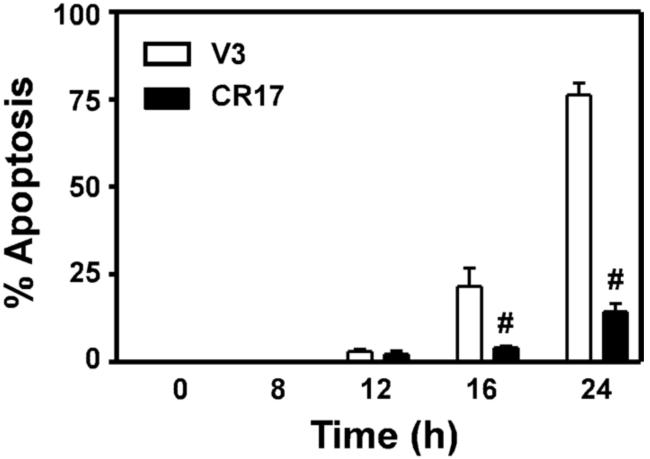
To determine whether GCL overexpression altered Hepa cell sensitivity to As3+-induced apoptosis, V3 and CR17 cells were treated with 20 uM As3+ for various time periods and analyzed for apoptotic cell death (Pierce et al., 2000). Similar to our findings in the parental Hepa-1c1c7 cell line (Fig. 1), treatment of Hepa-V3 cells with 20 uM As3+ resulted in a time-dependent induction of apoptosis as judged by cytoplasmic shrinkage, membrane blebbing, and the appearance of condensed nuclei on cell staining with DAPI, with ~75% of the cells displaying apoptotic nuclear morphology at 24 h (Fig. 3, open bars). In contrast, Hepa-CR17 cells were highly resistant to As3+-induced apoptosis, with only ~15% of cells exhibiting apoptotic nuclear morphology at 24 h (Fig. 3, closed bars).
Figure 3. Enhanced GSH biosynthetic capacity inhibits As3+-induced apoptosis.
Hepa-V3 and Hepa-CR17 cells were treated with 20 uM As3+ for the time periods indicated. Cells were stained with DAPI and apoptosis was quantified by assessing apoptotic nuclear morphology by fluorescence microscopy. Data presented are averages +/− SEM of at least three experiments. # p < 0.001, indicates a significant difference compared to As3+-induced apoptosis in Hepa-V3 cells at that time point.
GCL overexpression attenuates As3+-induced cytochrome c release and caspase-9 activation
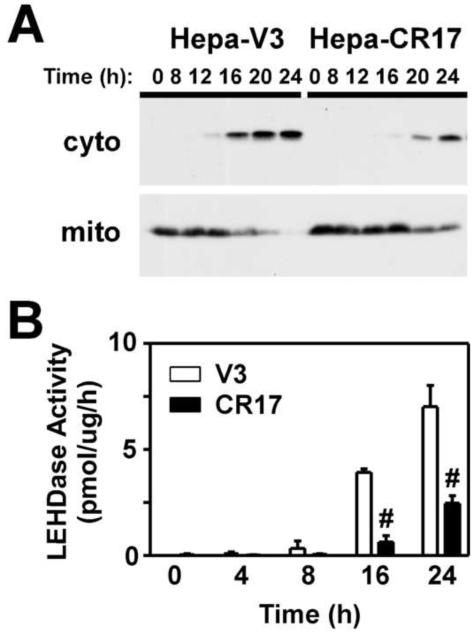
As3+-induced apoptosis occurs via activation of the intrinsic cell death pathway resulting in mitochondrial dysfunction, release of cytochrome c and other apoptotic factors, and activation of caspase-9 (Larochette et al., 1999; Costantini et al., 2000). In an attempt to elucidate the molecular mechanism(s) mediating the protective effects of GCL overexpression, we initially examined whether increased GSH biosynthesis suppressed As3+-induced apoptosis at the level of the mitochondria by assessing As3+-induced translocation of cytochrome c in the Hepa-V3 and CR17 cell lines. Cells were treated with As3+ and cytosolic and mitochondrial fractions isolated and analyzed for cytochrome c content by immunoblotting. As shown in Figure 4A, As3+ treatment caused the time-dependent translocation of cytochrome c from the mitochondrial fraction to the cytosolic fraction in Hepa-V3 cells. In contrast, As3+-induced cytochrome c translocation in the Hepa-CR17 cell line was attenuated and delayed. As cytochrome c leads to caspase-9 activation via apoptosome formation (Riedl and Salvesen, 2007), we determined whether suppression of cytochrome c release was associated with a concomitant decrease in As3+-induced activation of caspase-9. As3+ caused the time-dependent activation of LEHDase activity in the Hepa-V3 cell line, which was significantly reduced in Hepa-CR17 cells (Fig. 4B). These findings support a model in which enhanced GSH biosynthetic capacity suppresses As3+-induced apoptosis by preventing cytochrome c release from the mitochondria and caspase-9 activation.
