Abstract
Background
Autonomic nervous system (ANS) dysfunction and reduced heart rate variability (HRV) have been reported in a wide variety of psychiatric disorders, but have not been well characterized in bipolar mania. We recorded cardiac activity and assessed HRV in acutely hospitalized manic bipolar (BD) and schizophrenia (SCZ) patients compared to age- and gender-matched healthy comparison (HC) subjects.
Method
HRV was assessed using time domain, frequency domain, and nonlinear analyses in 23 manic BD, 14 SCZ, and 23 HC subjects during a 5 minute rest period. Psychiatric symptoms were assessed by administration of the Brief Psychiatric Rating Scale (BPRS) and the Young Mania Rating Scale (YMRS).
Results
Manic BD patients demonstrated a significant reduction in HRV, parasympathetic activity, and cardiac entropy compared to HC subjects, while SCZ patients demonstrated a similar, but non-significant, trend towards lower HRV and entropy. Reduction in parasympathetic tone was significantly correlated with higher YMRS scores and the unusual thought content subscale on the BPRS. Decreased entropy was associated with increased aggression and diminished personal hygiene on the YMRS scale.
Conclusion
Cardiac function in manic BD individuals is characterized by decreased HRV,
Keywords: Heart rate variability, mania, bipolar disorder, schizophrenia, entropy
1. Introduction
Dysregulation of the autonomic nervous system (ANS) is a common characteristic of a variety of psychiatric disorders, including depression, schizophrenia, and panic disorder (Friedman and Thayer, 1998a; Karavidas et al., 2007; Valkonen-Korhonen et al., 2003). Heart rate variability (HRV) describes the variation in heartbeat intervals and is a reliable and quantitative measure of ANS activity. The rhythm of the heart is primarily determined by the depolarization rate of the pacemaker tissue located in the cardiac sinoatrial node (SA), which is modulated by both the parasympathetic and sympathetic branches of the ANS (Malik and Camm, 1995). The complex beat-to-beat variation in heart rate is thus dependent upon the balance between sympathetic activation mediated by SA noradrenergic receptors and tonic inhibition mediated by cholinergic input from the vagus nerve.
HRV represents a sensitive measure of autonomic system function (Low and Pfeifer, 1997) and can be utilized as a tool to assess the effect of psychopathology and disease on the balance between sympathetic and parasympathetic input to the heart. Healthy subjects exhibit a high degree of HRV, reflecting the ability to adapt quickly to physical or psychological demands of the environment. Lower levels of HRV are associated with cardiac damage, including myocardial infarction, impaired ventricular ejection, and sudden cardiac death (Singer and Ori, 1995). Decreased HRV may also indicate a maladaptive response to stressors in the environment, characterized by parasympathetic suppression and/or sympathetic overactivation (Berntson et al., 1997; Carney et al., 2005).
Assessment of HRV has traditionally been classified into time domain and frequency domain analyses. Time domain measures of HRV are quantified from the sinus RR intervals, or the differences in the length of time between consecutive R peaks that signal the ventricular depolarization that occurs with each heartbeat (Cowan, 1995). The most common measure of time domain HRV is the standard deviation of all of the RR intervals, or SDNN. Additional variables are derived from the differences between adjacent RR intervals, including the RMSSD (the root mean square of successive RR differences, where each difference is squared, summed, the results are averaged, then the square root obtained), and the pNN50 (the percentage of adjacent RR intervals that differ by more than 50 milliseconds).
Frequency domain analysis, also referred to as the power spectral density analysis, separates the heart rate signal into distinct frequency bands and quantifies their relative intensity, called power (Cowan, 1995). Low frequency (LF) range power (0.04–0.15 Hz) is mediated by both sympathetic and parasympathetic activity, while high frequency range power (HF) (0.15-0.4 Hz) is mediated primarily by parasympathetic activity. Given the lack of specificity of the LF signal, information obtained from this variable is of limited value; however, in the context of measuring the HF signal and assessing the relationship of LF to HF power (the LF/HF ratio), the comparative balance of the two branches of the ANS on cardiac activity can be assessed. While time domain measures such as SDNN reflect both sympathetic and parasympathetic activity, RMSSD and pNN50 are strongly correlated with the HF signal and are believed to be more specific indicators of parasympathetic activity (Kleiger et al., 1991), providing another measure of vagal tone.
Mood disorders are strongly associated with changes in cardiovascular function (Johnson and Grippo, 2006) and alterations in HRV. A number of studies have reported decreases in HRV in depressed individuals (Agelink et al., 2002; Dalack and Roose., 1990; Rechlin et al., 1994a), though others have not observed differences (Yeragani et al., 1991). Cardiac patients with more severe depression exhibit less HRV compared to those with less severe depression (Krittayaphong et al., 1997) and successful treatment of depression tends to be accompanied by an increase in HRV (Balogh et al., 1993; Chambers and Allen, 2002). In the last few years, vagal nerve stimulation (VNS) has been reported to successfully improve affect in treatment-resistant depression (George et al., 2005), suggesting a strong link between sympathovagal dysregulation and symptoms of depression.
Anxiety disorders have long been associated with autonomic abnormalities, including low HRV, decreased cardiac vagal tone, and elevated sympathetic heart rate control (Friedman and Thayer, 1998b). Impaired HRV characterized by decreased SDNN, diminished HF power, and an elevated LF/HF ratio has been associated with trait anxiety (Miu et al., 2008), phobic anxiety (Kawachi et al., 1995), generalized anxiety disorder (Thayer et al., 1996), anticipatory anxiety (Melzig et al., 2008) and panic disorder (Yeragani et al., 1998). Furthermore, HRV alterations in individuals with anxiety disorders have been observed during relatively calm baseline states as well as acute episodes of anxiety (e.g., during pharmacological manipulations such as isoproterenol or lactate administration) (Friedman and Thayer, 1998b).
A growing number of reports have also observed HRV dysfunction in schizophrenia (SCZ) (Bar et al., 2005; Boettger et al., 2006; Valkonen-Korhonen et al., 2003). Two recent studies reported that unmedicated SCZ patients exhibited decreased RMSSD, pNN50, and HF power compared to normal comparison subjects, but did not show differences in LF power (Bar et al., 2005; Malaspina et al., 1997; Valkonen-Korhonen et al., 2003). These data suggest this disorder is characterized by a decrease in parasympathetic activity independent of medication effects. Other groups have observed that decreased vagal tone in SCZ patients is significantly correlated with an increase in psychotic symptoms as assessed by the Positive and Negative Syndrome Scale (PANSS) (Okada et al., 2003; Toichi et al., 1999).
While alterations in HRV have been characterized in psychiatric disorders such as major depression, SCZ and panic disorder, the effect of bipolar disorder (BD) on HRV has not been studied extensively. One report examined HRV in a population of euthymic BD subjects and reported a decrease in SDNN, a decrease in the LF/HF ratio, and an increase in HF power compared to healthy comparison subjects (Cohen et al., 2003), indicating an increase in vagal tone. Although some BD subjects were taking lithium or a combination of lithium and other psychotropic medications, such as valproate or carbamazapine, there was no apparent effect of medication on HRV. As the authors acknowledge, these data are difficult to interpret, as a decrease in SDNN is typically associated with a decrease rather than an increase in parasympathetic activity. While this report describes HRV in a sample of euthymic BD, to our knowledge, HRV has never been described in a population of BD individuals who are in a manic state at the time of testing.
