Abstract
The integrity of actin cytoskeletal organization in aqueous humor outflow pathway is thought to play a critical role in modulation of aqueous humor outflow through the trabecular meshwork. Our understanding of the regulation of actin cytoskeletal dynamics in outflow pathway, however, is very limited. To explore the potential importance of Neural Wiskott-Aldrich syndrome protein (N-WASP), a critical regulator of actin polymerization/nucleation in aqueous humor outflow pathway, the effects of Wiskostatin, a selective pharmacological inhibitor of N-WASP, on aqueous humor outflow facility were evaluated using enucleated porcine eyes and a constant pressure perfusion system. Further, drug induced effects on actin cytoskeletal organization, cell adhesions, myosin II phosphorylation, matrix metalloproteinase (MMP) activity, and cytoskeletal protein profile in porcine trabecular meshwork (TM) cells were determined by immunofluorescence, zymography, and mass spectrometry. Aqueous humor outflow facility was increased significantly and progressively in the Wiskostatin perfused porcine eyes. The Wiskostatin perfused eyes appear to exhibit increased giant vacuoles in the inner wall of aqueous plexi and deformation of aqueous plexi. The Wiskostatin treated TM cells demonstrated extensive vacuoles in their cytosol, and both actin stress fibers and focal adhesions were decreased in a reversible manner. The drug treated TM cells also revealed decreased myosin II and actin in the cytoskeletal enriched triton insoluble fraction but did not affect myosin II phosphorylation or MMP-2 activity. These data demonstrate that the chemical inhibition of N-WASP increases aqueous humor outflow facility in association with decreased actomyosin interaction and cell adhesive interactions revealing the importance of N-WASP in homeostasis of aqueous humor outflow.
Keywords: Actin cytoskeleton, N-WASP, Wiskostatin, Aqueous humor outflow, Trabecular meshwork
1. Introduction
Glaucoma, characterized by optic nerve degeneration and retinal ganglion cell death, is a leading cause of blindness worldwide. In primary open angle glaucoma (POAG), the most prevalent type of this disease, intraocular pressure (IOP) is commonly elevated as a result of increased resistance of the conventional aqueous humor outflow process (Quigley, 1993; Weinreb and Khaw, 2004). In humans this pathway is a predominant route of aqueous humor outflow (Tan et al., 2006), and the increased resistance in POAG is thought to be related to functional and structural abnormalities in the juxtacanalicular region (JCT) of the trabecular meshwork (TM) and/or Schlemm's canal (SC). Although retinal ganglion cell survival represents a primary goal for the treatment of this disease, lowering IOP is the only option available to treat glaucoma, at the present time (Weinreb and Khaw, 2004). Hence, understanding the molecular mechanism(s) of both normal and perturbed aqueous humor outflow in glaucoma is necessary and important.
TM, JCT and SC cells are believed to influence aqueous outflow facility by altering their morphology, cell adhesive interactions and extracellular environment, leading to changes in the geometry of the aqueous outflow pathway, and paracellular and transcellular permeability (Epstein et al., 1999; Johnson et al., 1992; Rao and Epstein, 2007; Tian et al., 2009; Tian et al., 2000). The TM cells have smooth muscle-like contractile and relaxation properties regulated by actomyosin interaction (Coroneo et al., 1991; Rao and Epstein, 2007). The involvement of the actin cytoskeleton in glaucoma pathobiology was suggested by observing disordered actin fibers in glaucomatous outflow tissue specimens (Read et al., 2006). The influence of actin cytoskeletal integrity on aqueous humor outflow facility has also been well documented in both in vivo and ex-vivo studies (Rao and Epstein, 2007; Tian et al., 2009; Tian et al., 2000). Moreover, inhibitors of various kinases including Rho kinase (ROCK), protein kinase C (PKC), and myosin light chain kinase (MLCK), which regulate myosin light chain phosphorylation and actomyosin interaction, have been demonstrated to increase aqueous outflow facility and lower IOP (Honjo et al., 2001; Khurana et al., 2003; Rao et al., 2001; Tanihara et al., 2008; Tian et al., 2009). Although cellular relaxation, altered cell morphology, and loss of cell adhesive interactions are commonly observed in outflow pathway cells treated with actin depolymerizing agents and kinase inhibitors (Rao and Epstein, 2007; Tian et al., 2009; Tian et al., 2000), the cellular and molecular basis for the potential involvement of the actin cytoskeleton and the regulatory proteins of actin cytoskeleton in the homeostasis of aqueous humor outflow function have not been well characterized.
Neural Wiskott-Aldrich syndrome protein (N-WASP), a member of the WASP family of proteins, is a key regulator of actin polymerization and nucleation; its activity is regulated by phosphatidylinositol bisphosphate (PIP2) and the small GTPase Cdc42, which induces a conformational switch that initiates actin polymerization (Bompard and Caron, 2004; Takenawa and Suetsugu, 2007). N-WASP in its active confirmation interacts with actin-related protein-2/3 (ARP2/3) and activates ARP2/3 complex to induce actin nucleation and polymerization (Bompard and Caron, 2004; Takenawa and Suetsugu, 2007). Actin nucleation and polymerization in turn plays a crucial role in various cellular functions, such as membrane protrusion, migration, endocytosis, vesicle and organelle trafficking, podosome formation, transcription, and exocytosis (Goley and Welch, 2006; Oikawa and Takenawa, 2009; Takenawa and Suetsugu, 2007; Wu et al., 2006). Recently, Wiskostatin, a small molecular chemical compound, has been identified as a selective and reversible inhibitor of N-WASP (Peterson et al., 2004), and this compound keeps N-WASP in its autoinhibited form and thereby prevents N-WASP membrane association and ultimately actin polymerization.