Figure 4. Overexpression of GCL inhibits As3+-induced cytochrome c release and caspase-9 activation.
Hepa-V3 and Hepa-CR17 cells were treated with 20 uM As3+ for the time periods indicated. (A) Cytosolic and mitochondrial fractions were isolated by a digitonin lysis procedure (Single et al., 1998) and cytochrome c expression in the fractions was assessed by immunoblotting. (B) Caspase-9-like activity was measured utilizing the fluorogenic substrate Ac-LEHD-AMC. The data presented in B are averages +/− SEM of at least three experiments. # p < 0.001, indicates a significant difference compared to As3+-induced caspase activity in Hepa-V3 cells at that time point.
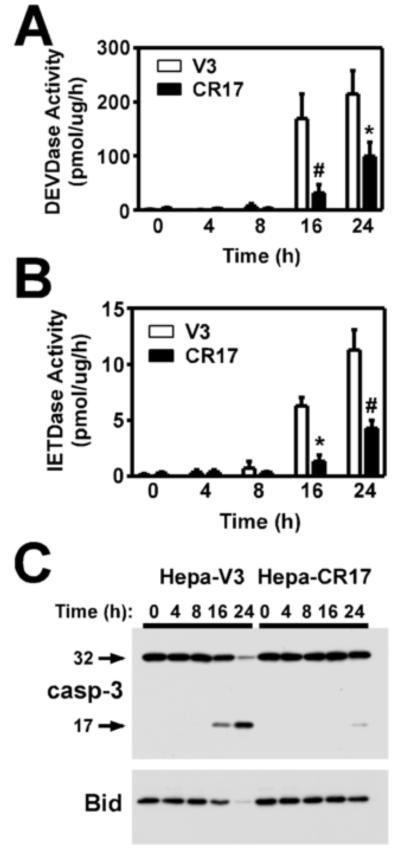
GCL overexpression attenuates As3+-induced activation of caspases-3/8
Caspase-9 leads to the activation of effector caspases, such as caspase-3, that mediate execution of the apoptotic program (Riedl and Salvesen, 2007). Caspase-3 also serves in an amplification loop via feedback activation of caspase-8, which can then enhance mitochondrial dysfunction and apoptotic cell death via cleavage and activation of Bid (Slee et al., 1999). To further elucidate the molecular mechanism(s) mediating the protective effects of GCL overexpression, we examined whether increased GSH biosynthesis suppressed activation of these caspase pathways. Similar to parental Hepa-1c1c7 cells, As3+ induced the time-dependent activation of both caspase-3 and -8 activities in the Hepa-V3 cell line (Fig. 5A and 5B, open bars). In contrast, As3+-induced activation of these caspases was significantly reduced in the CR17 cell line (Fig. 5A and 5B, closed bars). The decrease in As3+-induced DEVDase activity in the CR17 cell line correlated with reduced processing and cleavage of the 32 kDa pro-caspase-3 to its 17 kDa active fragment (Fig. 5C, upper panel). Similarly, the cleavage and loss of Bid, an established endogenous caspase-8 target protein (Li et al., 1998), was dramatically reduced in the Hepa-CR17 cell line (Fig. 5C, bottom panel). Importantly, cytochrome c release, caspase activation, and the cleavage of cellular caspase targets correlated both temporally and in magnitude with the onset of As3+-induced apoptotic morphology and cell death in the V3 and CR17 cell lines. In aggregate, these findings indicate that enhanced GSH biosynthetic capacity suppresses As3+-induced mitochondrial dysfunction, cytochrome c release, and activation of the caspase cascade.
Figure 5. Overexpression of GCL inhibits As3+-induced caspase-3/8 activation.
Hepa-V3 and Hepa-CR17 cells were treated with 20 uM As3+ for the time periods indicated. (A) Caspase-3-like activity was measured utilizing the fluorogenic substrate Ac-DEVD-AMC, (B) caspase-8-like activity was measured using Ac-IETD-AMC, and (C) the cleavage and/or processing of pro-caspase-3 and Bid were assessed by immunoblotting. The data presented in A/B are averages +/− SEM of at least three experiments. * p < 0.01, # p < 0.001, indicates a significant difference compared to As3+-induced caspase activity in Hepa-V3 cells at that time point.