Fluctuation in heart rate occurs over a wide frequency and demonstrates a broad, irregular variability that suggests that the mechanisms involved in cardiovascular regulation interact in a nonlinear manner (Makikallio et al., 2002). Over the past decade, there has been an increasing emphasis on applying nonlinear methods of analysis to characterize cardiac function, including the use of detrended fluctuation analyses, approximate entropy, sample entropy, Poincare plots, and symbolic dynamics (Todder et al., 2005; Voss et al., 2006; Huikuri et al., 2009). Some evidence suggests that nonlinear analyses are superior predictors of cardiac dysfunction, including ventricular tachycardia and sudden cardiac death (Baumert et al., 2004; Hoyer et al., 2006), when compared to the traditional time and frequency domain analyses. Assessment of nonlinear measures of HRV such as entropy and Lyapunov exponents have indicated a decreased pattern of complexity, or less variability, in depressed or SCZ patients (Yeragani et al., 2002; Bar et al., 2007; Boettger et al., 2006; Boettger et al., 2008; Kim et al., 2004) compared to healthy comparison subjects. One report (Todder et al., 2005) performed a series of nonlinear analyses on the same HRV dataset obtained from euthymic BD subjects as described above (Cohen et al., 2003), but did not observe any group differences.
Over the past two decades, we have applied nonlinear dynamical measures to examine the pattern of motor activity in rodents using the concept of phase space, a mathematical representation of a system where the variables are treated as coordinates (Paulus et al., 1990). More recently, we have used this methodology to derive a measure of dynamical entropy h from acceleration data to determine if patterns of human motor activity are more predictable or more variable over time (Young et al., 2007).
The objective of the current study was to compare HRV in acutely hospitalized BD patients in a manic state, acutely hospitalized SCZ patients and healthy comparison subjects (HC). While BD and SCZ are considered to be distinct disorders, they do share genetic (Pope and Yurgelun-Todd, 1990), epidemiological (Buckley, 2008), and symptom (Pini et al., 2004) characteristics and may be mediated by similar neurobiological mechanisms (Citrome et al., 2005). Thus, one aim of the current investigation was to determine if both disorders are associated with similar alterations in HRV as compared to HC subjects. HRV was quantified using time and frequency domain measures, while nonlinear complexity was assessed with the dynamic entropy h measure originally developed in our lab to describe patterns of motor activity (Paulus et al., 1990; Young et al., 2007) and sample entropy, a more traditional index of heart rate dynamics (Huikuri et al., 2009).
2. Methods
2.1. Participants
Acutely hospitalized patients between the age of 18 and 55 with SCID (Structured Clinical Interview for DSM-IV) diagnosed Bipolar Disorder, Current Episode Manic (n = 23), or schizophrenia (n = 14) were recruited from the inpatient unit at the University of California San Diego Medical Center to participate in this study. 23 healthy comparison subjects who had never met SCID criteria for an Axis I disorder were recruited from advertisements in the San Diego community. BD and SCZ patients were tested within an average of 96 hours of admission to the psychiatric service at the UCSD–Medical Center while they were highly symptomatic. Psychiatric diagnosis was determined by a trained clinician and acute symptoms were assessed by administration of the Brief Psychiatric Rating Scale (BPRS) and the Young Mania Rating Scale (YMRS). Comparison and patient groups were matched for age, gender, and Body Mass Index (Table 1) and BD and SCZ subjects were also matched for the onset and duration of illness. HC and BD females were matched for menstrual cycle phase, but this information could not be obtained from several SCZ female subjects. HC subjects did have significantly more education compared to BD and SCZ subjects (p < 0.05). A prior report (Perry et al., 2009, in press) has described alterations in motor activity in a subset of subjects included in the current study, but HRV in these individuals has not been previously reported. All participants provided written informed consent to the current protocol approved by the UCSD institutional review board.
Table 1.
Demographic and illness factors for healthy comparison subjects (HC, n = 23), bipolar manic patients (BD, n = 23) and schizophrenia patients (SCZ, n = 14). Menstrual cycle history was obtained for HC and BD, but not all of the SCZ females. Data are represented as means ± S.E.M.
| Parameter | HC | BD | SCZ | Group Differences |
|---|---|---|---|---|
| Age (years) | 29.4 ± 2.0 | 32.9 ± 2.6 | 32.9 ± 2.9 | ns |
| Gender | 13 M, 10 F | 14 M, 9 F | 9 M, 5 F | ns |
| Body Mass Index | 25.0 ± 1.2 | 28.2 ± 1.6 | 24.6 ± 1.2 | ns |
| Education (years) | 14.8 ± 0.3 | 13.4 ± 0.4 | 12.9 ± 0.4 | BD, SCZ < HC, p < 0.05 |
| Smokers/Non-smokers | 5 / 18 | 12 / 11 | 8 / 6 | ns |
| Menstrual cycle (days) | 13.3 ± 2.7 | 8.5 ± 4.6 | ---------- | ns |
| Age of onset of Illness | ---------- | 21.9 ± 1.6 | 23.9 ± 2.2 | ns |
| Duration of Illness (years) | ---------- | 11.9 ± 2.3 | 9.0 ± 2.4 | ns |
| Days in current treatment | ---------- | 2.9 ± 0.5 | 6.6 ± 3.0 | ns |
| Illness Subtype | ---------- | Current Episode Manic | 9 paranoid, 3 disorganized, 2 undifferentiated | ---------- |
| YMRS Score | ---------- | 27.4 ± 1.7 | 19.9 ± 2.0 | BD > SCZ, p < 0.01 |
| BPRS Score | ---------- | 40.4 ± 2.0 | 37.1 ± 2.1 | ns |
Participants were excluded if: 1) they met criteria for Axis I or II disorders aside from the primary diagnosis of BD and SCZ for the appropriate patient groups, 2) had any neurological conditions or head trauma, 3) ever received treatment with electroconvulsive therapy, 4) had a history of stroke, heart attack, or cardiac disease, 5) had drug abuse or dependence in the past six months, or 6) had a positive result for cocaine, amphetamine, opiates, or PCP on a toxicology screen.
The majority of BD and SCZ patients were treated with atypical antipsychotic and/or mood stabilizing medications (Table 2) while participating in this study; 1 BD subject and 3 SCZ subjects were unmedicated at the time of testing. BD subjects were typically treated with a combination of mood-stabilizing and atypical antipsychotic medications while the majority of the SCZ subjects were prescribed an antipsychotic medication alone. The most common medications were risperidone (n = 19), valproate (n = 11), and lithium (n = 7). Doses of psychotropic medication did not significantly differ between BD and SCZ patients.
Table 2.
Patient medication for BD (n = 23) and SCZ (n = 14) subjects. Doses are represented as means ± S.E.M.
| Medication Type | BD patients (n) | SCZ patients (n) |
|---|---|---|
| Antipsychotic alone | 4 | 9 |
| Mood stabilizer alone | 1 | 0 |
| Antipsychotic plus mood stabilizer | 17 | 2 |
| Not medicated | 1 | 3 |
| On Risperidone | 11 | 8 |
| On Valproate | 9 | 2 |
| On Lithium | 7 | 0 |
| Risperidone Dose (mg) | 3.2 ± 0.52 | 3.6 ± 0.59 |
| Valproate Dose (mg) | 2055.5 ± 100.2 | 2250 ± 250 |
| Lithium Dose (mg) | 1100 ± 192.7 | ------- |
2.2. HRV Assessment
Subjects were fitted with the LifeShirt (LS), a continuous monitoring system that collects physiological data through various sensors, including electrical activity of the myocardium via 3 electrocardiogram (ECG) leads (Vivometrics, 2002). The sensor array of the LS is embedded in a sleeveless Lycra undergarment that can be worn comfortably for extended periods. An on-board PDA continuously encrypts and stores the patient's physiological data on a compact flash memory card. VivoLogic™, proprietary PC-based software, decrypts the data and provides the viewing of high-resolution waveforms and trends over time. Summary reports present processed data in concise, graphical, and numeric formats exported in ASCII format for analysis in other software programs.