In this study, we determined the effects of Wiskostatin on aqueous humor outflow facility and its cellular responses in cultured TM cells to explore the potential role of N-WASP in aqueous humor outflow function. Enucleated porcine eyes perfused with Wiskostatin demonstrated increased aqueous outflow facility, which was associated with structural and morphological changes in aqueous humor outflow pathway. These data reveal that N-WASP may be involved in modulation of aqueous humor outflow facility via trabecular meshwork pathway, and provide novel insights into the participation of different cytoskeletal regulatory proteins in aqueous humor outflow homeostasis.
2. Materials and Methods
2.1. Materials
Wiskostatin was purchased from Biomol International, LP (Plymouth Meeting, PA). N-WASP polyclonal antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Enhanced chemiluminescence (ECL) Plus detection reagents were from Amersham Pharmacia Biotech (Piscataway, NJ). Cell culture media and fetal bovine serum were obtained from Gibco-BRL (Gaithersburg, MD). Tetrarhodamine isothiocyanate (TRITC) –phalloidin, monoclonal antibody against β-actin, and monoclonal antibody against vinculin were purchased from Sigma-Aldrich (St. Louis, MO). Phospho-MLC2 polyclonal antibody was purchased from Cell Signaling Technology (Beverly, MA). Myosin IIA and IIB polyclonal antibodies were from Covance (Princeton, NJ).
2.2. Perfusion and Histological examination
Freshly obtained enucleated porcine eyes from a local abattoir were perfused with 50 μM Wiskostatin (initially dissolved in DMSO) in perfusion medium containing Dulbeco phosphate-buffered saline (DPBS) and 5.5 mM D-glucose at 25°C using a Grant constant pressure perfusion system as described previously (Rao et al., 2005). After establishing the initial baseline outflow measurements at 15 mmHg and 25°C, the anterior chambers of the eyes were exchanged with drug and perfused with drug continuously for 5 hr. Outflow measurements were recorded at hourly intervals. Drug effects were expressed as percentage change in outflow facility (compared to baseline values) over 5 hr, in drug treated versus sham treated (DMSO) paired controls (contra lateral eyes). Data were analyzed by a paired two-tailed Student's t-test to determine significance. At the end of a 5 hr perfusion period, sham control and drug-treated fellow eyes were fixed and subjected to light and electron microscopic analysis as described in our previous study (Rao et al., 2001).
2.3. Cell culture and Viability Assay
Porcine TM (PTM) cells were isolated as we described earlier from pig eyes obtained from a local abattoir (Rao et al., 2001). PTM cells were used between passages 3 to 5. All experiments were conducted using confluent cultures and cells were cultured at 37°C under 5% CO2, in Dulbecco's modified Eagle Medium (DMEM) containing 10% FBS and Penicillin –Streptomycin-Glutamine. For cell viability assays, drug-treated PTM cells were incubated with fluorescein diacetate and propidium iodide as described previously and cell viability and toxicity was assessed by following the fluorescein diacetate green fluorescence and propidium iodide red staining, respectively (Rao et al., 2001).
2.4. Immunofluorescence staining
Immunofluorescence staining was performed as we described previously (Rao et al., 2001). Briefly, the drug-treated PTM cells (cultured on gelatin-coated glass coverslips) were fixed with 3.7% formaldehyde for 12 minutes after morphologic examination by phase-contrast microscopy (IM35; Carl Zeiss, Thornwood, NY). Cells were then washed with cytoskeletal buffer (10 mM 2-[N-morpholino] ethane sulfonic acid (MES), 150 mM NaCl, 5 mM EGTA, 5 mM MgCl2, and 5 mM glucose pH 6.1), permeabilized with 0.5% Triton X-100, and blocked with 10% serum buffer. For F-actin staining, cells were incubated with TRITC-phalloidin (1:500) for 20 minutes. For detection of focal adhesions and myosin II, cells were immunostained with mouse anti-vinculin (1:200) and rabbit anti-myosin IIA antibody (1:500) for 2 hrs respectively, followed by incubation with secondary antibody conjugated with Alexa 488, and counterstaining of nuclei with 4′,6-diamidino-2-phenylindole (DAPI) for 30 minutes.
2.5. MLC phosphorylation
MLC phosphorylation status of PTM cells was determined as described by Garcia et al.(Garcia et al., 1995). Briefly, confluent cell cultures of both control and drug treated were extracted with cold 10% trichloroacedic acid and 0.5M dithiothreitol, and cell precipitates obtained after centrifugation at 13,000 rpm were dissolved in 8 M urea buffer using a sonicator, separated on glycerol slab gels, and transferred onto nitrocellulose filters. The filters were then subjected to immunoblot analysis using a rabbit antiphospho-MLC2 antibody (1:1000) followed by development with peroxidase-conjugated goat anti-rabbit IgG and an ECL plus detection system.
2.6. Gelatin Zymography
Matrix metalloproteinase-2 (MMP-2) activity was evaluated by gelatin zymography as described previously (Sanka et al., 2007). Briefly, PTM cells (5 × 105) which were serum starved for 24 hrs were treated with 15 μM of Wiskostatin for one hr and the conditioned media were concentrated by ultracentrifugal filter devices (Amicon; Millipore, Billerica, MA) with 10-kDa cutoff and subjected to zymogram gelatin gel electrophoresis (Invitrogen, Carlsbad, CA) as per manufacture's protocol.
2.7. Isolation of TM cell Triton X-100 resistance fractions
PTM cells were cultured to confluence in plastic Petridishes and treated with 15 μM of Wiskostatin for 1 hr after culturing with 1% serum for 24 hr. The Triton X-100 insoluble fraction was isolated as we described earlier (Rao et al., 2008). Briefly, cells were scraped on ice and lysed in solubilization buffer (SB) containing 10 mM Piperazine-N,N-bis[2-ethanesulfonic acid] dipotassium salt, 50 mM KCl, 10 mM Ethylene glycol-bis (β-aminoethyl ether) N,N,N,N-tetracetic acid (EGTA), 3 mM MgCl2, 2 M glycerol, 2 mM NaF, 1 mM Na3VO4, and protease inhibitors (25 μg/ml each of aprotinin, leupeptin, and pepstatin), using a sonicator. Cell lysates were centrifuged for 15 min at 800g. The resulting supernatants were then centrifuged at 35,000g for 30 min, after which, the pellets obtained were washed in SB and re-homogenized in SB containing 1% Triton X-100. The detergent treated samples were then centrifuged at 35,000g for 30 min and the insoluble cytoskeletal fraction (pellet) was washed once again and suspended in extraction buffer containing 20 mM Tris–HCl, 300 mM NaCl, 30 mM MgCl2, 1 mM EGTA, 1 mM 1,4-dithiothreitol (DTT) and protease inhibitors. All procedures were performed at 4 °C. The protein content of triton insoluble fraction was determined using the Bio-Rad protein reagent (Cat. # 500-0006. Bio-Rad Laboratories, Inc. Hercules, CA).