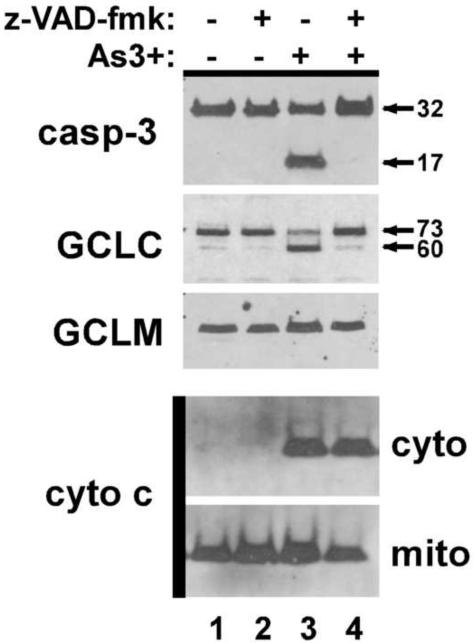
Caspase inhibition does not prevent As3+-induced cytochrome c release
The results in Figure 1 demonstrate that As3+-induced apoptosis in Hepa-1c1c7 cells is dependent on caspase activation. However, while As3+-induced cytochrome c release and caspase activation are both suppressed in Hepa-CR17 cells, it is not clear which of these events mediates the protective effects of GCL overexpression. To determine whether enhanced GSH biosynthesis protects against As3+-induced apoptosis by preventing cytochrome c release or suppressing caspase activity, we examined the effects of the pan-caspase inhibitor z-VAD-fmk on As3+-induced cytochrome c release. Pretreatment with zVAD-fmk abolished cellular caspase activity as judged by the inhibition pro-caspase-3 cleavage and processing and cleavage of GCLC, a known caspase-3 target protein (Franklin et al., 2002) (Fig. 6, upper panels). However, in contrast to GCL overexpression, caspase inhibition had no effect on As3+-induced cytochrome c release (bottom panels). These differential effects suggest that enhanced GSH biosynthetic capacity attenuates As3+-induced apoptosis by preventing cytochrome c release at a site upstream of caspase activation.
Figure 6. Caspase inhibition does not prevent As3+-induced cytochrome c release.
Parental Hepa-1c1c7 cells were pretreated with 50 uM zVAD-fmk for 1 h prior to treatment with 20 uM As3+ for 24 h as indicated. (top panels) Whole cell extracts were prepared and caspase-3, GCLC, and GCLM expression was assessed by immunoblotting. (bottom panels) Cells were harvested and fractionated as described in the Methods section and cytochrome c expression in the cytosolic (cyto) and mitochondrial (mito) fractions was assessed by immunoblotting.
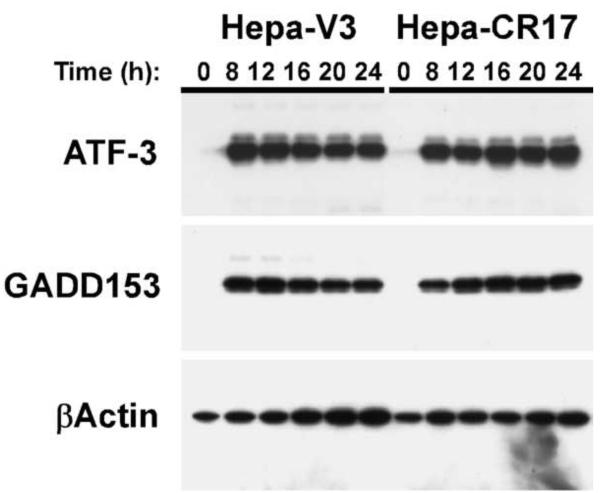
GCL overexpression does not abolish As3+-induced protein expression
As3+ metabolism involves GSH conjugation reactions and it is possible that GCL overexpression reduces As3+-induced apoptosis via transporter-mediated efflux of As3+ as a GSH conjugate (Leslie et al., 2004). To test this hypothesis, we examined whether other As3+-induced cellular responses were also attenuated in the Hepa-CR17 cell line. As3+ induces the expression of ATF-3 and GADD153 in a variety of cell types (Guyton et al., 1996; Fawcett et al., 1999). Immunoblot analysis of ATF-3 and GADD153 expression indicates that As3+ treatment causes a similar potent and prolonged induction of ATF-3 and GADD153 protein expression in both the Hepa-V3 and Hepa-CR17 cell lines (Fig. 7). Thus, while GCL overexpression inhibits As3+-induced apoptosis, it does not abolish all As3+-induced cellular responses.
Figure 7. Overexpression of GCL does not abolish As3+-induced protein expression.
Hepa-V3 and Hepa-CR17 cells were treated with 20 uM As3+ for the time periods indicated. Whole cell extracts were prepared and ATF-3, GADD153, and βActin expression were analyzed by immunoblotting.