The measurement of cardiac parameters such as HRV via the LS has been shown to be equivalent to standard heart rate laboratory measures such as Biopac (Heilman and Porges, 2007; Kent et al., 2009). Heilman and colleagues (2007) assessed the accuracy of the LS (sampling ECG at 200 Hz) compared to Biopac (sampling ECG at 1000 Hz) when quantifying heart rate and HRV in subjects during short periods (10 minutes) of rest and exercise. The authors observed that the LS system performed equivalently to Biopac during both conditions and concluded that the LS algorithm for R-wave detection and timing precision were sufficiently robust to provide highly accurate cardiac data.
LS ECG data was obtained from three self-adhering electrodes placed on the skin of the upper chest and lateral abdomen. Digitized ECG data were analyzed to detect the R-wave peaks of the QRS complex and RR interval artifacts 15% of reference RR duration were manually removed using linear interpolation. Ectopic beats were identified and removed using the Vivologic automated ectopic beat identification procedure. Only normal RR intervals were included in the analysis.
ECG recordings were obtained from participants during a 5 minute interval in a seated position at complete rest between 10 a.m. and 5 p.m. The 5 minute test period was selected because it is a standard length of HRV assessment (Malik and Camm, 1995) and also minimizes the difficulty of requiring manic BD subjects to remain at rest for extended periods of time. Participants were instructed to relax and sit quietly during the test period and were required to refrain from smoking for at least 30 minutes prior to the test session. Given that HRV can be affected by abnormally elevated heart rate (HR) (Malik and Camm, 1995) as occurs during exercise, participant HR was monitored during the session. All participants exhibited a baseline HR below 110 beats-per-minute (bpm), with the exception of 1 SCZ subject with an average HR of 113 bpm.
2.3. Data Collection
HRV data were analyzed in both time and frequency domains. In the time domain, HR, SDNN, RMSSD, and pNN50 were calculated. Spectral analyses were also performed to quantitate the LF/HF ratio and values of LF and HF power. LF and HF were normalized to total power (TP) and analyzed as normalized low frequency power: (LFn, LF/(TP - Very Low Frequency)) and normalized high frequency power (HFn, LF/(TP - Very Low Frequency)). Because the total power of the spectral signal can differ greatly from individual to individual, it has been recommended that LF and HF data be presented in values normalized to the total area under the curve (Pagani et al., 1986).
The power spectrum of the HRV signal was assessed using nonparametric Fast Fourier Transform (FFT) and the Welch periodogram method. In brief, per standard procedures (Vivometrics, 2002), RR interval data were resampled at 4 Hz and partitioned into 60 second segments that overlapped by 30 seconds. Each segment was linearly detrended, Hanning windowed, and then the FFT was applied to each segment. Successive power spectral density functions were calculated, and then subsequently averaged across segments to obtain reliable estimates. The area under the curve was calculated for each frequency range and integrated using a simple rectangular rule.
To assess the nonlinear complexity of the heart rate pattern, we applied the dynamic entropy h measure to the RR interval data also generated by the LS. Each RR interval (typically ranging from 500 ms to 1000 ms) was treated as a single data point and an entropy value was generated from the sequence of RR interval data obtained during the 5 minute rest period using the same methodology previously applied to human motor activity (Perry et al., 2009, in press; Minassian et al., 2009).
In brief, the calculation of dynamical entropy is based on determining the minimal length of a subsequence that renders this sequence unique in a data sequence, e.g. the subsequence “01” but not the subsequence “11” is unique in the sequence “1110110”, i.e., there is no other “01” subsequence but there are three “11” subsequences (in the first, second, and fifth position of this data sequence). For example, data are divided into four equal sized bins and transformed into sequences of bin numbers, where a small RR interval value followed by a large RR interval value would correspond to a sequence (1,4). Using a method developed by Grassberger (Schurmann and Grassberger, 1996), the unique sub-sequence lengths of the RR interval data are obtained. The unique subsequence length was determined for each sequence starting at the ith data point and the local dynamical entropy is computed via log (number of data points)/[unique subsequence length]. Lower values of h (low entropy) suggest highly predictable or ordered RR sequences across time, while higher values (high entropy) suggest a greater variety, or disorder, in the RR sequences across time. This measure thus provides us with an additional method to assess cardiac variability in conjunction with the more traditional time and frequency domain analyses.
A second measure of nonlinear complexity, sample entropy (SampEn) was also included in the data analyses. SampEn quantifies the probability that sequences of patterns in a data set that are initially closely related remain close on the next incremental comparison, within a specified tolerance (Lake et al., 2002). Similar to entropy h, higher values of SampEn indicate less predictability and more complexity in the data, while lower values suggest greater regularity in the time series (Richman and Moorman, 2000). Previous reports have indicated that sample entropy can be accurately estimated from a set of 100 to 5000 data points when the length of sequences to be compared (m) is set at 1 or 2 and the tolerance level (r) for determining a difference between data points is set between 0.1 and 0.25 of the standard deviation of the total data set (Pincus and Huang, 1992; Groome et al., 1999). As the value of r increases, the probability of sequence matches approaches 1 and the value of SampEn can tend towards zero, reducing the ability to detect features of interest in the examined dataset; in contrast, some physiological processes may be less detectable with lower values of m (Lake et al., 2002).
Preliminary analyses with the HRV data obtained from manic BD and SCZ patients indicated that SampEn values approached or effectively reached zero when the value of r was set at or above 0.2; thus the r value for the current dataset was set at 0.1 and SampEn was subsequently calculated for m values of 1, 2, and 3 as shown below. SampEn was computed using Matlab software with tools available on the PhysioNet website (Goldberger et al., 2000).
2.4. Statistical Analyses
Mean values for HR, SDNN, RMSSD, pNN50, HFn, LFn, the LF/HF ratio and the RR entropy h were calculated for the 5 minute rest period. Group differences on the time and frequency HRV measures were tested using a multivariate analysis of variance (MANOVA), followed up by univariate ANOVAs for each parameter. A univariate ANOVA was performed for the entropy h and the SampEn data. Post-hoc differences were assessed using Bonferroni-adjusted multiple t-test comparisons. Bivariate Pearson r correlations were performed to assess relationships between the time and frequency domain and nonlinear HRV measures.
All analyses included education and smoking as covariates, given the differences in these measures between the groups. While the difference in the smoker/non-smoker ratio did not reach significance between the groups, there were a higher number of smoking subjects in both patient groups compared to healthy comparison subjects (Table 1). Given the effect of nicotine intake on cardiac activity (Thong et al., 2004), we felt it appropriate to control for the potential effect of smoking history in the assessment of the HRV data.
Bivariate Pearson r correlations were performed to assess relationships between illness duration, symptom rating scales (18 items for BPRS, 11 items for YMRS) and HRV measures. Independent samples t-tests were conducted to assess the potential effect of psychotropic medications (risperidone, valproate, and lithium) on HRV. In addition, patients treated with combination of mood-stabilizing and atypical antipsychotic medications were also compared to patients treated with antipsychotic medication alone. To reduce the probability of a Type 1 error associated with a large number of statistical analyses, the level of significance for Pearson r and independent samples t-test comparisons was set at p < 0.025, rather than p < 0.05.