2.8. In-gel Trypsin Digestion and Mass Spectrometry Analysis
The triton insoluble fractions of TM cells derived from drug treatment and control were separated on SDS–PAGE containing 8% acrylamide after mixing with sample loading buffer and boiling for 3 minutes. After electrophoresis, gels were stained with Gel Code Blue (Thermo Scientific, Rockford, IL) and the destained gels were photographed. The distinct protein bands were cut out from the gel and subjected to in-gel trypsin digestion as described earlier (Rao et al., 2008). The trypsin digested protein peptides were identified by a combination of peptide mass finger printing analyses (matrix assisted laser desorption/ionisation-time of flight-time of flight mass spectrometry-MALDI-TOF-TOF MS) and MS/MS (Tandem mass spectrometry) as described previously (Rao et al., 2008).
3. Results
3.1. Increased aqueous outflow facility with Wiskostatin perfusion
Enucleated porcine eyes were perfused with 50 μM of Wiskostatin at a constant pressure of 15 mm Hg. Baseline rates of outflow facility in the control eyes and drug-perfused eyes were 0.67 ± 0.11 and 0.56 ± 0.16 μl/min/mm Hg, respectively (n=9). Following the drug perfusion, outflow facility was observed to increase significantly by 24% over control eyes (P < 0.05) at 1 hr. The effect was progressive and reached to 44% increase over control eyes at 5 hrs (Fig. 1). Fellow paired control eyes showed an expected washout response (≈ 30-40%) in outflow facility over the corresponding initial baseline values. This perfusion experiment demonstrated a time dependent increase in aqueous outflow facility with Wiskostatin treatment and the difference in aqueous facility between control and drug treated eyes was significantly different (minimum at P<0.05) starting from one hr post drug perfusion (Fig. 1).
Figure 1.
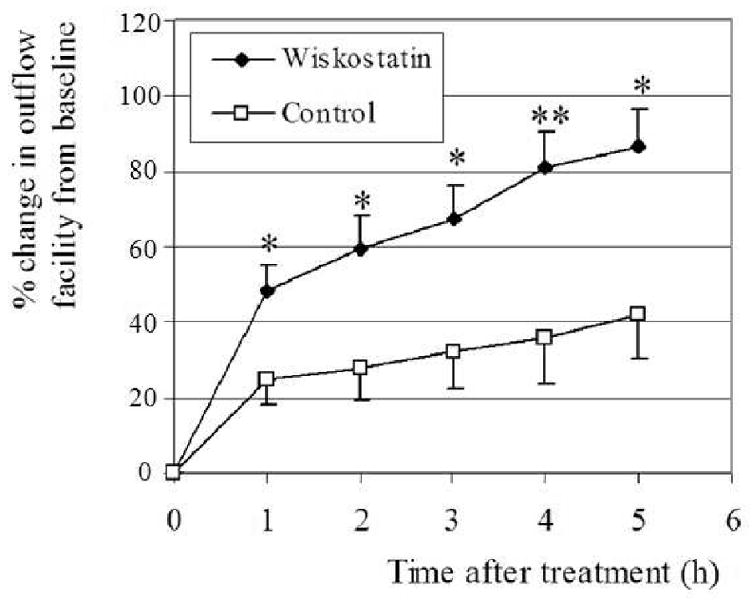
Effects of Wiskostatin perfusion on aqueous humor outflow facility in enucleated porcine eyes. After recording the baseline facility, eyes were exchanged with Wiskostatin (50 μM) and perfused with drug solution at a constant pressure of 15 mm Hg at 25 °C. Following the drug perfusion, outflow facility from the baseline facility was observed to increase progressively and significantly over control eyes. Values are mean ± SE, n = 9, *p < 0.05, **p < 0.01.
3.2. Wiskostatin-induced structural changes in the outflow pathway tissues
After 5 hr perfusion with Wiskostatin, the porcine eyes were fixed and outflow pathway tissues were examined histologically. Six specimens were prepared from different quadrants of 2 eyes in each group. Light microscope examination revealed that 3 out of 6 specimens were found to have clear oval shaped aqueous plexi (equivalent of human Schlemm's canal), in the control group. In contrast, none in 6 specimens had a clear oval shaped aqueous plexus in the Wiskostatin perfused group. Instead, some of them had deformed aqueous plexi, into which JCT appeared to be herniated (Fig. 2). Interestingly, giant vacuoles in the endothelial cells of aqueous plexi were found to be more prominent in the Wiskostatin treated group compared to controls (Fig. 2, arrows). Meanwhile, these changes were not associated with any noticeable cell loss or accumulation of cell debris in the TM of eyes perfused with Wiskostatin, indicating no tissue-destructive effects from the Wiskostatin treatment.
Figure 2.
