Abstract
Objective
Lynch Syndrome (LS) is characterized by a high lifetime incidence of colorectal cancer and gynecologic malignancies such as endometrial and ovarian cancer. Identification of LS families is important as it allows for heightened cancer screening which decreases colorectal cancer mortality. The original 1996 Bethesda guidelines included two gynecologic populations that should be further evaluated for LS: those with endometrial cancer before the age of 45 and those with two LS-related cancers (i.e. synchronous endometrial and ovarian cancer). Our study aims to estimate the prevalence of LS in these two populations.
Methods
We utilized a diagnostic algorithm that included immunohistochemistry for mismatch repair protein expression followed by selective evaluation for microsatellite instability and MLH1 gene promoter methylation.
Results
Among 72 eligible patients, 9 (12%) had molecular findings consistent with LS: 6/50 (12%) in the early-onset endometrial cancer group and 3/22 (14%) in the synchronous primary cancer group. In an additional 3 cases, MLH1 silencing was due to promoter methylation: 1/50 (2%) in the early-onset endometrial cancer group and 2/22 (9%) in the synchronous primary cancer group. Of the 9 women with molecular criteria suggesting LS, only three had pedigrees meeting the Amsterdam criteria.
Conclusions
A diagnostic algorithm can identify patients with LS and those who warrant further genetic testing. Our findings reinforce the recommendation that women diagnosed with endometrial cancer before age 45 and women with synchronous endometrial and ovarian cancer be screened for LS, irrespective of family history.
Introduction
Lynch Syndrome (LS), also known as Hereditary Nonpolyposis Colorectal Cancer (HNPCC), results from the autosomal dominant inheritance of a mutated DNA mismatch repair (MMR) gene. Clinically, LS families have up to an 80% risk of developing colorectal cancer, a 60% risk of developing endometrial cancer and a 12% risk of developing ovarian cancer [1, 2]. Cancers of the stomach, pancreas, upper urinary tract, biliary tract and small intestine are also reported in LS families [3]. Identification of LS in affected individuals has important implications for screening in individuals as well as family members, as close screening and surveillance has been shown to reduce the mortality of colorectal cancer by over 60% [4].
The initial (1991) and revised (1998) Amsterdam criteria were developed to identify families at high risk for LS [5, 6]. These criteria required colorectal or other LS-associated cancers in three first-degree relatives, occurring in at least two successive generations, and in one individual under the age of 50. These criteria were recognized to have poor sensitivity in identifying individuals carrying a LS gene mutation. Therefore, the Bethesda Guidelines were introduced to broaden testing recommendations and to identify a greater proportion of affected individuals. The original 1996 Bethesda Guidelines recommended molecular testing for LS in six groups of patients, including two gynecologic cancer populations: those with endometrial cancer diagnosed before 45 years of age and those with two LS-related cancers (i.e. synchronous endometrial and ovarian cancers) [7]. The Bethesda guidelines were revised in 2002 to enhance the sensitivity and specificity of the original recommendations, but they failed to specify which gynecologic cancers should undergo further testing [8].
The majority of LS results from an inherited germline mutation in one of three mismatch repair (MMR) genes, MLH1, MSH2, or MSH6 [9, 10]. Deficient MMR protein activity leads to DNA microsatellite instability (MSI) and absent immunohistochemical protein expression in tumor tissue [11]. The pattern of abnormal staining provides guidance as to which of the MMR genes is likely to harbor a germline mutation [12]. However, epigenetic silencing of the MLH1 gene by promoter methylation can also result in defective MMR protein activity [13]. This is a somatic and non-heritable event, and does not warrant further evaluation for LS.
This study was designed to utilize a diagnostic algorithm to estimate the prevalence of LS in two gynecologic populations for whom screening is recommended by the 1996 Bethesda guidelines; women less than 45 years of age at diagnosis with endometrial cancer and women with synchronous endometrial and ovarian cancers.
Materials and Methods
Patient Population
After Institutional Review Board approval, 72 patients were identified from a pathology database at Cedars-Sinai Medical Center in Los Angeles, CA. Group 1 included 50 patients with endometrial cancer diagnosed before 45 years of age and group 2 included 22 patients with synchronous endometrial and ovarian cancers (no age restriction). Cases were selected between March 1994 and August 2008 based on the availability of pathologic materials for analysis. During that equivalent time period, there were 1197 total patients diagnosed with endometrial carcinoma (all ages, all histologies); 100 (8.3%) cases occurred in women younger than age of 45. Among these 100 cases, 75 (75%) contained a diagnosis of endometrial carcinoma alone and 25 (25%) were associated with co-existing adnexal disease (synchronous or metastatic). H&E stained slides of formalin-fixed paraffin embedded tissue were retrieved from the surgical pathology files, reviewed, diagnoses confirmed, and appropriate tumor and control tissue blocks selected for study by a gynecologic pathologist. Cases were not included in this series if too little tumor tissue existed for analysis, if the original blocks were from an outside institution, or if tumor blocks could not be retrieved. Cases that contained both endometrial and ovarian carcinomas were included if a diagnosis of synchronous rather than metastatic disease was favored by the gynecologic pathologist. Retrospective chart reviews were performed to collect demographic and clinical information.