Discussion
Inorganic As3+ is a known human carcinogen and the development of relevant cultured cell models has permitted examination of the molecular events associated with As3+-induced transformation. Chronic low level exposure to As3+ leads to malignant transformation in vitro which is associated with the development of As3+ adaptation, whereby cells become resistant to acute As3+ toxicity (Liu et al., 2001a; Brambila et al., 2002; Leslie et al., 2004; Coppin et al., 2008). Microarray studies indicate that this adaptation involves the up-regulation of numerous detoxification and antioxidant enzymes, including proteins involved in GSH biosynthesis, metabolism, and efflux (Chen et al., 2001a; Liu et al., 2001a; Lu et al., 2001; Hamadeh et al., 2002; Liu et al., 2004). In this study, we directly examined whether increased cellular GSH biosynthetic capacity alone could inhibit As3+-induced apoptosis and account for this resistant phenotype. For these studies we employed Hepa-1c1c7 cells stably transfected with both GCL subunits, which resulted in a 2-3-fold increase in GCLC and GCLM protein expression and an ~2.5 fold increase in GCL activity compared to vector-transfected cells (Fig. 2, (Botta et al., 2004)). These relative increases correlate well with those observed in As3+-transformed cells in culture (Coppin et al., 2008). However, in contrast to As3+-transformed cells which often contain large increases in GSH levels (Qu et al., 2001; Brambila et al., 2002; Chien et al., 2004; Coppin et al., 2008), GCL overexpression only led to a modest increase in GSH levels. Importantly, GCL overexpression nearly abolished As3+-induced apoptosis in Hepa-1c1c7 cells. While cellular GSH levels can dictate cellular sensitivity to As3+-induced apoptosis, it has been postulated that increased GCL activity and cellular GSH biosynthetic capacity rather than elevated GSH levels mediates the development of apoptotic resistance in transformed cells (Yang et al., 2002). Thus, reestablishing cellular GSH homeostasis after acute As3+ exposure via rapid resynthesis of depleted GSH stores may play a more significant role in mediating the cytoprotective effects of GCL overexpression than elevation of static GSH levels per se. The ability of GCL overexpression to prevent As3+-induced apoptosis while having only a modest effect on cellular GSH levels provides support for this hypothesis.
The ability of enhanced GSH biosynthetic capacity to suppress As3+-induced apoptosis could be derivative of either the antioxidant or metabolic/detoxification properties of GSH. GSH could directly scavenge As3+-induced ROS or act indirectly as a co-factor in glutathione peroxidase-mediated reduction of hydrogen or lipid peroxides (Griffith and Mulcahy, 1999). Alternatively, suppression of As3+-induced toxicity could be mediated via GST-mediated GSH conjugation and detoxification of As3+ (Griffith and Mulcahy, 1999). Indeed, transporter-mediated efflux of As3+ as a GSH conjugate is thought to contribute to As3+ adaptation (Liu et al., 2001a; Leslie et al., 2004; Coppin et al., 2008). However, As3+-induced ATF-3 and GADD153 expression were not suppressed in Hepa-CR17 cells, suggesting that the anti-apoptotic effects of GCL overexpression were not due to GSH-mediated metabolism/detoxification of As3+, which would inhibit all As3+-mediated cellular responses. Thus, while GCL overexpression inhibits As3+-induced apoptosis, it does not abolish all As3+-induced signaling events that may be required for malignant transformation (Hamadeh et al., 2002; Trouba et al., 2002). However, it is not known whether acquired tolerance to As3+ occurs during, or in response to As3+-induced transformation. This is an important distinction when attempting to ascertain whether this resistant phenotype provides a selective growth advantage and contributes to As3+-induced malignant transformation.
As3+ initiates activation of the apoptotic machinery via direct effects on mitochondrial membrane proteins and subsequent release of pro-apoptotic factors from the mitochondria (Larochette et al., 1999; Costantini et al., 2000). While both enhanced GSH biosynthetic capacity and caspase inhibition prevented As3+-induced apoptosis, only GCL overexpression inhibited cytochrome c release. This is consistent with previous studies demonstrating that caspase activation is not necessary for cytochrome c release during chemical-induced apoptosis (Sun et al., 1999). These findings also suggest enhanced GSH biosynthesis inhibits As3+-induced apoptosis at the level of cytochrome c release and not via inhibition of downstream caspase activation. Furthermore, while As3+ does not cause a significant depletion of mitochondrial GSH (Bustamante et al., 2005), As3+-induced mitochondrial dysfunction is thought to be in equilibrium with mitochondrial GSH levels (Costantini et al., 1996). GSH biosynthesis occurs in the cytoplasm and mitochondrial GSH levels are maintained by high affinity transport of cytosolic GSH across the mitochondrial inner membrane (Lash, 2006). While mitochondrial pools of GSH are distinct from cytosolic pools and are preserved even when cytosolic GSH levels are significantly depleted (Soderdahl et al., 2003), mitochondrial GSH levels are nonetheless sensitive to changes in cytosolic GSH biosynthesis (Chen et al., 2007). Recent studies indicate that Bcl-2 also regulates an essential mitochondrial GSH pool that plays a critical role in maintaining mitochondrial redox homeostasis (Zimmermann et al., 2007). Importantly, mitochondria have been identified as the source of As3+-induced ROS production (Pourahmad et al., 2003) and GSH plays a critical co-factor role in glutathione peroxidase-mediated reduction of hydrogen peroxide and lipid peroxides within the mitochondria (Orrenius et al., 2007). GSH also serves as a co-factor for mitochondrial localized glutaredoxin 2 that reduces protein disulfide and mixed disulfides (Orrenius et al., 2007). Thus, GSH may serve to maintain mitochondrial redox homeostasis and counteract the deleterious effects of As3+-induced mitochondrial-generated ROS, preventing mitochondrial dysfunction and release of pro-apoptotic factors such as cytochrome c. Interestingly, mitochondrial damage and subsequent ROS production may also play a causal role in As3+-induced genotoxicity and carcinogenesis (Liu et al., 2001b; Liu et al., 2005).