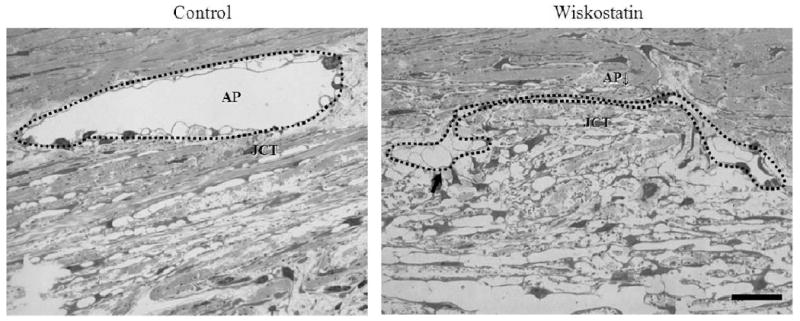
Light microscope-based histological integrity of outflow tissues perfused with or without Wiskostatin. After 5 hr perfusion, 3 in 6 specimens from different quadrants of 2 eyes were found to have clear oval shaped aqueous plexi (AP) in the control group. In contrast, none in 6 specimens had a clear oval shaped aqueous plexi in Wiskostatin perfused group. Instead, some of them had deformed aqueous plexi (marked with a dotted line), into which JCT was found to be herniated and with prominent giant vacuoles (indicated with arrows) in the endothelial cells of aqueous plexi compared to controls. Scale bar: 20 μm.
In agreement with light microscopy-based examination, drug treated samples viewed under transmission electron microscope exhibited more giant vacuoles in the inner wall of aqueous plexi compared to control specimens. In one of the drug perfused eyes, the integrity of inner wall of aqueous plexi was found to have a break in the lining of the inner wall endothelial cells (Fig. 3, indicated with arrowhead).
Figure 3.
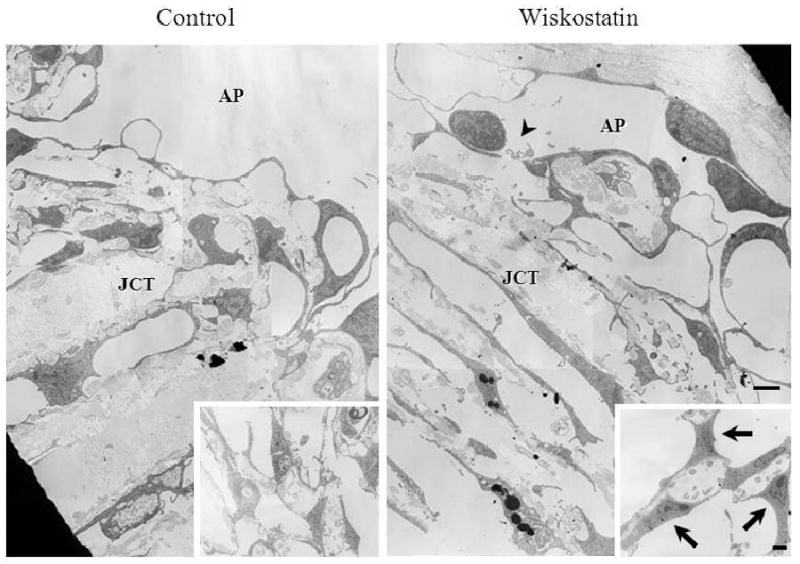
Transmission electron micrographs of outflow tissue perfused with or without Wiskostatin. Drug perfused samples appear to have enlarged and more giant vacuoles in the inner wall of aqueous plexi compared to control specimens. A small break in the lining of endothelial cells (arrowhead), and enlarged TM cells (arrows) in the corneoscleral meshwork (insets) were found in a Wiskostatin perfused eye. Scale bar: 2 μm.
3.3. Effects of Wiskostatin on PTM Cell Morphology and Cytoskeletal Organization
Before testing the effects of wiskostain on PTM cells, PTM and HTM cells were confirmed to express N-WASP based on immunoblot analysis (data not shown). To determine the effect of Wiskostatin at the cellular level, the confluent PTM cell cultures were treated with different concentrations of Wiskostatin (5, 15, and 30 μM) after maintaining the cells in 1% serum media for 24 hr, and monitored the changes in cell morphology. PTM cells treated with 15 and 30 μM of Wiskostatin demonstrated vacuoles in their cytosol at 1 hr after treatment (Fig. 4, indicated with arrows). At higher concentration (30 μM and more), PTM cells presented more extensive vacuoles, cell–cell separation and cell rounding. These changes were reversible within 24 hrs of drug withdrawal, and the drug treated TM cells did not exhibit any significant increase in cell toxicity over control cells based on live cell imaging of fluorescein diacetate and propidium iodide incorporation after 4 hr treatment with drug (data not shown).
Figure 4.
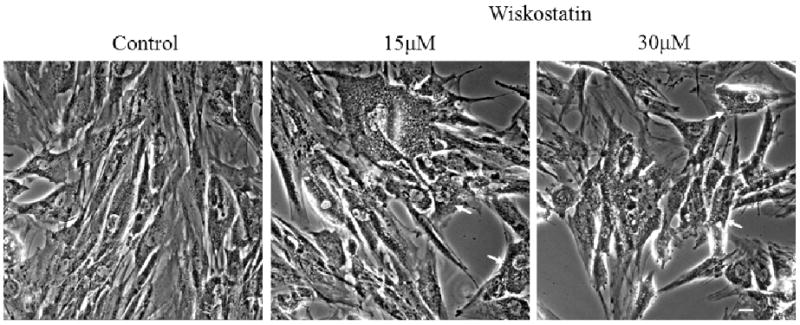
Phase contrast micrographs of cultured PTM cells treated with Wiskostatin. The confluent cultured PTM cells were treated with different concentrations of Wiskostatin as indicated after maintaining them in 1% serum media for 24 hrs. Wiskostatin-treated PTM cells presented extensive vacuoles (indicated with white arrows) and cell– cell separation in a dose dependent manner. Scale bar: 10 μm.
Since N-WASP has been shown to be a critical determinant of actin cytoskeletal dynamics, changes in actin cytoskeletal integrity and cell adhesions induced by Wiskostatin were assessed by immunofluorescence analysis. The Wiskostatin-treated PTM cells demonstrated that both actin stress fibers stained with phalloidin and focal adhesions stained with vinculin antibody were decreased in their staining dose dependently after 1 hr treatment (Fig. 5). The decrease in actin cell stress fibers was more evident in the cell cytoplasm relative to cell cortical region.