Molecular Analysis for Lynch Syndrome
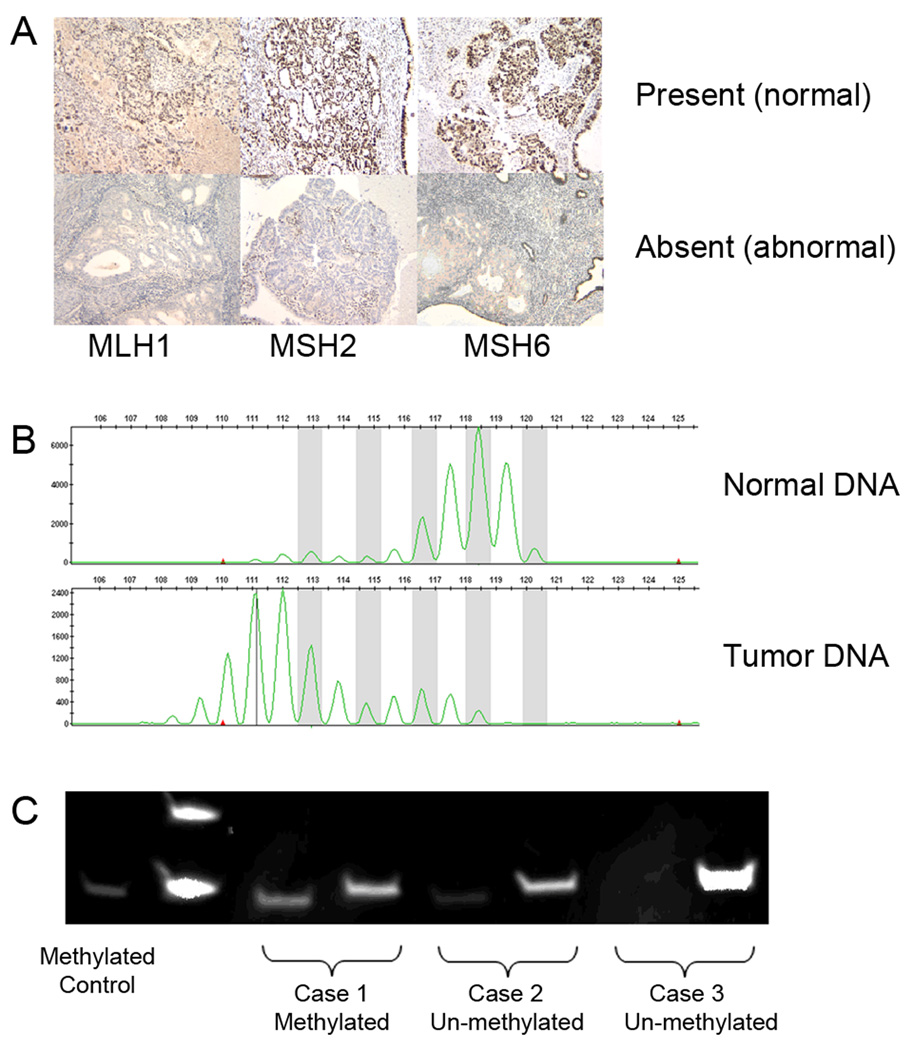
Serial sections of the selected paraffin embedded tumor and control tissue blocks from all 72 patients were immunostained for MMR proteins: MLH1, MSH2, and MSH6. The immunostained slides were reviewed by a gynecologic pathologist and characterized as absent, weak, or present based on the intensity of nuclear staining for MLH1, MSH2, and MSH6. Figure 1A illustrates present (normal) and absent (abnormal) staining patterns.
Figure 1.
A. Immunohistochemistry results for MLH1, MSH2, and MSH6 The top row demonstrates normal nuclear staining for each mismatch repair protein. The bottom row demonstrates absent staining which is consistent with abnormal mismatch repair protein function. B. Representative example of microsatellite instability in endometrial cancer DNA (bottom) compared to matched normal DNA (top) in one of the NCI-recommended microsatellite markers. In this case, there is a shift in the peaks, representing an error in the DNA replication process and contraction of this microsatellite region. C. Methylation specific PCR results. Lane 1 contains the methylated control. For each case, two PCR reactions were performed with primers specific for the methylated MLH1 promoter (loaded on the left) and with primers specific for the unmethylated MLH1 promoter (loaded on the right). Cases 1 and 2 demonstrate tumors with MLH1 silencing due to MLH1 gene promoter methylation, while case 3 represents and case that may be due to an inherited germline MLH1 gene mutation.