3. Results
3.1. Time and Frequency Domain analyses
The MANOVA performed for HRV data during the 5 minute rest period indicated a significant effect of group [F(14,100) = 3.40, p < 0.001], but no significant effect of either education or smoking, variables that were included as covariates in this analysis. Subsequent univariate ANOVAs revealed a main effect of group on HR [F(2,55) = 16.31, p < 0.001], RMSSD [F(2, 55) = 3.60, p < 0.05], pNN50 [F(2,55) = 4.62, p < 0.05], HFn [F(2, 55) = 7.06, p < 0.01], LF/HF ratio [F(2, 55) = 5.07, p < 0.05], and a marginal effect on LFn [F(2, 55) = 3.16, p = 0.05] (Table 3). SDNN was lower in both patient groups compared to healthy comparison subjects, but the differences did not reach significance. Posthoc tests showed that BD patients exhibited a significant increase in the LF/HF ratio (p < 0.05), and a significant decrease in the HFn power (p < 0.01), RMSSD (p < 0.05), and pNN50 (p < 0.05) compared to healthy comparison subjects, indicating a decrease in parasympathetic activity. Both BD and SCZ patients exhibited an increase in HR (BD, p < 0.001; SCZ, p < 0.001) compared to healthy comparison subjects, but the SCZ patients did not differ significantly from the other two groups on any other HRV measure.
Table 3.
Autonomic parameters for healthy comparison subjects (HC, n = 23), BD (n = 23) and SCZ (n = 14) during the 5 minute rest period. SampEn tolerance level r for determining a difference between RR intervals was set at 10% of the standard deviation of the data set. Data are represented as means ± S.E.M. Asterisks indicate significant differences as compared to the healthy comparison group; * p < 0.05; ** p < 0.01; *** p < 0.001.
| Parameter | HC | BD | SCZ |
|---|---|---|---|
| Time Domain | |||
| HR (beats/min) | 65.1 ± 2.5 | 85.3 ± 2.5*** | 87.3 ± 3.7*** |
| SDNN (ms) | 65.8 ± 5.6 | 46.7 ± 7.4 | 44.1 ± 6.6 |
| RMSSD (ms) | 49.0 ± 5.8 | 26.7 ± 5.7* | 31.3 ± 6.4 |
| pNN50 (%) | 0.25 ± 0.04 | 0.08 ± 0.03* | 0.15 ± 0.05 |
| Frequency Domain | |||
| HFn | 0.37 ± 0.03 | 0.20 ± 0.02** | 0.31 ± 0.05 |
| LFn | 0.55 ± 0.03 | 0.65 ± 0.02 | 0.55 ± 0.05 |
| LF/HF ratio | 2.08 ± 0.38 | 4.88 ± 0.83* | 2.66 ± 0.48 |
| Nonlinear Analysis | |||
| entropy h | 0.34 ± 0.01 | 0.30 ± 0.01** | 0.32 ± 0.01 |
| SampEn (m = 1) | 0.19 ± 0.03 | 0.08 ± 0.03* | 0.10 ± 0.03 |
| SampEn (m = 2) | 0.18 ± 0.03 | 0.08 ± 0.02 | 0.09 ± 0.03 |
| SampEn (m = 3) | 0.16 ± 0.03 | 0.07 ± 0.02 | 0.08 ± 0.02 |
3.2. Nonlinear HRV analysis
Univariate ANOVA of the dynamical entropy h calculated from RR interval sequences indicated a main effect of group during the 5 minute rest period [F(2,55) = 5.19, p < 0.01] (Table 3). Education and smoking were included as covariates, but had no significant effect on entropy. Posthoc tests showed that BD patients exhibited decreased entropy h, signifying less variability in the RR interval, compared to healthy comparison subjects (p < 0.01). SCZ patients showed a decline in entropy h relative to the healthy comparison group, but did not differ significantly from the other two groups. Subsequent correlations between entropy h and HRV frequency data across all groups revealed that RR entropy was negatively correlated with LFn (r = -0.58, p < 0.001) and the LF/HF ratio (r = -0.65, p < 0.001) and positively correlated with HFn (r = 0.64, p < 0.001).
Given that the entropy h measure is designed to assess the predictability of activity over time, in theory, no significant group differences should exist if the data points are placed in random order. To test this idea, the RR data points for each individual subject were completely randomized using a Microsoft Excel random number generator and the entropy analyses were repeated for the three subject groups. As expected, we did not observe any significant effect of group on entropy with the randomized data [F(2,55) = 0.33, p = 0.72, ns], and the entropy h means for the three groups (comparison, 0.442; BD, 0.452; SCZ, 0.449) were approximately equivalent. These results suggest that the entropy h parameter is capable of detecting differences in predictability or variability in the current HRV data set.
Univariate ANOVA of SampEn calculated from RR interval sequences indicated a main effect of group during the 5 minute rest period when sequence length m was set to 1 [F(2,55) = 3.20, p < 0.05], a marginally significant effect with m = 2 [F(2,55) = 3.18, p = 0.05], and a strong trend towards a group effect when m = 3 [F(2,55) = 2.84, p = 0.06] (Table 3). Education and smoking were included as covariates, but had no significant effect on SampEn. Post-hoc tests showed that BD patients exhibited significantly decreased SampEn compared to healthy comparison subjects when m = 1 (p < 0.05) and exhibited a strong trend towards decreased SampEn when m = 2 (p = 0.053) and m = 3 (p = 0.08). SCZ patients showed a decline in SampEn relative to comparison subjects, but did not differ significantly from the other two groups.
Entropy h was significantly correlated with SampEn across the subject groups for m = 1 (r = 0.60, p < 0.001), m = 2 (r = 0.61, p < 0.001), and m = 3 (r = 0.59, p < 0.001). Similar to entropy h, SampEn was also negatively correlated with LFn [m = 1 (r = -0.43, p < 0.01); m = 2 (r = -0.43, p < 0.001); m = 3 (r = -0.42, p < 0.001)], negatively correlated with the LF/HF ratio [m = 1 (r = -0.53, p < 0.001); m = 2 (r = -0.52, p < 0.001); m = 3 (r = -0.43, p < 0.001)], and positively correlated with HFn [m = 1 (r = 0.67, p < 0.001); m = 2 (r = 0.66, p < 0.001); m = 3 (r = 0.69, p < 0.001)].
3.3. Clinical Assessment
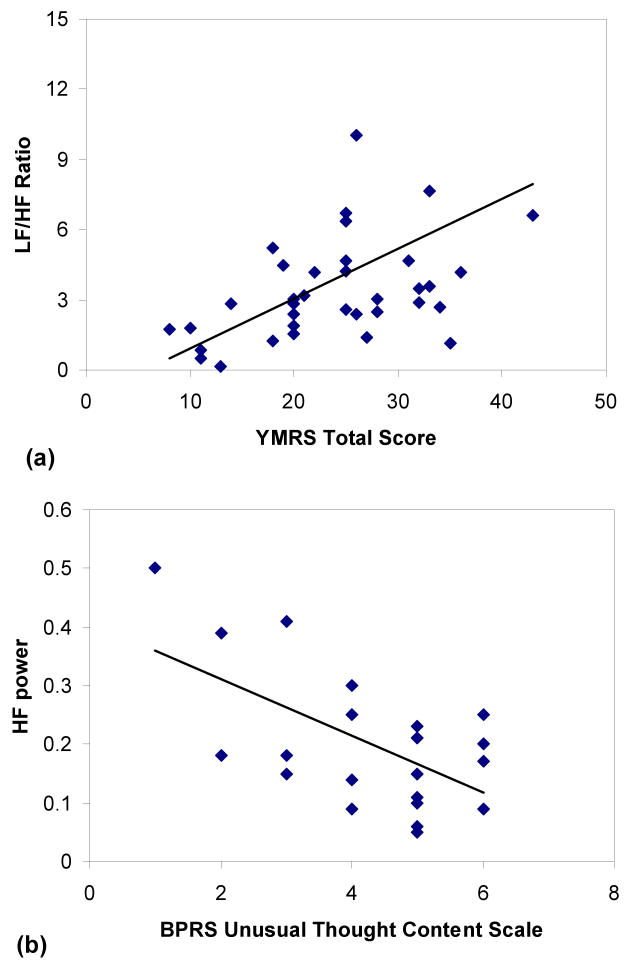
We did not observe any significant correlation between the duration of illness, age of onset, or the number of days on the current medication regimen and any HRV measure in either the BD or SCZ patients. As shown in Table 1, although there was no difference in the total BPRS score between the patient groups, BD subjects showed significantly higher total YMRS scores compared to the SCZ group (p < 0.01). The total YMRS score was correlated with a higher LF/HF ratio in both patient groups (r = 0.53, p < 0.01; Figure 1a) and the BD group alone (r = 0.48, p = 0.02), as well as with lower HFn power in the SCZ group (r = -.61, p = 0.02) during the 5 minute rest period. These data indicate that more severe manic symptoms were associated with a greater ratio of sympathetic to parasympathetic activity in all patients and a marked decrease (lower HFn) in parasympathetic cardiac tone in SCZ subjects.