Figure 5.
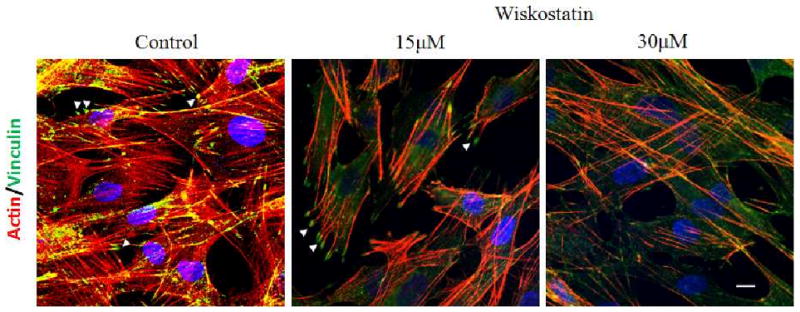
Effects of Wiskostatin on porcine TM cell actin cytoskeletal integrity and cell adhesions. Wiskostatin treated PTM cells demonstrated that both actin stress fibers stained with phalloidin (red) and focal adhesions stained against vinculin (green, indicated with arrow heads) were decreased in their staining dose dependently compared to control cells. Nuclei were counterstained with DAPI (blue). Scale bar: 10 μm.
3.4. Effects of Wiskostatin on PTM cell cytoskeletal protein composition
To further explore drug-induced changes in cytoskeletal proteins, the Triton X-100 resistant cytoskeletal fraction was isolated from the cultured PTM cells, and was subjected to SDS-PAGE. After staining with Gelcode blue dye, the amounts of two protein bands (indicated with arrows) were found to be markedly decreased in Wiskostatin treated specimens compared to controls (Fig. 6A, arrows). Using the MALDI-TOF-TOF Mass spectrometry, we identified these two bands as myosin IIA and γ-actin, indicating the specific effect of Wiskostatin on the actin-myosin complex in the cytoskeletal enriched fraction. The total content of myosin II (both IIA and IIB), and actin determined by immunoblot analysis, however, was found to be no different between the drug treated and control samples (data not shown) indicating drug induced impairment in assembly of actin and myosin II but not on the total protein content (Please see attached supplemental figure 1, which is intended for the review process only).
Figure 6.
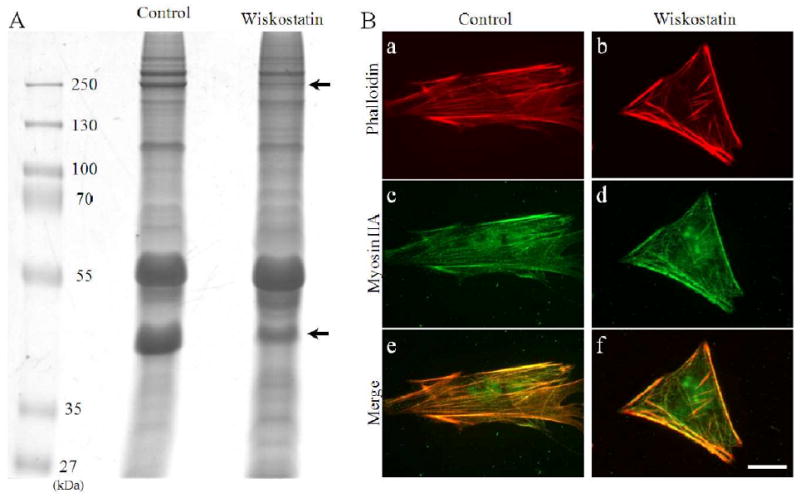
Effects of Wiskostatin on porcine TM cell actomyosin cytoskeleton. A. Triton X-100 resistant cytoskeletal fraction was collected from the insoluble fraction of cultured TM cells treated with Wiskostatin, and was subjected to SDS-PAGE. After staining with gelcode blue dye, the densities of two protein bands (indicated with arrows) were found to be decreased after treatment with Wiskostatin compared to control. Based on Mass spectrometry analysis, these protein bands were identified as myosin IIA (upper arrow) and γ-actin (lower arrow). B. The intracellular localization of actin stained with phalloidin (red, a, b) and myosin IIA (stained with anti-myosin IIA antibody) (green, c, d) in the TM cells by immunocytochemistry. In control cells, actin and myosin IIA were colocalized and distributed throughout the cell body including cortical regions (a, c, e). In contrast, in the Wiskostatin treated cells, though actin and myosin were still colocalized, their staining in cytoplasm, especially in the center, were reduced, while at the cortical region or cell border staining remained mostly unaltered in Wiskostatin treated cells (b, d, f). Scale bar: 20 μm.
We also evaluated the changes in the co-distribution of actin stress fibers and myosin IIA in the TM cells by immunocytochemistry. In control cells, actin and myosin IIA were colocalized and formed fibers throughout the cell body including the cortical region and the cytoplasmic region. In contrast, while actin and myosin were still colocalized to the cortical region of the cells as seen in control cells, the cytoplasmic region was found to have much reduced crosslinked actomyosin in the Wiskostatin treated cells (Fig. 6B).
3.5. Effects of Wiskostatin on Myosin Light-Chain Phosphorylation and MMP2 Activity in PTM Cells
Since various cytoskeletal reorganizing agents, such as Rho kinase inhibitors, protein kinase C inhibitors, and cholesterol-lowering statins, have been shown to affect actin and myosin interaction by decreasing MLC phosphorylation in TM cells (Khurana et al., 2003; Rao et al., 2001; Song et al., 2005), we also assessed MLC phosphorylation status in Wiskostatin-treated cells using immunoblot analysis. After 1 hr treatment with 15 μM Wiskostatin, no difference, was observed between control and Wiskostatin treated TM cells in MLC phosphorylation status indicating that the early changes observed in actomyosin organization in the drug treated cells were independent of altered myosin II activity (Fig. 7).