In summary, these findings indicate that enhanced GSH biosynthesis resulting from increased expression of the GCL subunits can inhibit As3+-induced apoptosis. This cytoprotective effect may play an important role in the development of apoptotic resistance during As3+-induced malignant transformation which is associated with increased GCL subunit expression and GSH homeostasis and can be reversed by inhibition of GCL activity (Liu et al., 2001a; Brambila et al., 2002). However, it is important to note that As3+ adaptation is associated with the up-regulation of a number of proteins involved in GSH metabolism that could also contribute to this resistant phenotype, including various GSTs and ABCC transporters (Chen et al., 2001a; Liu et al., 2001a; Lu et al., 2001; Hamadeh et al., 2002; Liu et al., 2004). In fact, acquired tolerance to As3+-induced toxicity can be reversed by inhibition of GCL, GST or ABCC1 transporter activity (Liu et al., 2001a; Brambila et al., 2002). Thus, while enhanced GSH biosynthesis may play an important role in apoptotic resistance in As3+-transformed cells, additional proteins involved in GSH metabolism and efflux likely contribute to this resistant phenotype.
Acknowledgements
This work was supported by NIH grants CA75316, CA90473, and ES07033 (CCF). James A. Thompson is a Mary Gates Scholar. The authors thank Dr. Terrance Kavanagh for numerous valuable discussions and for providing the Hepa-V3 and Hepa-CR17 cell lines and GCL antibodies used in these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
The authors declare that there are no conflicts of interest
References:
- Achanzar WE, Brambila EM, Diwan BA, Webber MM, Waalkes MP. Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. J Natl Cancer Inst. 2002;94:1888–1891. doi: 10.1093/jnci/94.24.1888. [DOI] [PubMed] [Google Scholar]
- Baker MA, Cerniglia GJ, Zaman A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal Biochem. 1990;190:360–365. doi: 10.1016/0003-2697(90)90208-q. [DOI] [PubMed] [Google Scholar]
- Bode AM, Dong Z. The paradox of arsenic: molecular mechanisms of cell transformation and chemotherapeutic effects. Crit Rev Oncol Hematol. 2002;42:5–24. doi: 10.1016/s1040-8428(01)00215-3. [DOI] [PubMed] [Google Scholar]
- Botta D, Franklin CC, White CC, Krejsa CM, Dabrowski MJ, Pierce RH, Fausto N, Kavanagh TJ. Glutamate-cysteine ligase attenuates TNF-induced mitochondrial injury and apoptosis. Free Radic Biol Med. 2004;37:632–642. doi: 10.1016/j.freeradbiomed.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Brambila EM, Achanzar WE, Qu W, Webber MM, Waalkes MP. Chronic arsenic-exposed human prostate epithelial cells exhibit stable arsenic tolerance: mechanistic implications of altered cellular glutathione and glutathione S-transferase. Toxicol Appl Pharmacol. 2002;183:99–107. [PubMed] [Google Scholar]
- Bustamante J, Nutt L, Orrenius S, Gogvadze V. Arsenic stimulates release of cytochrome c from isolated mitochondria via induction of mitochondrial permeability transition. Toxicol Appl Pharmacol. 2005;207:110–116. doi: 10.1016/j.taap.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Chen H, Liu J, Merrick BA, Waalkes MP. Genetic events associated with arsenic-induced malignant transformation: applications of cDNA microarray technology. Mol Carcinog. 2001a;30:79–87. doi: 10.1002/1098-2744(200102)30:2<79::aid-mc1016>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Chen H, Liu J, Zhao CQ, Diwan BA, Merrick BA, Waalkes MP. Association of cmyc overexpression and hyperproliferation with arsenite-induced malignant transformation. Toxicol Appl Pharmacol. 2001b;175:260–268. doi: 10.1006/taap.2001.9253. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang Y, Miller ML, Shen D, Shertzer HG, Stringer KF, Wang B, Schneider SN, Nebert DW, Dalton TP. Hepatocyte-specific Gclc deletion leads to rapid onset of steatosis with mitochondrial injury and liver failure. Hepatology. 2007;45:1118–1128. doi: 10.1002/hep.21635. [DOI] [PubMed] [Google Scholar]