Figure 1.
Total score on the Young Mania Rating Scale (YMRS) was significantly correlated with the LF/HF ratio in BD and SCZ patients (r = 0.53, p < 0.01) (a) and HF power was negatively correlated with the Brief Psychiatric Rating Scale (BPRS) assessment of unusual thought content (r = -.61, p < 0.01) (b) in BD patients.
Although the total BPRS score was not significantly related to HRV in either patient group, higher ratings of unusual thought content (BPRS Item 15) in BD subjects were correlated with higher LFn power (r = 0.53, p = 0.01) and lower HFn power (r = -.61, p < 0.01) (Figure 1b). Finally, there was a modest negative correlation between entropy h and the YMRS aggression (r = -.37, p = 0.02) and YMRS appearance (r = -.37, p = 0.02) ratings for all patients during the rest period, suggesting that a decrease in RR entropy was associated with more aggressive behavior and decreased personal hygiene.
To determine the potential effect of participant anxiety on cardiac function, we examined the relationship between HRV and the clinical rating of anxiety evaluated during the session (BPRS Item 2). There was no significant correlation between anxiety and any HRV measure for all patients combined or for either the BD or SCZ groups assessed separately. Similarly, we did not observe any significant correlation between psychomotor activity as assessed by Item 2 of the YMRS scale and any HRV measure for the patient groups, suggesting that alterations in autonomic function were independent of potential differences in physical activity.
3.4. Effect of Medication
Treatment with risperidone or valproate did not have a significant effect on HRV in either BD or SCZ patients, nor were there any significant differences between patients treated with antipsychotic medication alone compared to patients treated with a combination of antipsychotic and mood stabilizer medication. BD subjects treated with lithium (n = 7) showed some evidence of a decrease in the LF/HF ratio [t (21) = 2.37, p = 0.03] compared to BD patients not taking the drug (n = 16).
4. Discussion
The aim of this study was to examine HRV in acutely hospitalized manic BD and SCZ patients during a 5 minute rest period. Manic BD patients exhibited a decrease in HRV compared to HC subjects, and SCZ patients demonstrated a similar, albeit non-significant, trend. Manic BD subjects showed a significant increase in heart rate, a significant reduction in RMSSD and pNN50, and a trend towards a decrease in SDNN compared to HC subjects. Frequency domain analyses revealed that the BD group also exhibited a significant decrease in HFn power and an increase in the LF/HF ratio compared to the healthy comparison subjects. In addition, values of dynamical entropy h and SampEn were decreased in the BD group compared to HC, indicating a decrease in the complexity and variability of the RR pattern. Taken as a whole, these data indicate that manic BD individuals in this study demonstrated a consistent decrease in HRV as assessed by a variety of measures. This effect was mediated at least in part by suppression of parasympathetic input as indicated by lower HFn power, RMSSD, and pNN50.
These results differ from earlier reports describing HRV in euthymic BD (Cohen et al., 2003; Todder et al., 2005). Although euthymic BD subjects also exhibited decreased SDNN compared to healthy comparison subjects (Cohen et al,. 2003), they showed an increase in parasympathetic activity (higher HF power) in contradiction to the current findings. It is possible that a reduction in SDNN (a measure that reflects both sympathetic and parasympathetic activity) could represent a consistent trait exhibited during both the manic and euthymic phases of the disorder. In contrast, it is conceivable that suppression of parasympathetic tone, as reflected by a reduction in HF power, RMSSD and pNN50, could be state-dependent, observed primarily in the manic phase of the illness.
Our data indicate that manic BD subjects showed reduced HRV variability assessed by nonlinear measures entropy h and SampEn, in contrast to the lack of differences observed with nonlinear analyses in euthymic BD individuals (Todder et al., 2005). Higher levels of both entropy h and SampEn were associated with increased HF power and a reduction in the LF/HF ratio, indicating that a more predictable RR pattern is related to increased parasympathetic tone. In addition, these two measures of entropy were significantly correlated, suggesting that entropy h may be a reliable measure of HRV complexity. These results provide additional support for the concept that alterations in cardiac variability and predictability in BD may indeed be state dependent.
We did not observe any differences between SCZ and BD patients on HRV measures assessed in this study. Similar to BD subjects, SCZ subjects exhibited a strong trend towards lower RMSSD, pNN50, HFn power, entropy h, and SampEn compared to the HC group, but the only significant difference was an elevated heart rate. Interpretation of these data is limited by the consideration that we tested a relatively low number of SCZ subjects diagnosed with different subtypes of the disorder, including patients with paranoid, disorganized, and undifferentiated schizophrenia. While several studies have assessed HRV only in a paranoid SCZ population (Bar et al., 2005; Rechlin et al., 1994; Boettger et al., 2006), others have reported impaired HRV in subjects with similar disorders such as delusional disorder or schizoaffective disorder (Valkonen-Korhonen et al., 2003). Overall, however, our results provide tentative support for previous findings that observed decreased HRV in SCZ patients characterized by a reduction in parasympathetic activity (Boettger et al., 2006; Valkonen-Korhonen et al., 2003).
HRV measures were significantly correlated with manic symptoms quantified by the YMRS rating scale. Total YMRS score was significantly correlated with a higher LF/HF ratio in all patients and the BD group alone, while negatively correlated with HFn power in the SCZ group. Unusual thought content, defined as the expression of bizarre or delusional ideas, was also correlated with decreased HFn power and higher LFn power in BD subjects. Finally, we observed that a decrease in entropy was significantly correlated with higher levels of aggression and poor personal hygiene in all patients as assessed by the YMRS scale. These data suggest that more severe psychiatric symptoms and thought disorder are associated with dysfunction in the balance between cardiac sympathetic and parasympathetic tone. The results support previous findings that indicate loss of vagal function is associated with more severe psychotic symptoms (Bar et al., 2005; Toichi et al., 1999) and lend additional support to evidence suggesting state-dependent HRV dysfunction in psychiatric disorders.
As discussed earlier, cardiac function and HRV measures are profoundly impacted by anxiety (Friedman and Thayer, 1998b), and disorders such as BD are characterized by a high comorbidity for anxiety syndromes such as post-traumatic stress disorder (PTSD) and panic disorder (El-Mallakh et al., 2008). In the current study, BD and SCZ patients who met Axis I criteria for other comorbid psychiatric disorders, including PTSD and panic disorder, were excluded from participation to minimize this issue. In addition, we did not observe any significant correlation between participant anxiety during the session as assessed by the Brief Psychiatric Rating Scale and any HRV measure, suggesting that group differences in autonomic function were not simply mediated by anxiety responses. However, we cannot exclude the possibility that cardiac function could be influenced by the presence of anxiety symptoms and submit that this issue should be considered when interpreting the data. The role of anxiety could be examined in greater detail in future studies that compare HRV in BD subjects with and without comorbid anxiety disorders.