Figure. 7.
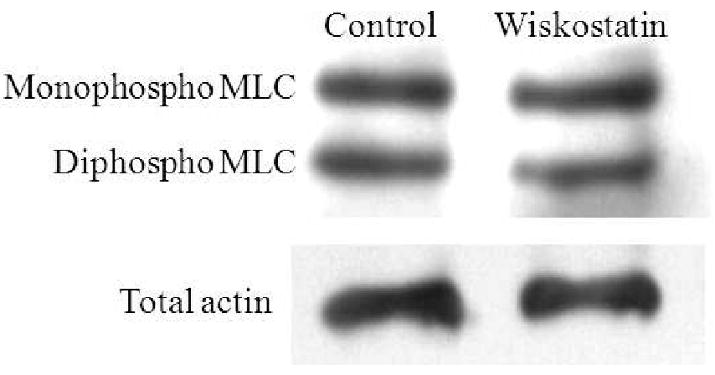
Effects of Wiskostatin on TM cell myosin light chain phosphorylation. PTM cells treated with Wiskostatin (15μM for one hr) were examined for changes in MLC phosphorylation. Wiskostatin treatment was found to have no influence on MLC phosphorylationin PTM cells.
We also evaluated MMP-2 activity in conditioned media derived from Wiskostatin treated and sham treated control cells by gelatin zymogram to explore the possibility of Wiskostatin influencing the MMP-2 activity as has been known for other actin cytoskeletal depolymerizing agents (Sanka et al., 2007). However, MMP-2 activity was found to be unaffected by Wiskostatin treatment for one hr (Fig. 8). These results were based on duplicates.
Figure 8.

Effects of Wiskostatin on TM cell MMP-2 activity.
The conditioned medium derived from the PTM cells treated with 15 μM Wiskostatin for one hr was tested for the MMP-2 activation by gelatin zymography and found to be no change in the MMP-2 activity.
4. Discussion
In the present study, we found that Wiskostatin increases conventional aqueous humor outflow with accompanying structural changes in the outflow pathway, such as deformed aqueous plexi, and prominent giant vacuoles. At a cellular level, Wiskostatin treatment induced vacuole formation, decreases in actomyosin stress fibers, focal adhesions and altered cell morphology in cultured TM cells. These observations collectively confirm the importance of N-WASP, a critical regulator of actin nucleation and polymerization in aqueous humor outflow.
We undertook this project with a goal of obtaining further insight into the role of actin cytoskeletal integrity in modulation of aqueous humor outflow function. Though it is well recognized that the polymerization status of actin and actin-myosin contractile activity in TM cells influence aqueous humor outflow via the conventional route, our knowledge of the various cellular mechanisms that control the actin cytoskeletal organization in the outflow pathway tissue is very limited (Epstein et al., 1999; Rao and Epstein, 2007; Tian et al., 2009; Tian et al., 2000). Actin dynamics is a complex process regulated by various actin interacting and signaling molecules (Goley and Welch, 2006; Hall, 2005; Takenawa and Suetsugu, 2007). N-WASP, which controls ARP2/3 complex activity by linking upstream signals, initiates actin polymerization and plays a critical role in several actin-dependent cellular processes including cell adhesion, migration, formation of podosomes, endosomes, and membrane trafficking (Bompard and Caron, 2004; Goley and Welch, 2006; Oikawa and Takenawa, 2009; Takenawa and Suetsugu, 2007). To explore N-WASP role in aqueous humor outflow, we targeted its activity using a small molecular pharmacological inhibitor, Wiskostatin (Peterson et al., 2004). Wiskostatin perfusion in enucleated porcine eyes caused a significant and progressive increase in aqueous outflow facility. Interestingly, this induced aqueous humor outflow facility appears to be associated with deformation of aqueous plexi, and increased giant vacuole formation in the inner wall of aqueous plexi.
Interestingly, Wiskostatin-induced changes in aqueous humor outflow pathway were found to be somewhat similar to Rho kinase inhibitor induced effects (Rao et al., 2001). While there were some similarities between the Wiskostatin and Rho kinase inhibitor induced changes in the histological integrity, particularly with respect to increased giant vacuoles (Rao et al., 2001), Wiskostatin did not decrease myosin light chain phosphorylation in the PTM cells. Moreover, compared to Rho kinase inhibitors (Rao et al., 2001), Wiskostatin demonstrated a milder response in reducing the actin stress fiber and focal adhesion staining and this response appears to be more prominent on the cytoplasmic actin cytoskeleton compared to cortical actin network. The reasons for Wiskostatin preferential effects on the cytoplasmic actin cytoskeleton relative to cortical actin cytosketon are not clear at present.
Additionally, unlike Rho kinase inhibitors, Wiskostatin caused a deformation of aqueous plexi and expansion or herniation of JCT into aqueous plexi. It is noteworthy that similar herniation structural changes to Wiskostatin were reported in monkey eyes after argon laser trabeculoplasty (Melamed and Epstein, 1987), which has been used for the treatment of open angle glaucoma for many years. In this laser model, the shift of flow from the impermeable scarred lasered sites to non-lasered areas was believed to be associated with an expansion of the JCT with resulting herniations into Schlemm's canal in the regions between the laser sites. Recently, such phenomenon was also observed in a human eye after laser trabeculoplasty (Johnson, 2007). Such herniation might provide an increased area for aqueous humor drainage, possibly by canceling the “funnel effect” of inner wall of SC (Johnson et al., 1992). As per funneling phenomena, it is assumed that there is an interactive effect between the nonuniform flow within the JCT and the low porosity and the effective area of pores of the inner wall endothelium of SC, and this cumulative interactive response finally influences the flow resistance of JCT and SC (Johnson et al., 1992). Therefore, any structural changes to JCT and SC regions are expected to alter the flow resistance by reducing the SC funnel effect. One of the Wiskostatin perfused eyes demonstrated a break in the inner wall of an aqueous plexus but that may be an isolated observation. However, we cannot rule out the possibility of subtle breaks in the inner wall of aqueous plexi also causing increased outflow. Other cytoskeletal drugs, including cytochalasin D, EDTA, latrunculin and ethacrynic acid, have been documented to alter the structure of the inner wall of SC accompanying induced increases in aqueous humor outflow facility (Epstein et al., 1987; Ethier et al., 2006; Hamanaka and Bill, 1987; Johnson, 1997; Sabanay et al., 2006).