Selected cases with absent or weak immunostaining for one or more of the MMR proteins were tested for MSI (representative example in figure 1B). DNA from tumor and normal tissue were extracted from paraffin-embedded tissues using the EX-WAX DNA extraction kit (Chemicon International; Temecula, CA) DNA from matched tumor and normal tissue was amplified for the five National Cancer Institute (NCI) recommended microsatellite markers, BAT25, BAT26, D17S250, D2S123, and D5S346 using fluromer-labeled primers [18]. MSI was determined by comparing each endometrial and ovarian cancer to paired normal DNA from the same individual. A tumor was designated MSI-High (MSI-H) if ≥2 of the 5 MSI markers demonstrated evidence of instability. Tumors with zero or one marker unstable were designated as microsatellite stable (MSS) or MSI-low (MSI-L), respectively [18, 19].
Tumors with absent MLH1 immunohistochemistry were further evaluated for MLH1 promoter methylation (representative examples in figure 1C). Tumor DNA was bisulfite treated using the Qiagen Epitect Kit (Qiagen; Valencia, CA) allowing for the conversion of unmethylated cytosines to uracil. For each tumor DNA sample, two separate PCR reactions were set up to amplify for methylated and unmethylated MLH1 gene promoters. PCR products were run on 20% Tris-HCl polyacrylamide gels in 1× TAE at 80V for 2 hours and visualized under ultraviolet light after staining and destaining with ethidium bromide.
Detailed descriptions of the laboratory protocols for immunohistochemistry, DNA extraction, MSI testing and methylation specific PCR, including primer sequences and protocol conditions, are provided as supplementary material (S1).
Diagnostic Algorithm
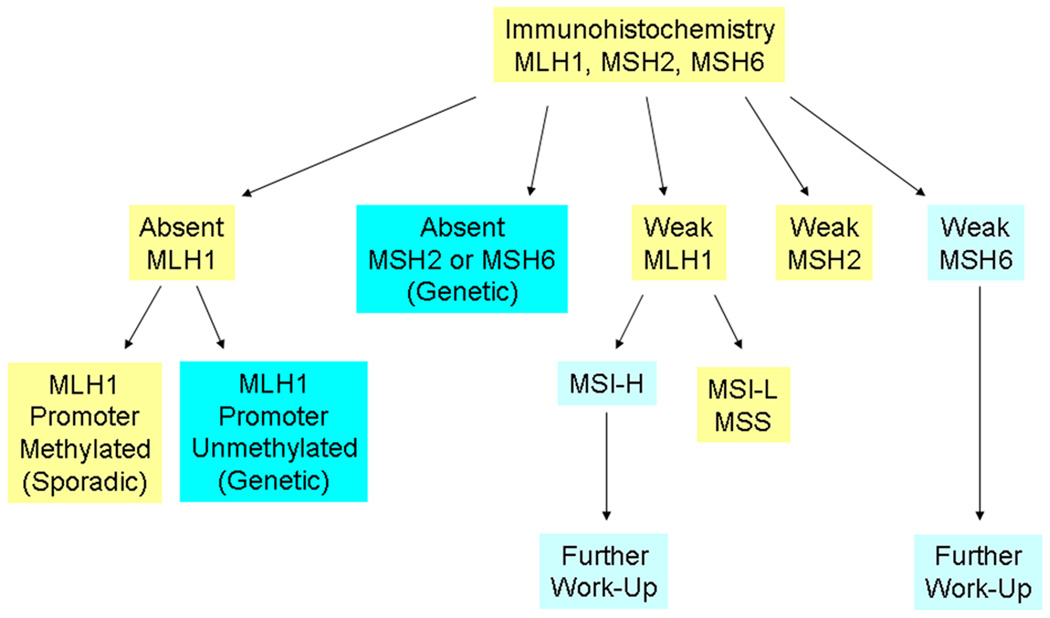
Figure 2 illustrates the diagnostic algorithm that we used to determine whether a tumor would be classified as genetic (arising from LS), sporadic, or requiring further work-up. Two patterns of molecular findings were considered to be diagnostic of Lynch syndrome: (1) absent MLH1 staining with an unmethylated MLH1 gene promoter and (2) absent MSH2 and/or MSH6 staining. Tumors with absent MLH1 staining and evidence of methylation of the MLH1 gene promoter were classified as sporadic. Tumors with weak immunohistochemical staining were triaged according to information from the literature. MLH1 staining by immunohistochemistry, in particular, can be problematic in predicting the presence of a germline MLH1 mutation [20, 21]. Tumors from MLH1 mutation carriers demonstrate absent staining in only 2/3 of cases and have been shown to exhibit weak positive MLH1 staining in 1/3 of cases [22]. Therefore, we triaged tumors with weak MLH1 staining to further evaluation by MSI testing and those with a pattern of MSI-H were considered appropriate for referral for genetic testing. In contrast, tumors from individuals with MSH2 germline mutations demonstrate absent MSH2 staining [22]. Therefore, we considered the finding of weak MSH2 staining to be clinically insignificant and not warranting further work-up. Tumors from patients with germline MSH6 mutations have been shown to demonstrate lower or absent levels of MSI [23]. Therefore, any abnormal MSH6 staining, irrespective of MSI status was considered appropriate for referral for genetic testing.