- Chien CW, Chiang MC, Ho IC, Lee TC. Association of chromosomal alterations with arsenite-induced tumorigenicity of human HaCaT keratinocytes in nude mice. Environ Health Perspect. 2004;112:1704–1710. doi: 10.1289/ehp.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circu ML, Aw TY. Glutathione and apoptosis. Free Radic Res. 2008;42:689–706. doi: 10.1080/10715760802317663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppin JF, Qu W, Waalkes MP. Interplay between cellular methyl metabolism and adaptive efflux during oncogenic transformation from chronic arsenic exposure in human cells. J Biol Chem. 2008 doi: 10.1074/jbc.M802942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini P, Chernyak BV, Petronilli V, Bernardi P. Modulation of the mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. J Biol Chem. 1996;271:6746–6751. doi: 10.1074/jbc.271.12.6746. [DOI] [PubMed] [Google Scholar]
- Costantini P, Jacotot E, Decaudin D, Kroemer G. Mitochondrion as a novel target of anticancer chemotherapy. J Natl Cancer Inst. 2000;92:1042–1053. doi: 10.1093/jnci/92.13.1042. [DOI] [PubMed] [Google Scholar]
- Elbekai RH, El-Kadi AO. Modulation of aryl hydrocarbon receptor-regulated gene expression by arsenite, cadmium, and chromium. Toxicology. 2004;202:249–269. doi: 10.1016/j.tox.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J. 1999;339(Pt 1):135–141. [PMC free article] [PubMed] [Google Scholar]
- Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med. 2009;30:86–98. doi: 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin CC, Krejsa CM, Pierce RH, White CC, Fausto N, Kavanagh TJ. Caspase-3-Dependent Cleavage of the Glutamate-L-Cysteine Ligase Catalytic Subunit during Apoptotic Cell Death. Am J Pathol. 2002;160:1887–1894. doi: 10.1016/S0002-9440(10)61135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin CC, Rosenfeld-Franklin ME, White C, Kavanagh TJ, Fausto N. TGFbeta1-induced suppression of glutathione antioxidant defenses in hepatocytes: caspase-dependent posttranslational and caspase-independent transcriptional regulatory mechanisms. Faseb J. 2003;17:1535–1537. doi: 10.1096/fj.02-0867fje. [DOI] [PubMed] [Google Scholar]
- Franklin CC, Srikanth S, Kraft AS. Conditional expression of mitogen-activated protein kinase phosphatase- 1, MKP-1, is cytoprotective against UV-induced apoptosis. Proc Natl Acad Sci U S A. 1998;95:3014–3019. doi: 10.1073/pnas.95.6.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW, Mulcahy RT. The enzymes of glutathione synthesis: gamma-glutamylcysteine synthetase. Adv Enzymol Relat Areas Mol Biol. 1999;73:209–267. doi: 10.1002/9780470123195.ch7. [DOI] [PubMed] [Google Scholar]
- Guyton KZ, Xu Q, Holbrook NJ. Induction of the mammalian stress response gene GADD153 by oxidative stress: role of AP-1 element. Biochem J. 1996;314(Pt 2):547–554. doi: 10.1042/bj3140547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamadeh HK, Trouba KJ, Amin RP, Afshari CA, Germolec D. Coordination of altered DNA repair and damage pathways in arsenite-exposed keratinocytes. Toxicol Sci. 2002;69:306–316. doi: 10.1093/toxsci/69.2.306. [DOI] [PubMed] [Google Scholar]
- Huang C, Ma WY, Li J, Goranson A, Dong Z. Requirement of Erk, but not JNK, for arsenite-induced cell transformation. J Biol Chem. 1999;274:14595–14601. doi: 10.1074/jbc.274.21.14595. [DOI] [PubMed] [Google Scholar]
- Kitchin KT, Ahmad S. Oxidative stress as a possible mode of action for arsenic carcinogenesis. Toxicol Lett. 2003;137:3–13. doi: 10.1016/s0378-4274(02)00376-4. [DOI] [PubMed] [Google Scholar]
- Larochette N, Decaudin D, Jacotot E, Brenner C, Marzo I, Susin SA, Zamzami N, Xie Z, Reed J, Kroemer G. Arsenite induces apoptosis via a direct effect on the mitochondrial permeability transition pore. Exp Cell Res. 1999;249:413–421. doi: 10.1006/excr.1999.4519. [DOI] [PubMed] [Google Scholar]
- Lash LH. Mitochondrial glutathione transport: physiological, pathological and toxicological implications. Chem Biol Interact. 2006;163:54–67. doi: 10.1016/j.cbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie EM, Haimeur A, Waalkes MP. Arsenic transport by the human multidrug resistance protein 1 (MRP1/ABCC1). Evidence that a tri-glutathione conjugate is required. J Biol Chem. 2004;279:32700–32708. doi: 10.1074/jbc.M404912200. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Li M, Cai JF, Chiu JF. Arsenic induces oxidative stress and activates stress gene expressions in cultured lung epithelial cells. J Cell Biochem. 2002;87:29–38. doi: 10.1002/jcb.10269. [DOI] [PubMed] [Google Scholar]