Another possible mechanism for increased outflow in Wiskostatin perfused eyes could be related to N-WASP involvement in exocytosis, endocytosis and phagocytosis (Gasman et al., 2004; Kitamura et al., 2003; Roth, 2007). Wiskostatin treated TM cells exhibited increased vacuolization. It is not clear whether this change in vacuolization influences cell secretory properties and possibly affects outflow facility. However, in contrast to observations in cultured TM cells, drug perfused outflow tissue did not reveal any notable increases in such vacuoles. N-WASP has been reported to be involved in podosome and invadopodia formation and extracellular matrix degradation (Lorenz et al., 2004; Oikawa and Takenawa, 2009; Spinardi and Marchisio, 2006). Recently, N-WASP was demonstrated to be localized to the podosomes of trabecular meshwork cells indicating its potential role in ECM turnover in TM tissue (Aga et al., 2008). In this study we did not examine for changes in podosomes. However, Wiskostatin treated TM cells did not exhibit any change in the MMP-2 activity as determined by zymography.
At the cellular level, the appearance of cytosolic vacuoles has not been reported with treatments with other cytoskeletal drugs as far as we know. This observation characterizes Wiskostatin as a unique compound, and may be a reflection of the multifunction of N-WASP, including effects on macropinocytosis, exocytosis, endocytosis, and phagocytosis (Takenawa and Suetsugu, 2007). Although the significance of the cytosolic vacuoles is unclear, this effect was reversible after withdrawal of Wiskostatin, and no changes were observed in the staining of the organelles, such as endosome, endoplasmic reticulum, lysosome, golgi, and mitochondria (unpublished data), suggesting that this observation is not due to cytotoxicity. Though Wiskostatin was not found to directly affect the MLC phosphorylation of PTM cells, this agent did reduce the content of actin and myosin II in the Triton X-100 insoluble fraction indicating that Wiskostatin influences actin and myosin II organization and assembly but not their total protein content. This effect of Wiskostatin on TM tissue is expected to change the mechanical or tensional properties which may in tern influence the structural integrity of outflow pathway.
In conclusion, Wiskostatin increased conventional aqueous humor outflow facility with accompanying structural changes that are likely related to the loss of actin myosin interaction and cell adhesion. These data identify N-WASP as an important regulator of TM cell actin cytoskeletal organization and a potential molecular target for the novel treatment of glaucoma.
Supplementary Material
The PTM cells treated with with Wiskostatin (15 μM for one hr) showed no change in the total content of myosin IIB and IIB in the total cell lysates as determined by immunoblot analysis.
Acknowledgments
We are thankful to Dr. Nikolai Skiba and Ying Hao for their help in mass spectrometer and electron microscope analyses, respectively. Technical help from Jianming Qiu in MLC phosphorylation is greatly appreciated. This work was supported in part by R01 grant (EY018590) from the NIH, and Research to Prevent Blindness.
Footnotes
Financial disclosures: There are no financial disclosures.
Conflict of interest: There are no conflicts to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aga M, Bradley JM, Keller KE, Kelley MJ, Acott TS. Specialized podosome- or invadopodia-like structures (PILS) for focal trabecular meshwork extracellular matrix turnover. Invest Ophthalmol Vis Sci. 2008;49:5353–65. doi: 10.1167/iovs.07-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bompard G, Caron E. Regulation of WASP/WAVE proteins: making a long story short. J Cell Biol. 2004;166:957–62. doi: 10.1083/jcb.200403127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coroneo MT, Korbmacher C, Flugel C, Stiemer B, Lutjen-Drecoll E, Wiederholt M. Electrical and morphological evidence for heterogeneous populations of cultured bovine trabecular meshwork cells. Exp Eye Res. 1991;52:375–88. doi: 10.1016/0014-4835(91)90032-a. [DOI] [PubMed] [Google Scholar]
- Epstein DL, Freddo TF, Bassett-Chu S, Chung M, Karageuzian L. Influence of ethacrynic acid on outflow facility in the monkey and calf eye. Invest Ophthalmol Vis Sci. 1987;28:2067–75. [PubMed] [Google Scholar]
- Epstein DL, Rowlette LL, Roberts BC. Acto-myosin drug effects and aqueous outflow function. Invest Ophthalmol Vis Sci. 1999;40:74–81. [PubMed] [Google Scholar]
- Ethier CR, Read AT, Chan DW. Effects of latrunculin-B on outflow facility and trabecular meshwork structure in human eyes. Invest Ophthalmol Vis Sci. 2006;47:1991–8. doi: 10.1167/iovs.05-0327. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol. 1995;163:510–22. doi: 10.1002/jcp.1041630311. [DOI] [PubMed] [Google Scholar]
- Gasman S, Chasserot-Golaz S, Malacombe M, Way M, Bader MF. Regulated exocytosis in neuroendocrine cells: a role for subplasmalemmal Cdc42/N-WASP-induced actin filaments. Mol Biol Cell. 2004;15:520–31. doi: 10.1091/mbc.E03-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7:713–26. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–5. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- Hamanaka T, Bill A. Morphological and functional effects of Na2EDTA on the outflow routes for aqueous humor in monkeys. Exp Eye Res. 1987;44:171–90. doi: 10.1016/s0014-4835(87)80002-7. [DOI] [PubMed] [Google Scholar]
- Honjo M, Tanihara H, Inatani M, Kido N, Sawamura T, Yue BY, Narumiya S, Honda Y. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001;42:137–44. [PubMed] [Google Scholar]