Figure 2.
Diagnostic algorithm used in our study. Dark blue boxes represent molecular findings consistent with Lynch Syndrome. Light blue boxes represent abnormal molecular findings that warrant further genetic testing for Lynch Syndrome.
Results
Patient characteristics are summarized in Table 1. Results of the molecular analysis for group 1 (50 early onset endometrial cancers) and group 2 (22 synchronous endometrial and ovarian cancers) are detailed in table 2 and table 3, respectively. Absence of staining for MLH1 or MSH2 was found in 7 (14%) tumors in group 1 and in 5 (23%) tumors in group 2. A strong correlation existed between negative IHC staining and MSI of tumor in both groups.
Table 1.
Patient Characteristics
| Group 1: Early-onset Endometrial CA (age< 45 yrs) N=50 |
Group 2: Synchronous Endometrial/Ovarian CA N=22 |
|
|---|---|---|
| Median age (range) | 39 (29–44) | 42 (31–52) |
| Median BMI (range) | 26.9 (18–62) | 26 (18–44) |
| Race/Ethnicity | ||
| Caucasian | 34 (68%) | 17 (77%) |
| Asian | 11 (22%) | 3 (14%) |
| Other | 5 (10%) | 2 (9%) |
| Endometrial CA Histology | ||
| Endometrioid | 45 (90%) | 19 (86%) |
| Adenosquamous | 1 (2%) | 1 (5%) |
| Mixed | 4 (8%) | 2 (9%) |
| Endometrial CA Stage | ||
| I | 37 (74%) | 18 (81%) |
| II | 1 (2%) | 1 (5%) |
| III | 5 (10%) | 2 (9%) |
| IV | 1 (2%) | 1 (5%) |
| Unknown | 6 (12%) | |
Table 2.
Early onset endometrial cancer (Group 1): immunohistochemistry and MSI testing results (n=50)
| Total | MSI-H | MSI-L | MSS | Unknown | |
|---|---|---|---|---|---|
| Absent MLH1 | 6 (12%) | 5 | 1 | 0 | 0 |
| Absent MSH2 | 1 (2%) | 1 | 0 | 0 | 0 |
|
Total absent IHC |
7 (14%) | ||||
| Weak MLH1 | 9 (18%) | 1 | 2 | 5 | 1 |
| Weak MSH6 | 3 (6%) | 2 | 0 | 1 | 0 |
| Total weak IHC | 12 (24%) | ||||
| Normal IHC | 31 (62%) |
MSI = microsatellite instability; MSI-H = MSI-high; MSI-L = MSI-low; MSS = microsatellite stable; IHC = immunohistochemistry
Table 3.
Synchronous endometrial and ovarian cancer (Group 2): immunohistochemistry and MSI testing results (n=22)
| Total | MSI-H | MSI-L | MSS | MSS | |
|---|---|---|---|---|---|
| Absent MLH1 | 3 (14%) | 3 | 0 | 0 | 0 |
| Absent MSH2 | 2 (9%) | 1 | 0 | 0 | 1 |
|
Total absent IHC |
5 (23%) | ||||
| Weak MLH1 | 4 (18%) | 0 | 2 | 1 | 1 |
| Total weak IHC | 4 (18%) | ||||
| Normal IHC | 13 (59%) |
MSI = microsatellite instability; MSI-H = MSI-high; MSI-L = MSI-low; MSS = microsatellite stable; IHC = immunohistochemistry
Those tumors with absent MLH1 immunohistochemistry were further evaluated for methylation of the MLH1 gene promoter. In group 1, among six cases with absent MLH1 immunostaining, five (83%) were unmethylated, providing strong support for an underlying etiology of LS in these early onset endometrial cancer cases. In contrast, in group 2, among three synchronous endometrial/ovarian cancer cases, two (67%) were methylated and determined to be of sporadic origin (Supplemental table S2).