- Liu J, Chen H, Miller DS, Saavedra JE, Keefer LK, Johnson DR, Klaassen CD, Waalkes MP. Overexpression of glutathione S-transferase II and multidrug resistance transport proteins is associated with acquired tolerance to inorganic arsenic. Mol Pharmacol. 2001a;60:302–309. doi: 10.1124/mol.60.2.302. [DOI] [PubMed] [Google Scholar]
- Liu J, Xie Y, Ward JM, Diwan BA, Waalkes MP. Toxicogenomic analysis of aberrant gene expression in liver tumors and nontumorous livers of adult mice exposed in utero to inorganic arsenic. Toxicol Sci. 2004;77:249–257. doi: 10.1093/toxsci/kfh055. [DOI] [PubMed] [Google Scholar]
- Liu SX, Athar M, Lippai I, Waldren C, Hei TK. Induction of oxyradicals by arsenic: implication for mechanism of genotoxicity. Proc Natl Acad Sci U S A. 2001b;98:1643–1648. doi: 10.1073/pnas.031482998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SX, Davidson MM, Tang X, Walker WF, Athar M, Ivanov V, Hei TK. Mitochondrial damage mediates genotoxicity of arsenic in mammalian cells. Cancer Res. 2005;65:3236–3242. doi: 10.1158/0008-5472.CAN-05-0424. [DOI] [PubMed] [Google Scholar]
- Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Liu J, LeCluyse EL, Zhou YS, Cheng ML, Waalkes MP. Application of cDNA microarray to the study of arsenic-induced liver diseases in the population of Guizhou, China. Toxicol Sci. 2001;59:185–192. doi: 10.1093/toxsci/59.1.185. [DOI] [PubMed] [Google Scholar]
- Miller WH, Jr., Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–3903. [PubMed] [Google Scholar]
- Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- Pi J, Diwan BA, Sun Y, Liu J, Qu W, He Y, Styblo M, Waalkes MP. Arsenic-induced malignant transformation of human keratinocytes: Involvement of Nrf2. Free Radic Biol Med. 2008;45:651–658. doi: 10.1016/j.freeradbiomed.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi J, Qu W, Reece JM, Kumagai Y, Waalkes MP. Transcription factor Nrf2 activation by inorganic arsenic in cultured keratinocytes: involvement of hydrogen peroxide. Exp Cell Res. 2003;290:234–245. doi: 10.1016/s0014-4827(03)00341-0. [DOI] [PubMed] [Google Scholar]
- Pierce RH, Campbell JS, Stephenson AB, Franklin CC, Chaisson M, Poot M, Kavanagh TJ, Rabinovitch PS, Fausto N. Disruption of Redox Homeostasis in Tumor Necrosis Factor-Induced Apoptosis in a Murine Hepatocyte Cell Line. Am J Pathol. 2000;157:221–236. doi: 10.1016/S0002-9440(10)64533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourahmad J, O'Brien PJ, Jokar F, Daraei B. Carcinogenic metal induced sites of reactive oxygen species formation in hepatocytes. Toxicol In Vitro. 2003;17:803–810. doi: 10.1016/s0887-2333(03)00123-1. [DOI] [PubMed] [Google Scholar]
- Qu W, Kasprzak KS, Kadiiska M, Liu J, Chen H, Maciag A, Mason RP, Waalkes MP. Mechanisms of arsenic-induced cross-tolerance to nickel cytotoxicity, genotoxicity, and apoptosis in rat liver epithelial cells. Toxicol Sci. 2001;63:189–195. doi: 10.1093/toxsci/63.2.189. [DOI] [PubMed] [Google Scholar]
- Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- Romach EH, Zhao CQ, Del Razo LM, Cebrian ME, Waalkes MP. Studies on the mechanisms of arsenic-induced self tolerance developed in liver epithelial cells through continuous low-level arsenite exposure. Toxicol Sci. 2000;54:500–508. doi: 10.1093/toxsci/54.2.500. [DOI] [PubMed] [Google Scholar]
- Schuliga M, Chouchane S, Snow ET. Upregulation of glutathione-related genes and enzyme activities in cultured human cells by sublethal concentrations of inorganic arsenic. Toxicol Sci. 2002;70:183–192. doi: 10.1093/toxsci/70.2.183. [DOI] [PubMed] [Google Scholar]
- Sens DA, Park S, Gurel V, Sens MA, Garrett SH, Somji S. Inorganic cadmium- and arsenite-induced malignant transformation of human bladder urothelial cells. Toxicol Sci. 2004;79:56–63. doi: 10.1093/toxsci/kfh086. [DOI] [PubMed] [Google Scholar]