HRV is affected by several physiological variables, including age, gender, body mass index, and the phase of the menstrual cycle in females (Yildirir et al., 2002; Vallejo et al., 2005). In the current study, there was no significant difference between menstrual phase in HC and BD female participants, but this information could not be obtained from several of the SCZ patients. Previous studies have reported an increase in relative sympathetic activity during the luteal phase compared to the follicular phase as indicated by a higher LF/HF ratio and reduced HF power (Yildirir et al., 2002; Sato et al., 1995). Thus, the unconfirmed variability of menstrual cycle in SCZ group females is one limitation of this study.
It is important to note that BD and SCZ patients in the current study were treated with medications with the potential to affect HRV. Many psychotropic drugs have the ability to alter cardiac function by directly affecting the myocardium, altering the conduction system of the heart, or by inducing tachycardia through the blockade of muscarinic and adrenergic receptors (Chong et al., 2001). To assess the potential effect of medication in the current study, we compared HRV data for patients treated with the most frequently administered drugs. We did not observe any significant effect of risperidone, valproate, or any difference between combination treatment with a mood stabilizer and antipsychotic compared to an antipsychotic alone. However, BD individuals treated with lithium (n = 7) did exhibit a decrease in the LF/HF ratio compared to BD subjects not taking the drug (n = 16). Considering that BD subjects exhibited a significant increase in the LF/HF ratio and lower HFn power compared to HC subjects in our study, this effect of lithium could not account for the group differences we observe. Given that lithium is known to decrease SA depolarization (Chong et al., 2001), it is possible that administration of this drug blunted sympathetic activation of SA node, thus reducing the relative percentage of LF power.
A number of studies have reported that impaired HRV is associated with more severe psychiatric illness as determined by clinical measures that include the BPRS and PANSS (Bar et al., 2005; Toichi et al., 1999; Okada et al., 2003; Agelink et al., 1998) and these findings are supported by the current data. While these measures are designed to assess an individual's current state of mind, they do involve eliciting retrospective information from the subject that could be biased by poor memory or mood state during administration (Kimhy et al., 2009). Another limitation is that HRV assessment in psychiatric patients is typically conducted in a resting or stationary position, which has the benefit of minimizing the confound of motor activity on cardiac function, but the drawback of limited applicability to daily functioning in “real-world” environments. Recent studies have attempted to circumvent these difficulties by using the experience sampling method (ESM), where individuals record their condition throughout the day as prompted randomly by a watch or PDA (Myin-Germeys et al., 2001). For example, Kimhy and colleagues (2009) recently used the ESM method to demonstrate that momentary increases in stress level were associated with reduced cardiac parasympathetic activity in SCZ inpatients. Future studies could also apply similar methods to assess HRV during “real-world” circumstances in BD.
The precise mechanism of ANS dysregulation in psychiatric disorders still remains unclear, complicated by the large number of cortical, subcortical, and brainstem structures that coordinate autonomic function. Recent reports suggest that reduced HRV may represent evidence of an inhibitory deficit that is mirrored by impaired cognitive and behavioral inhibition (Thayer and Lane, 2009). Individuals with low resting HRV demonstrate greater difficulty in inhibiting inappropriate responses on the Stroop task and the Continuous Performance Task compared to those with higher levels of HRV (Hansen et al., 2003; Johnsen et al., 2003). In addition, lower SDNN and RMSSD were associated with impulsivity in suicidal depressed patients (Booij et al., 2006) and lower SDNN was correlated with increased craving for alcohol in recovering alcoholics (Ingjaldsson et al., 2003).
Several recent reports have suggested that the prefrontal cortex (PFC) could play a critical role in ANS dysregulation (Hansen et al., 2004; Thayer and Lane, 2007; Thayer and Lane, 2009). Disorders such as depression, SCZ, and BD are characterized by a decrease in PFC activity and concomitant deficits in executive function and inhibition (Thayer and Lane, 2009). Inactivation and reduced blood flow in the PFC has also been associated with decreased HRV (Ahern et al., 2001; Lane et al., 2009). A recent review (Thayer and Lane, 2009) has proposed that ANS dysregulation is driven by the failure of the PFC to inhibit the amygdala, a region that mediates cardiovascular and autonomic responses to stress. Although the function of structures such as the amygdala and PFC has been studied extensively, further research is needed to clarify neurological abnormalities that could mediate ANS dysregulation associated with psychiatric illness.
In conclusion, manic BD patients exhibited a significant decrease in HRV compared to age- and gender-matched healthy comparison subjects as assessed by time, frequency, and nonlinear measures. An increase in the ratio of sympathetic to parasympathetic activity was associated with more severe manic symptoms and unusual thought content, indicating that HRV alterations in BD may be state-dependent. The results of this study are limited by a small sample size in the SCZ group and the administration of psychotropic medication, although no confounding effects of treatment were observed. However, we did not observe any significant difference in HRV between manic BD and SCZ patients, suggesting that a similar mechanism may mediate ANS dysregulation in both disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agelink MW, Boz C, Ullrich H, Andrich J. Relationship between major depression and heart rate variability. Clinical consequences and implications for antidepressive treatment. Psychiatry Research. 2002;113:139–149. doi: 10.1016/s0165-1781(02)00225-1. [DOI] [PubMed] [Google Scholar]
- Agelink MW, Malessa R, Kamcili E, Zeit T, Lemmer W, Bertling R, et al. Cardiovascular autonomic reactivity in schizophrenics under neuroleptic treatment: A potential predictor of short-term outcome? Neuropsychobiology. 1998;38:19–24. doi: 10.1159/000026512. [DOI] [PubMed] [Google Scholar]
- Ahern GL, Sollers JJ, Lane RD, Labiner DM, Herring AM, Weinand ME, et al. Heart rate and heart rate variability changes in the intracarotid sodium amobarbital test. Epilepsia. 2001;42:912–921. doi: 10.1046/j.1528-1157.2001.042007912.x. [DOI] [PubMed] [Google Scholar]
- Balogh S, Fitzpatrick DF, Hendricks SE, Paige SR. Increases in heart rate variability with successful treatment in patients with major depressive disorder. Psychopharmacology Bulletin. 1993;29:201–206. [PubMed] [Google Scholar]
- Bar KJ, Boettger MK, Koschke M, Schulz S, Chokka P, Yeragani VK, et al. Non-linear complexity measures of heart rate variability in acute schizophrenia. Clinical Neurophysiology. 2007;118:2009–2015. doi: 10.1016/j.clinph.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Letzsch A, Jochum T, Wagner G, Greiner W, Sauer H. Loss of efferent vagal activity in acute schizophrenia. Journal of Psychiatric Research. 2005;39:519–527. doi: 10.1016/j.jpsychires.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Baumert M, Baier V, Haueisen J, Wessel N, Meyerfeldt U, Schirdewan A, et al. Forecasting of life threatening arrhythmias using the compression entropy of heart rate. Methods of Information in Medicine. 2004;43:202–206. [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Boettger S, Hoyer D, Falkenhahn K, Kaatz M, Yeragani VK, Bar KJ. Altered diurnal autonomic variation and reduced vagal information flow in acute schizophrenia. Clinical Neurophysiology. 2006;117:2715–2722. doi: 10.1016/j.clinph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Boettger S, Hoyer D, Falkenhahn K, Kaatz M, Yeragani VK, Bar KJ. Nonlinear broad band dynamics are less complex in major depression. Bipolar Disorders. 2008;10:276–284. doi: 10.1111/j.1399-5618.2007.00503.x. [DOI] [PubMed] [Google Scholar]