Supplemental table S3 details the molecular findings in four cases with synchronous endometrial and ovarian cancers with absent MMR protein staining (group 2). The two tumor sites tend to show similar molecular characteristics. In each of the four patients, the same MMR protein was affected in both of their tumors; and in two of the patients, methylation of the MLH1 promoter was demonstrated in both tumors. Cases 1 and 2 are consistent with possible LS, while cases 3 and 4 demonstrate evidence of epigenetic MLH1 silencing through promoter methylation. A fifth case (not included in the table) underwent staining only for the endometrial cancer, which demonstrated absent MSH2 and MSH6 staining. Based on this finding, the patient was referred to genetic testing and was found to have a deleterious mutation in MSH2.
Table 4 summarizes our findings. When considering the entire diagnostic algorithm that includes immunohistochemistry, MSI testing, and evaluation for MLH1 promoter methylation, molecular criteria supporting a genetic etiology of LS were found in 12% (6/50) of early onset endometrial cancer patients (group 1) and in 14% (3/22) of synchronous endometrial/ovarian cancer patients (group 2). Epigenetic silencing of MLH1 by promoter methylation was a more prominent feature of synchronous cases (9%) than early endometrial cases (2%). Suspicious, but non-diagnostic molecular abnormalities were found in an additional 10% of early endometrial (group 1) and 4% of synchronous cases (group 2), warranting further evaluation and genetic work-up.
Table 4.
Summary results based on our diagnostic algorithm
| Group 1: Early onset endometrial cancer |
Group 2: Synchronous endometrial and ovarian cancer |
|
|---|---|---|
| Genetic, suggests LS | 6/50 (12%) | 3/22 (14%) |
| Absent MLH1, unmethylated | ||
| Absent MSH2/MSH6 | ||
| Sporadic | 1/50 (2%) | 2/22 (9%) |
| Absent MLH1, methylated | ||
| Needs further evaluation | 5/50 (10%) | 1/22 (4%) |
| Weak MLH1, MSI-H or unknown |
||
| Weak MSH6, any MSI status | ||
LS = Lynch Syndrome; MSI = microsatellite instability; MSI-H = MSI-high
Among the nine patients with molecular criteria for LS, only three met the Amsterdam II criteria based on family history and only one additional patient had a first degree relative with a history of a LS-associated tumor (Supplemental table S4). Three of the nine patients have undergone commercial genetic testing, and all were found to carry deleterious germline LS mutations: patient 1 carries the MSH2 IVS5 +3A>T mutation, patient 5 carries the MLH1 K416X mutation, and patient 8 has a deletion of exons 1 to 6 in the MSH2 gene.
Discussion
Among two populations of gynecologic oncology patients that are recommended to undergo genetic testing by the 1996 Bethesda guidelines, we found 12% (6/50) of the early-onset endometrial cancer group and 14% (3/22) of the synchronous endometrial and ovarian cancer group to have tumors with molecular characteristics suggestive of LS. Only three of these nine patients had a pedigree pattern that met the revised Amsterdam criteria for LS.
Screening by IHC for MMR proteins followed by selective MSI testing and evaluation for MLH1 promoter methylation may provide a useful algorithm for triage of patient samples toward genetic testing to identify a deleterious mutation in a MMR gene. We demonstrate a high concordance between absent MMR protein IHC and the MSI-H phenotype and conclude that further MSI testing is not necessary in these cases.
The prediction of MLH1 mutations by IHC has been problematic in the past, due to the occurrence of MLH1 missense mutations that result in a deficient, but antigenically-active protein [20]. We approached this problem by using MSI testing as a triage tool for further work-up of tumors with weak MLH1 protein staining [22]. An alternative approach would be to add the PMS2 antibody to the IHC panel. Addition of PMS2 increases the sensitivity of IHC in predicting MLH1 mutation to 92%; up from 85% with the three-antibody panel composed of MLH1, MSH2 and MSH6 [21]. MLH1 dimerizes with PMS2 and mutations of MLH1 will often cause a concurrent loss of the two proteins [24]. Our study potentially underestimates the prevalence of LS by the omission of this fourth antibody in our screening panel.