- Shertzer HG, Vasiliou V, Liu RM, Tabor MW, Nebert DW. Enzyme induction by Lbuthionine (S,R)-sulfoximine in cultured mouse hepatoma cells. Chem Res Toxicol. 1995;8:431–436. doi: 10.1021/tx00045a015. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Hochadel JF, Fulmer BA, Waalkes MP. Effect of glutathione depletion and metallothionein gene expression on arsenic-induced cytotoxicity and c-myc expression in vitro. Toxicol Sci. 1998;45:204–211. doi: 10.1006/toxs.1998.2539. [DOI] [PubMed] [Google Scholar]
- Siitonen T, Alaruikka P, Mantymaa P, Savolainen ER, Kavanagh TJ, Krejsa CM, Franklin CC, Kinnula V, Koistinen P. Protection of acute myeloblastic leukemia cells against apoptotic cell death by high glutathione and gamma-glutamylcysteine synthetase levels during etoposide-induced oxidative stress. Ann Oncol. 1999;10:1361–1367. doi: 10.1023/a:1008382912096. [DOI] [PubMed] [Google Scholar]
- Single B, Leist M, Nicotera P. Simultaneous release of adenylate kinase and cytochrome c in cell death. Cell Death Differ. 1998;5:1001–1003. doi: 10.1038/sj.cdd.4400462. [DOI] [PubMed] [Google Scholar]
- Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderdahl T, Enoksson M, Lundberg M, Holmgren A, Ottersen OP, Orrenius S, Bolcsfoldi G, Cotgreave IA. Visualization of the compartmentalization of glutathione and protein-glutathione mixed disulfides in cultured cells. Faseb J. 2003;17:124–126. doi: 10.1096/fj.02-0259fje. [DOI] [PubMed] [Google Scholar]
- Somji S, Zhou XD, Garrett SH, Sens MA, Sens DA. Urothelial cells malignantly transformed by exposure to cadmium (Cd(+2)) and arsenite (As(+3)) have increased resistance to Cd(+2) and As(+3)-induced cell death. Toxicol Sci. 2006;94:293–301. doi: 10.1093/toxsci/kfl108. [DOI] [PubMed] [Google Scholar]
- Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem. 1999;274:5053–5060. doi: 10.1074/jbc.274.8.5053. [DOI] [PubMed] [Google Scholar]
- Thompson JA, White CC, Cox DP, Chan JY, Kavanagh TJ, Fausto N, Franklin CC. Distinct Nrf1/2-independent mechanisms mediate As 3+-induced glutamate-cysteine ligase subunit gene expression in murine hepatocytes. Free Radic Biol Med. 2009;46:1614–1625. doi: 10.1016/j.freeradbiomed.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, White CC, Krejsa CM, Diaz D, Woods JS, Eaton DL, Kavanagh TJ. Induction of glutamate-cysteine ligase (gamma-glutamylcysteine synthetase) in the brains of adult female mice subchronically exposed to methylmercury. Toxicol Lett. 1999;110:1–9. doi: 10.1016/s0378-4274(99)00133-2. [DOI] [PubMed] [Google Scholar]
- Trouba KJ, Geisenhoffer KM, Germolec DR. Sodium arsenite-induced stress-related gene expression in normal human epidermal, HaCaT, and HEL30 keratinocytes. Environ Health Perspect. 2002;110(Suppl 5):761–766. doi: 10.1289/ehp.02110s5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CC, Viernes H, Krejsa CM, Botta D, Kavanagh TJ. Fluorescence-based microtiter plate assay for glutamate-cysteine ligase activity. Anal Biochem. 2003;318:175–180. doi: 10.1016/s0003-2697(03)00143-x. [DOI] [PubMed] [Google Scholar]
- Wild AC, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression: insights into transcriptional control of antioxidant defenses. Free Radic Res. 2000;32:281–301. doi: 10.1080/10715760000300291. [DOI] [PubMed] [Google Scholar]
- Yang Y, Dieter MZ, Chen Y, Shertzer HG, Nebert DW, Dalton TP. Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm(−/−) knockout mouse. Novel model system for a severely compromised oxidative stress response. J Biol Chem. 2002;277:49446–49452. doi: 10.1074/jbc.M209372200. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Yamauchi H, Fan Sun G. Chronic health effects in people exposed to arsenic via the drinking water: dose-response relationships in review. Toxicol Appl Pharmacol. 2004;198:243–252. doi: 10.1016/j.taap.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Zhao CQ, Young MR, Diwan BA, Coogan TP, Waalkes MP. Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proc Natl Acad Sci U S A. 1997;94:10907–10912. doi: 10.1073/pnas.94.20.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann AK, Loucks FA, Schroeder EK, Bouchard RJ, Tyler KL, Linseman DA. Glutathione binding to the Bcl-2 homology-3 domain groove: a molecular basis for Bcl-2 antioxidant function at mitochondria. J Biol Chem. 2007;282:29296–29304. doi: 10.1074/jbc.M702853200. [DOI] [PMC free article] [PubMed] [Google Scholar]