- Booij L, Swenne CA, Brosschot JF, Haffmans PM, Thayer JF, Van der Does AJ. Tryptophan depletion affects heart rate variability and impulsivity in remitted depressed patients with a history of suicidal ideation. Biological Psychiatry. 2006;60:507–514. doi: 10.1016/j.biopsych.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Buckley PF. Update on the treatment and management of schizophrenia and bipolar disorder. CNS Spectrums. 2008;13:1–10. 11–12. doi: 10.1017/s1092852900028212. quiz. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosomatic Medicine. 2005;67(Suppl 1):S29–33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- Chambers AS, Allen JJ. Vagal tone as an indicator of treatment response in major depression. Psychophysiology. 2002;39:861–864. doi: 10.1111/1469-8986.3960861. [DOI] [PubMed] [Google Scholar]
- Chong SA, Mythily, Mahendran R. Cardiac effects of psychotropic drugs. Annals of the Academy of Medicine, Singapore. 2001;30:625–631. [PubMed] [Google Scholar]
- Citrome L, Goldberg JF, Stahl SM. Toward convergence in the medication treatment of bipolar disorder and schizophrenia. Harvard Review of Psychiatry. 2005;13:28–42. doi: 10.1080/10673220590923164. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Kotler M, Mittelman I, Osher Y, Bersudsky Y. Impaired heart rate variability in euthymic bipolar patients. Bipolar Disorders. 2003;5:138–143. doi: 10.1034/j.1399-5618.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- Cowan MJ. Measurement of heart rate variability. Western Journal of Nursing Research. 1995;17:32–48. 101–111. doi: 10.1177/019394599501700104. discussion. [DOI] [PubMed] [Google Scholar]
- Dalack GW, Roose SP. Perspectives on the relationship between cardiovascular disease and affective disorder. The Journal of Clinical Psychiatry. 1990;51(Suppl):4–9. 10–11. discussion. [PubMed] [Google Scholar]
- El-Mallakh RS, Hollifield M. Comorbid anxiety in bipolar disorder alters treatment and prognosis. Psychiatric Quarterly. 2008;79:139–150. doi: 10.1007/s11126-008-9071-5. [DOI] [PubMed] [Google Scholar]
- Friedman BH, Thayer JF. Anxiety and autonomic flexibility: a cardiovascular approach. Biological Psychology. 1998a;49:303–323. doi: 10.1016/s0301-0511(98)00051-9. [DOI] [PubMed] [Google Scholar]
- Friedman BH, Thayer JF. Autonomic balance revisited: panic anxiety and heart rate variability. Journal of Psychosomatic Research. 1998b;44:133–151. doi: 10.1016/s0022-3999(97)00202-x. [DOI] [PubMed] [Google Scholar]
- George MS, Rush AJ, Marangell LB, Sackeim HA, Brannan SK, Davis SM, et al. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biological Psychiatry. 2005;58:364–373. doi: 10.1016/j.biopsych.2005.07.028. [DOI] [PubMed] [Google Scholar]
- Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov P, Mark RG, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101:E215–220. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- Groome LJ, Mooney DM, Holland SB, Smith LA, Atterbury JL, Loizou PC. Human fetuses have nonlinear cardiac dynamics. Journal of Applied Physiology. 1999;87:530–537. doi: 10.1152/jappl.1999.87.2.530. [DOI] [PubMed] [Google Scholar]
- Hansen AL, Johnsen BH, Sollers JJ, 3rd, Stenvik K, Thayer JF. Heart rate variability and its relation to prefrontal cognitive function: the effects of training and detraining. European Journal of Applied Physiology. 2004;93:263–272. doi: 10.1007/s00421-004-1208-0. [DOI] [PubMed] [Google Scholar]
- Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. International Journal of Psychophysiology. 2003;48:263–274. doi: 10.1016/s0167-8760(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Heilman KJ, Porges SW. Accuracy of the LifeShirt (Vivometrics) in the detection of cardiac rhythms. Biological Psychology. 2007;75:300–305. doi: 10.1016/j.biopsycho.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Friedrich H, Zwiener U, Pompe B, Baranowski R, Werdan K, et al. Prognostic impact of autonomic information flow in multiple organ dysfunction syndrome patients. International Journal of Cardiology. 2006;108:359–369. doi: 10.1016/j.ijcard.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Huikuri HV, Perkiomaki JS, Maestri R, Pinna GD. Clinical impact of evaluation of cardiovascular control by novel methods of heart rate dynamics. Philosophical Transactions Series A, Mathematical, Physical, and Engineering Sciences. 2009;367:1223–1238. doi: 10.1098/rsta.2008.0294. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry. 2003;54:1427–1436. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Johnsen BH, Thayer JF, Laberg JC, Wormnes B, Raadal M, Skaret E, et al. Attentional and physiological characteristics of patients with dental anxiety. Journal of Anxiety Disorders. 2003;17:75–87. doi: 10.1016/s0887-6185(02)00178-0. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Grippo AJ. Sadness and broken hearts: neurohumoral mechanisms and comorbidity of ischemic heart disease and psychological depression. Journal of Physiology and Pharmacology. 2006;57(Suppl 11):5–29. [PubMed] [Google Scholar]
- Karavidas MK, Lehrer PM, Vaschillo E, Vaschillo B, Marin H, Buyske S, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Applied Psychophysiology and Biofeedback. 2007;32:19–30. doi: 10.1007/s10484-006-9029-z. [DOI] [PubMed] [Google Scholar]
- Kawachi I, Sparrow D, Vokonas PS, Weiss ST. Decreased heart rate variability in men with phobic anxiety (data from the Normative Aging Study) American Journal of Cardiology. 1995;75:882–885. doi: 10.1016/s0002-9149(99)80680-8. [DOI] [PubMed] [Google Scholar]
- Kent L, O'Neill B, Davison G, Nevill A, Elborn JS, Bradley JM. Validity and reliability of cardiorespiratory measurements recorded by the LifeShirt during exercise tests. Respiratory Physiology & Neurobiology. 2009;167:162–167. doi: 10.1016/j.resp.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Kim JH, Yi SH, Yoo CS, Yang SA, Yoon SC, Lee KY, et al. Heart rate dynamics and their relationship to psychotic symptom severity in clozapine-treated schizophrenic subjects. Progress in Neuro-psychopharmacology & Biological Psychiatry. 2004;28:371–378. doi: 10.1016/j.pnpbp.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Kimhy D, Delespaul P, Ahn H, Cai S, Shikhman M, Lieberman JA, et al. Concurrent Measurement of “Real-World” stress and arousal in individuals with psychosis: assessing the feasibility and validity of a novel methodology. Schizophrenia Bulletin. 2009 May 8; doi: 10.1093/schbul/sbp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiger RE, Bigger JT, Bosner MS, Chung MK, Cook JR, Rolnitzky LM, et al. Stability over time of variables measuring heart rate variability in normal subjects. The American Journal of Cardiology. 1991;68:626–630. doi: 10.1016/0002-9149(91)90355-o. [DOI] [PubMed] [Google Scholar]
- Krittayaphong R, Cascio WE, Light KC, Sheffield D, Golden RN, Finkel JB, et al. Heart rate variability in patients with coronary artery disease: differences in patients with higher and lower depression scores. Psychosomatic Medicine. 1997;59:231–235. doi: 10.1097/00006842-199705000-00004. [DOI] [PubMed] [Google Scholar]
- Lake DE, Richman JS, Griffin MP, Moorman JR. Sample entropy analysis of neonatal heart rate variability. American Journal of Physiology Regulatory, Integrative, and Comparative Physiology. 2002;283:R789–797. doi: 10.1152/ajpregu.00069.2002. [DOI] [PubMed] [Google Scholar]