We chose to use IHC as the primary screening tool based on studies that suggest similar effectiveness of this method when compared to screening by MSI [25, 26]. Addition of an IHC panel of MMR proteins to the pathological evaluation of a tumor is relatively easy for the clinical pathologist and the pattern of MMR protein staining abnormalities can direct genetic testing towards the gene most likely to be affected [21]. Furthermore, IHC is more likely than MSI testing to detect a MSH6 deficient tumor that may be characterized by low or absent MSI [23]. However, IHC can miss cases resulting from a deleterious missense mutation that encodes a functionally-deficient but antigenically-intact protein [27]. Furthermore, while most cases of LS are due to mutations in MLH1, MSH2, MSH6, or PMS2; cases arising from an as of yet undefined mutated gene would not be detected by the IHC antibody panel [28]. Notably, the concordance rate between IHC and MSI testing is only 92%, with both tests missing some cases that would be detected by the other [29, 30].
The relatively high rate of MLH1 promoter methylation found in the synchronous primary cancer group suggests the benefit of adding MLH1 methylation analysis to the diagnostic algorithm. Those cases found to have MLH1 promoter methylation would not require additional genetic testing for LS. Studies in colorectal cancer have demonstrated the utility of adding BRAF V600E mutation analysis to determine the sporadic nature of tumors with decreased MLH1 expression [31–33]. Sparse data are available to this approach to the work-up of endometrial cancer. However, one recent report suggests the BRAF V600E mutation is not found in sporadic endometrial carcinomas [34].
In our diagnostic algorithm, we classified two patterns of abnormalities to be virtually diagnostic of LS: (1) absent MLH1 staining and non-methylated MLH1 gene promoter and (2) absent MSH2 and/or MSH6 staining [35, 36]. Three of the nine patients classified as LS based on these patterns of molecular abnormalities underwent commercial genetic testing and all three (100%) were confirmed to carry a deleterious mutation. The highly predictive nature of these molecular findings raises the issue that IHC for MMR proteins could be interpreted as a genetic test. As such, the clinician should consider whether appropriate informed consent protocols should be in place before immunohistochemical testing is performed.
We did not perform germline testing on all patients in this study, nor did we study a population-based sample. Both of these limitations could result in either overestimation or underestimation of LS among our two study populations. Nevertheless, using our diagnostic algorithm, we found 12% of patients with endometrial cancers before the age of 45 to have molecular findings consistent with LS, which aligns with findings from prior studies. In patients diagnosed with endometrial cancer before 50 years of age, three studies utilizing germline gene sequencing reported LS in 4.9%[37], 8.6% [38], and 9% [39]. We found 14% of patients with synchronous endometrial and ovarian cancers to have molecular findings suggestive of LS. Our results are slightly higher than the those of two retrospective studies (also based on tumor molecular profiling) that suggested LS incidence rates of 3 – 7% [40, 41]. However, in a study utilizing germline mutation analysis in early-onset endometrial cancer patients less than 50 years of age, one of nine (11%) patients with a synchronous primary ovarian cancer had a LS mutation [39]. Our study also demonstrates a substantial proportion of MMR and MSI abnormalities in synchronous cases to result from MLH1 promoter methylation.
Among patients with synchronous endometrial and ovarian cancers, the tumors at both sites often showed similar IHC staining patterns and/or similar patterns of MLH1 promoter methylation, possibly reflecting either a still undefined genetic or environmental field effect that impacts tumor development at both sites. We included only tumors where the clinical impression of synchronous malignancies was favored, but the possibility that the two tumor sites represent a metastasis from one site to the other must also be considered. Nevertheless, the concordance of molecular findings in tumor pairs raises the feasibility of restricting molecular testing to the endometrial cancer in these patients.
The optimal population of endometrial cancer patients for referral to genetic testing has yet to be defined. In this study, we evaluated patients diagnosed with endometrial cancer before the age of 45 as recommended by the 1996 Bethesda guidelines. However, evidence suggests that a cutoff age of 45 will miss a large proportion of LS patients. In a population-based study, the median age of diagnosis among ten LS mutation carriers was 54.6 years (range 39–69), with six of the ten probands more than 50 years of age at the time of endometrial cancer diagnosis [37]. The use of family history as a triage tool may also miss LS cases. Berends et al [38] reported that among early-onset endometrial cancer patients (before the age of 50), 23% were found to have a germline LS mutation if they had a first-degree relative with a LS-associated cancer. In a population-based study of unselected endometrial cancer patients, seven of ten LS mutation carriers did not fulfill either the Amsterdam criteria or the Bethesda guidelines for screening [37].
Identification of LS individuals and families is important because it has been shown to decrease colorectal cancer mortality with the institution of heightened cancer screening protocols [4]. The use of immunohistochemistry followed by selective MSI and MLH1 promoter methylation studies may represent a useful algorithm for the identification of patients who should undergo analysis for a germline MMR gene mutation. We did not find family history to be a useful triage tool. Based on our findings, we would recommend screening for both gynecologic cancer populations identified by the original Bethesda guidelines, irrespective of family history. However, the optimal age cut-off for LS screening has not yet been defined and remains to be determined with future study.