- Johnson DH. The effect of cytochalasin D on outflow facility and the trabecular meshwork of the human eye in perfusion organ culture. Invest Ophthalmol Vis Sci. 1997;38:2790–9. [PubMed] [Google Scholar]
- Johnson DH. Histologic findings after argon laser trabeculoplasty in glaucomatous eyes. Exp Eye Res. 2007;85:557–62. doi: 10.1016/j.exer.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Johnson M, Shapiro A, Ethier CR, Kamm RD. Modulation of outflow resistance by the pores of the inner wall endothelium. Invest Ophthalmol Vis Sci. 1992;33:1670–5. [PubMed] [Google Scholar]
- Khurana RN, Deng PF, Epstein DL, Vasantha Rao P. The role of protein kinase C in modulation of aqueous humor outflow facility. Exp Eye Res. 2003;76:39–47. doi: 10.1016/s0014-4835(02)00255-5. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Shibagaki K, Takata K, Tsuchiya D, Taniguchi T, Gebicke-Haerter PJ, Miki H, Takenawa T, Shimohama S. Involvement of Wiskott-Aldrich syndrome protein family verprolin-homologous protein (WAVE) and Rac1 in the phagocytosis of amyloid-beta(1-42) in rat microglia. J Pharmacol Sci. 2003;92:115–23. doi: 10.1254/jphs.92.115. [DOI] [PubMed] [Google Scholar]
- Lorenz M, Yamaguchi H, Wang Y, Singer RH, Condeelis J. Imaging sites of N-wasp activity in lamellipodia and invadopodia of carcinoma cells. Curr Biol. 2004;14:697–703. doi: 10.1016/j.cub.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Melamed S, Epstein DL. Alterations of aqueous humour outflow following argon laser trabeculoplasty in monkeys. Br J Ophthalmol. 1987;71:776–81. doi: 10.1136/bjo.71.10.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa T, Takenawa T. PtdIns(3,4)P2 instigates focal adhesions to generate podosomes. Cell Adh Migr. 2009;3:195–7. doi: 10.4161/cam.3.2.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JR, Bickford LC, Morgan D, Kim AS, Ouerfelli O, Kirschner MW, Rosen MK. Chemical inhibition of N-WASP by stabilization of a native autoinhibited conformation. Nat Struct Mol Biol. 2004;11:747–55. doi: 10.1038/nsmb796. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Open-angle glaucoma. N Engl J Med. 1993;328:1097–106. doi: 10.1056/NEJM199304153281507. [DOI] [PubMed] [Google Scholar]
- Rao PV, Deng P, Sasaki Y, Epstein DL. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp Eye Res. 2005;80:197–206. doi: 10.1016/j.exer.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42:1029–37. [PubMed] [Google Scholar]
- Rao PV, Ho T, Skiba NP, Maddala R. Characterization of lens fiber cell triton insoluble fraction reveals ERM (ezrin, radixin, moesin) proteins as major cytoskeletal-associated proteins. Biochem Biophys Res Commun. 2008;368:508–14. doi: 10.1016/j.bbrc.2008.01.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VP, Epstein DL. Rho GTPase/Rho kinase inhibition as a novel target for the treatment of glaucoma. BioDrugs. 2007;21:167–77. doi: 10.2165/00063030-200721030-00004. [DOI] [PubMed] [Google Scholar]
- Read AT, Chan DW, Ethier CR. Actin structure in the outflow tract of normal and glaucomatous eyes. Exp Eye Res. 2006;82:974–85. doi: 10.1016/j.exer.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Roth MG. Integrating actin assembly and endocytosis. Dev Cell. 2007;13:3–4. doi: 10.1016/j.devcel.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Latrunculin B effects on trabecular meshwork and corneal endothelial morphology in monkeys. Exp Eye Res. 2006;82:236–46. doi: 10.1016/j.exer.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Sanka K, Maddala R, Epstein DL, Rao PV. Influence of actin cytoskeletal integrity on matrix metalloproteinase-2 activation in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007;48:2105–14. doi: 10.1167/iovs.06-1089. [DOI] [PubMed] [Google Scholar]
- Song J, Deng PF, Stinnett SS, Epstein DL, Rao PV. Effects of cholesterol-lowering statins on the aqueous humor outflow pathway. Invest Ophthalmol Vis Sci. 2005;46:2424–32. doi: 10.1167/iovs.04-0776. [DOI] [PubMed] [Google Scholar]
- Spinardi L, Marchisio PC. Podosomes as smart regulators of cellular adhesion. Eur J Cell Biol. 2006;85:191–4. doi: 10.1016/j.ejcb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- Tan JC, Peters DM, Kaufman PL. Recent developments in understanding the pathophysiology of elevated intraocular pressure. Curr Opin Ophthalmol. 2006;17:168–74. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- Tanihara H, Inatani M, Honjo M, Tokushige H, Azuma J, Araie M. Intraocular pressure-lowering effects and safety of topical administration of a selective ROCK inhibitor, SNJ-1656, in healthy volunteers. Arch Ophthalmol. 2008;126:309–15. doi: 10.1001/archophthalmol.2007.76. [DOI] [PubMed] [Google Scholar]
- Tian B, Gabelt BT, Geiger B, Kaufman PL. The role of the actomyosin system in regulating trabecular fluid outflow. Exp Eye Res. 2009;88:713–7. doi: 10.1016/j.exer.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Geiger B, Epstein DL, Kaufman PL. Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci. 2000;41:619–23. [PubMed] [Google Scholar]
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–20. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- Wu X, Yoo Y, Okuhama NN, Tucker PW, Liu G, Guan JL. Regulation of RNA-polymerase-II-dependent transcription by N-WASP and its nuclear-binding partners. Nat Cell Biol. 2006;8:756–63. doi: 10.1038/ncb1433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The PTM cells treated with with Wiskostatin (15 μM for one hr) showed no change in the total content of myosin IIB and IIB in the total cell lysates as determined by immunoblot analysis.