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. Neural correlates of heart rate variability during emotion. NeuroImage. 2009;44:213–222. doi: 10.1016/j.neuroimage.2008.07.056. [DOI] [PubMed] [Google Scholar]
- Low PA, Pfeifer MA. Standardization of Autonomic function. In: Low PA, editor. Clinical Autonomic Disorders. New York: Little, Brown and Company; 1997. pp. 287–295. [Google Scholar]
- Makikallio TH, Tapanainen JM, Tulppo MP, Huikuri HV. Clinical applicability of heart rate variability analysis by methods based on nonlinear dynamics. Cardiac Electrophysiology Review. 2002;6:250–255. doi: 10.1023/a:1016381025759. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Bruder G, Dalack GW, Storer S, Van Kammen M, Amador X, et al. Diminished cardiac vagal tone in schizophrenia: associations to brain laterality and age of onset. Biological Psychiatry. 1997;41:612–617. doi: 10.1016/s0006-3223(96)00161-8. [DOI] [PubMed] [Google Scholar]
- Malik M, Camm AJ. Heart Rate Variability. New York: Futura Publishing; 1995. [Google Scholar]
- Melzig CA, Weike AI, Hamm AO, Thayer JF. Individual differences in fear-potentiated startle as a function of resting heart rate variability: implications for panic disorder. International Journal of Psychophysiology. 2009;71:109–117. doi: 10.1016/j.ijpsycho.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Geyer MA, Paulus MP, Young JW, Perry W. The quantitative assessment of motor activity in mania and schizophrenia. Journal of Affective Disorders. 2009 May 11; doi: 10.1016/j.jad.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miu AC, Heilman RM, Miclea M. Reduced heart rate variability and vagal tone in anxiety: trait versus state, and the effects of autogenic training. Autonomic Neuroscience. 2009;145:99–103. doi: 10.1016/j.autneu.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, Delespaul PA, deVries MW. Schizophrenia patients are more emotionally active than is assumed based on their behavior. Schizophrenia Bulletin. 2000;26:847–854. doi: 10.1093/oxfordjournals.schbul.a033499. [DOI] [PubMed] [Google Scholar]
- Okada T, Toichi M, Sakihama M. Influences of an anticholinergic antiparkinsonian drug, parkinsonism, and psychotic symptoms on cardiac autonomic function in schizophrenia. Journal of Clinical Psychopharmacology. 2003;23:441–447. doi: 10.1097/01.jcp.0000088901.24613.b8. [DOI] [PubMed] [Google Scholar]
- Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circulation Research. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA, Gold LH, Mandell AJ. Application of entropy measures derived from the ergodic theory of dynamical systems to rat locomotor behavior. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:723–727. doi: 10.1073/pnas.87.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus M, Young JW, Kincaid MJ, Ferguson EJ, et al. From Mice to Men: A reverse translational study of dysfunctional exploration in psychiatric disorders. Archives of General Psychiatry. 2009 doi: 10.1001/archgenpsychiatry.2009.58. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus SM, Huang W. Approximate entropy: Statistical properties and applications. Communication in Statistics-Theory and Methods. 1992;21:3061–3077. [Google Scholar]
- Pini S, de Queiroz V, Dell'Osso L, Abelli M, Mastrocinque C, Saettoni M, et al. Cross-sectional similarities and differences between schizophrenia, schizoaffective disorder and mania or mixed mania with mood-incongruent psychotic features. European Psychiatry. 2004;19:8–14. doi: 10.1016/j.eurpsy.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Yurgelun-Todd D. Schizophrenic individuals with bipolar first-degree relatives: analysis of two pedigrees. The Journal of Clinical Psychiatry. 1990;51:97–101. [PubMed] [Google Scholar]
- Rechlin T, Weis M, Spitzer A, Kaschka WP. Are affective disorders associated with alterations of heart rate variability? Journal of Affective Disorders. 1994;32:271–275. doi: 10.1016/0165-0327(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. American Journal of Physiology Heart and Circulatory Physiology. 2000;278:H2039–2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Sato N, Miyake S, Akatsu J, Kumashiro M. Power spectral analysis of heart rate variability in healthy young women during the normal menstrual cycle. Psychosomatic Medicine. 1995;57:331–335. doi: 10.1097/00006842-199507000-00004. [DOI] [PubMed] [Google Scholar]
- Schurmann T, Grassberger P. Entropy estimation of symbol sequences. Chaos. 1996;6:414–427. doi: 10.1063/1.166191. [DOI] [PubMed] [Google Scholar]
- Singer DH, Ori Z. Changes in heart rate variability associated with sudden cardiac death. In: Malik M, Camm AJ, editors. Heart Rate Variability. New York: Futura Publishing Company; 1995. pp. 429–448. [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biological Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biological Psychology. 2007;74:224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Thong T, Yung IO, Zajdel DPP, Ellingson RM, McNames J, Aboy M, et al. Heart rate variability analysis of effect of nicotine using periodograms. Conference Proceedings IEEE Engineering in Medicine and Biology Society. 2004;1:294–297. doi: 10.1109/IEMBS.2004.1403150. [DOI] [PubMed] [Google Scholar]
- Todder D, Bersudsky Y, Cohen H. Nonlinear analysis of RR interval in euthymic bipolar disorder. Autonomic Neuroscience: Basic & Clinical. 2005;117:127–131. doi: 10.1016/j.autneu.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Toichi M, Kubota Y, Murai T, Kamio Y, Sakihama M, Toriuchi T, et al. The influence of psychotic states on the autonomic nervous system in schizophrenia. International Journal of Psychophysiology. 1999;31:147–154. doi: 10.1016/s0167-8760(98)00047-6. [DOI] [PubMed] [Google Scholar]
- Valkonen-Korhonen M, Tarvainen MP, Ranta-Aho P, Karjalainen PA, Partanen J, Karhu J, et al. Heart rate variability in acute psychosis. Psychophysiology. 2003;40:716–726. doi: 10.1111/1469-8986.00072. [DOI] [PubMed] [Google Scholar]
- Vallejo M, Marquez MF, Borja-Aburto VH, Cardenas M, Hermosillo AG. Age, body mass index, and menstrual cycle influence young women's heart rate variability --a multivariable analysis. Clinical Autonomic Research. 2005;15:292–298. doi: 10.1007/s10286-005-0272-9. [DOI] [PubMed] [Google Scholar]
- Vivometrics. The Lifeshirt System ™. Ventura, CA: 2002. [Google Scholar]
- Voss A, Baier V, Schulz S, Bar KJ. Linear and nonlinear methods for analyses of cardiovascular variability in bipolar disorders. Bipolar Disorders. 2006;8:441–452. doi: 10.1111/j.1399-5618.2006.00364.x. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Pohl R, Balon R, Ramesh C, Glitz D, Jung I, et al. Heart rate variability in patients with major depression. Psychiatry Research. 1991;37:35–46. doi: 10.1016/0165-1781(91)90104-w. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Sobolewski E, Igel G, Johnson C, Jampala VC, Kay J, et al. Decreased heart-period variability in patients with panic disorder: a study of Holter ECG records. Psychiatry Research. 1998;78:89–99. doi: 10.1016/s0165-1781(97)00136-4. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Rao KA, Smitha MR, Pohl RB, Balon R, Srinivasan K. Diminished chaos of heart rate time series in patients with major depression. Biological Psychiatry. 2002;51:733–744. doi: 10.1016/s0006-3223(01)01347-6. [DOI] [PubMed] [Google Scholar]
- Yildirir A, Kabakci G, Akgul E, Tokgozoglu L, Oto A. Effects of menstrual cycle on cardiac autonomic innervation as assessed by heart rate variability. Ann Noninvasive Electrocardiol. 2002;7:60–63. doi: 10.1111/j.1542-474X.2001.tb00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Minassian A, Paulus MP, Geyer MA, Perry W. A reverse-translational approach to bipolar disorder: rodent and human studies in the Behavioral Pattern Monitor. Neuroscience and Biobehavioral Reviews. 2007;31:882–896. doi: 10.1016/j.neubiorev.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]