Supplementary Material
Acknowledgements
Research Support provided by the Gynecologic Cancer Foundation/Lee Kaplan Ovarian Cancer Research Grant Award (C.S.W.), the General Clinical Research Center Grant No. M01-RR00425 (C.S.W.), the American Cancer Society California Division-Early Detection Professorship Grant No. SIOP-06-258-01-CCE (B.Y.K.), the Women’s Cancer Research Institute (C.S.W. and B.Y.K.), and NIH grant 2 R01 CA026038-30 (H.P.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: There are no financial disclosures from any authors.
This is an original manuscript. Data were presented in abstract form at the Society of Gynecologic Oncologists 40th Annual Meeting on Women’s Cancers, San Antonio, TX, on February 9th, 2009.
References
- 1.Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6(1):105–110. doi: 10.1093/hmg/6.1.105. [DOI] [PubMed] [Google Scholar]
- 2.Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81(2):214–218. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Watson P, Lynch HT. Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer. 1993;71(3):677–685. doi: 10.1002/1097-0142(19930201)71:3<677::aid-cncr2820710305>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Jarvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118(5):829–834. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 5.Vasen HF, Mecklin JP, Khan PM, et al. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34(5):424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 6.Vasen HF, Watson P, Mecklin JP, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116(6):1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Bigas MA, Boland CR, Hamilton SR, et al. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89(23):1758–1762. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- 8.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96(4):261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peltomaki P, de la Chapelle A. Mutations predisposing to hereditary nonpolyposis colorectal cancer. Adv Cancer Res. 1997;71:93–119. doi: 10.1016/s0065-230x(08)60097-4. [DOI] [PubMed] [Google Scholar]
- 10.Kolodner RD, Tytell JD, Schmeits JL, et al. Germ-line msh6 mutations in colorectal cancer families. Cancer Res. 1999;59(20):5068–5074. [PubMed] [Google Scholar]
- 11.Marra G, Boland CR. Hereditary nonpolyposis colorectal cancer: the syndrome, the genes, and historical perspectives. J Natl Cancer Inst. 1995;87(15):1114–1125. doi: 10.1093/jnci/87.15.1114. [DOI] [PubMed] [Google Scholar]
- 12.Vasen HF, Hendriks Y, de Jong AE, et al. Identification of HNPCC by molecular analysis of colorectal and endometrial tumors. Dis Markers. 2004;20(4–5):207–213. doi: 10.1155/2004/391039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kane MF, Loda M, Gaida GM, et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997;57(5):808–811. [PubMed] [Google Scholar]
- 14.Eifel P, Hendrickson M, Ross J, et al. Simultaneous presentation of carcinoma involving the ovary and the uterine corpus. Cancer. 1982;50(1):163–170. doi: 10.1002/1097-0142(19820701)50:1<163::aid-cncr2820500131>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 15.Pearl ML, Johnston CM, Frank TS, et al. Synchronous dual primary ovarian and endometrial carcinomas. Int J Gynaecol Obstet. 1993;43(3):305–312. doi: 10.1016/0020-7292(93)90520-7. [DOI] [PubMed] [Google Scholar]
- 16.Gitsch G, Hanzal E, Jensen D, et al. Endometrial cancer in premenopausal women 45 years and younger. Obstet Gynecol. 1995;85(4):504–508. doi: 10.1016/0029-7844(95)00001-8. [DOI] [PubMed] [Google Scholar]
- 17.Zaino R, Whitney C, Brady MF, et al. Simultaneously detected endometrial and ovarian carcinomas--a prospective clinicopathologic study of 74 cases: a gynecologic oncology group study. Gynecol Oncol. 2001;83(2):355–362. doi: 10.1006/gyno.2001.6400. [DOI] [PubMed] [Google Scholar]
- 18.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58(22):5248–5257. [PubMed] [Google Scholar]
- 19.Dietmaier W, Wallinger S, Bocker T, et al. Diagnostic microsatellite instability: definition and correlation with mismatch repair protein expression. Cancer Res. 1997;57(21):4749–4756. [PubMed] [Google Scholar]
- 20.Salahshor S, Koelble K, Rubio C, et al. Microsatellite Instability and hMLH1 and hMSH2 expression analysis in familial and sporadic colorectal cancer. Lab Invest. 2001;81(4):535–541. doi: 10.1038/labinvest.3780262. [DOI] [PubMed] [Google Scholar]
- 21.Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn. 2008;10(4):293–300. doi: 10.2353/jmoldx.2008.080031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangold E, Pagenstecher C, Friedl W, et al. Tumours from MSH2 mutation carriers show loss of MSH2 expression but many tumours from MLH1 mutation carriers exhibit weak positive MLH1 staining. J Pathol. 2005;207(4):385–395. doi: 10.1002/path.1858. [DOI] [PubMed] [Google Scholar]
- 23.Berends MJ, Wu Y, Sijmons RH, et al. Molecular and clinical characteristics of MSH6 variants: an analysis of 25 index carriers of a germline variant. Am J Hum Genet. 2002;70(1):26–37. doi: 10.1086/337944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadyrov FA, Dzantiev L, Constantin N, et al. Endonucleolytic function of MutLalpha in human mismatch repair. Cell. 2006;126(2):297–308. doi: 10.1016/j.cell.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 25.Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352(18):1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 26.Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26(35):5783–5788. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peltomaki P, Vasen H. Mutations associated with HNPCC predisposition -- Update of ICG-HNPCC/INSiGHT mutation database. Dis Markers. 2004;20(4–5):269–276. doi: 10.1155/2004/305058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part II. The utility of microsatellite instability testing. J Mol Diagn. 2008;10(4):301–307. doi: 10.2353/jmoldx.2008.080062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shia J, Ellis NA, Klimstra DS. The utility of immunohistochemical detection of DNA mismatch repair gene proteins. Virchows Arch. 2004;445(5):431–441. doi: 10.1007/s00428-004-1090-5. [DOI] [PubMed] [Google Scholar]
- 30.Mueller J, Gazzoli I, Bandipalliam P, et al. Comprehensive Molecular Analysis of Mismatch Repair Gene Defects in Suspected Lynch Syndrome (Hereditary Nonpolyposis Colorectal Cancer) Cases. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-09-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen LH, Lindebjerg J, Byriel L, et al. Strategy in clinical practice for classification of unselected colorectal tumours based on mismatch repair deficiency. Colorectal Dis. 2008;10(5):490–497. doi: 10.1111/j.1463-1318.2007.01378.x. [DOI] [PubMed] [Google Scholar]
- 32.Loughrey MB, Waring PM, Tan A, et al. Incorporation of somatic BRAF mutation testing into an algorithm for the investigation of hereditary non-polyposis colorectal cancer. Fam Cancer. 2007;6(3):301–310. doi: 10.1007/s10689-007-9124-1. [DOI] [PubMed] [Google Scholar]
- 33.Rajagopalan H, Bardelli A, Lengauer C, et al. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418(6901):934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- 34.Kawaguchi M, Yanokura M, Banno K, et al. Analysis of a correlation between the BRAF V600E mutation and abnormal DNA mismatch repair in patients with sporadic endometrial cancer. Int J Oncol. 2009;34(6):1541–1547. doi: 10.3892/ijo_00000283. [DOI] [PubMed] [Google Scholar]
- 35.Ewald J, Rodrigue CM, Mourra N, et al. Immunohistochemical staining for mismatch repair proteins, and its relevance in the diagnosis of hereditary non-polyposis colorectal cancer. Br J Surg. 2007;94(8):1020–1027. doi: 10.1002/bjs.5704. [DOI] [PubMed] [Google Scholar]
- 36.Buttin BM, Powell MA, Mutch DG, et al. Increased risk for hereditary nonpolyposis colorectal cancer-associated synchronous and metachronous malignancies in patients with microsatellite instability-positive endometrial carcinoma lacking MLH1 promoter methylation. Clin Cancer Res. 2004;10(2):481–490. doi: 10.1158/1078-0432.ccr-1110-03. [DOI] [PubMed] [Google Scholar]
- 37.Hampel H, Frankel W, Panescu J, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66(15):7810–7817. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- 38.Berends MJ, Wu Y, Sijmons RH, et al. Toward new strategies to select young endometrial cancer patients for mismatch repair gene mutation analysis. J Clin Oncol. 2003;21(23):4364–4370. doi: 10.1200/JCO.2003.04.094. [DOI] [PubMed] [Google Scholar]
- 39.Lu KH, Schorge JO, Rodabaugh KJ, et al. Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25(33):5158–5164. doi: 10.1200/JCO.2007.10.8597. [DOI] [PubMed] [Google Scholar]
- 40.Shannon C, Kirk J, Barnetson R, et al. Incidence of microsatellite instability in synchronous tumors of the ovary and endometrium. Clin Cancer Res. 2003;9(4):1387–1392. [PubMed] [Google Scholar]
- 41.Soliman PT, Broaddus RR, Schmeler KM, et al. Women with synchronous primary cancers of the endometrium and ovary: do they have Lynch syndrome? J Clin Oncol. 2005;23(36):9344–9350. doi: 10.1200/JCO.2005.03.5915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.