Summary
Hypoxia-inducible factor 1 (HIF-1) plays a key role in the reprogramming of cancer metabolism by activating transcription of genes encoding glucose transporters and glycolytic enzymes, which take up glucose and convert it to lactate; pyruvate dehydrogenase kinase 1, which shunts pyruvate away from the mitochondria; and BNIP3, which triggers selective mitochondrial autophagy. The shift from oxidative to glycolytic metabolism allows maintenance of redox homeostasis and cell survival under conditions of prolonged hypoxia. Many metabolic abnormalities in cancer cells increase HIF-1 activity. As a result, a feed-forward mechanism can be activated that drives HIF-1 activation and may promote tumor progression.
Introduction
Metastatic cancer is characterized by reprogramming of cellular metabolism leading to increased uptake of glucose for use as both an anabolic and catabolic substrate. Increased glucose uptake is such a reliable feature that it is utilized clinically to detect metastases by positron emission tomography using 18F-fluorodeoxyglucose (FDG-PET) with a sensitivity of ~90% [1]. As with all aspects of cancer biology, the details of metabolic reprogramming differ widely among individual tumors. However, the role of specific signaling pathways and transcription factors in this process is now understood in considerable detail. This review will focus on the involvement of hypoxia-inducible factor 1 (HIF-1) in both mediating metabolic reprogramming and responding to metabolic alterations. The placement of HIF-1 both upstream and downstream of cancer metabolism results in a feed-forward mechanism that may play a major role in the development of the invasive, metastatic, and lethal cancer phenotype.
O2 concentrations are significantly reduced in many human cancers compared to the surrounding normal tissue. The median PO2 in breast cancers is ~10 mm Hg, as compared to ~65 mm Hg in normal breast tissue [2]. Reduced O2 availability induces HIF-1, which regulates the transcription of hundreds of genes [3*,4*] that encode proteins involved in every aspect of cancer biology, including: cell immortalization and stem cell maintenance; genetic instability; glucose and energy metabolism; vascularization; autocrine growth factor signaling; invasion and metastasis; immune evasion; and resistance to chemotherapy and radiation therapy [5].
HIF-1 is a transcription factor that consists of an O2-regulated HIF-1α and a constitutively expressed HIF-1β subunit [6]. In well-oxygenated cells, HIF-1α is hydroxylated on proline residue 402 (Pro-402) and/or Pro-564 by prolyl hydroxylase domain protein 2 (PHD2), which uses O2 and α-ketoglutarate as substrates in a reaction that generates CO2 and succinate as byproducts [7]. Prolyl-hydroxylated HIF-1α is bound by the von Hippel-Lindau tumor suppressor protein (VHL), which recruits an E3-ubiquitin ligase that targets HIF-1α for proteasomal degradation (Figure 1A). Asparagine 803 in the transactivation domain is hydroxylated in well-oxygenated cells by factor inhibiting HIF-1 (FIH-1), which blocks the binding of the coactivators p300 and CBP [7]. Under hypoxic conditions, the prolyl and asparaginyl hydroxylation reactions are inhibited by substrate (O2) deprivation and/or the mitochondrial generation of reactive oxygen species (ROS), which may oxidize Fe(II) present in the catalytic center of the hydroxylases [8].
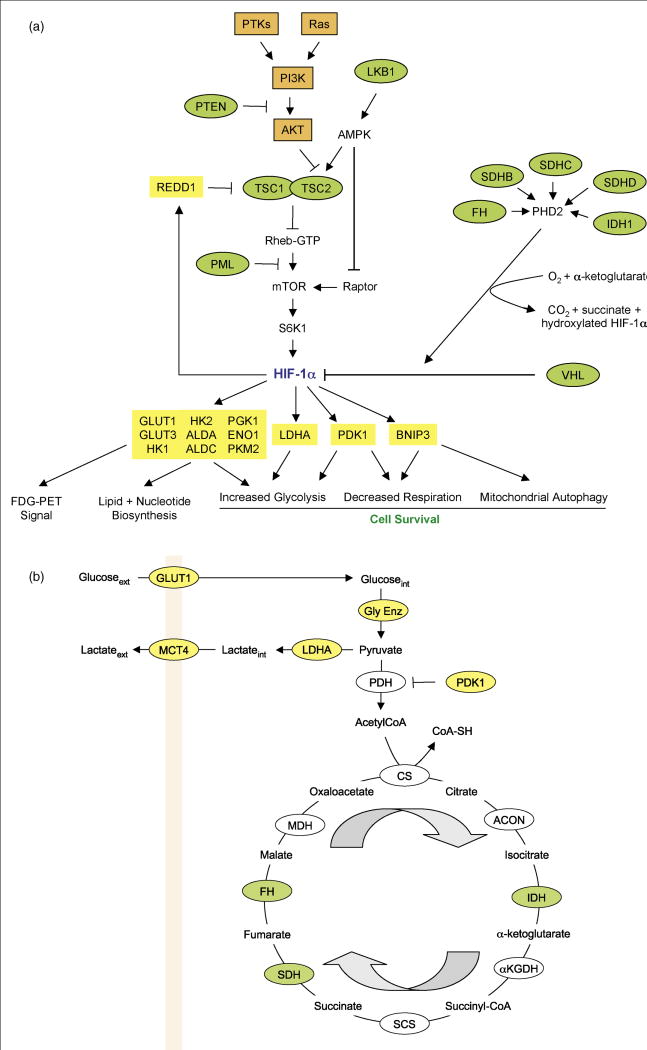
Figure 1.
HIF-1 and metabolism. (A) Regulation of HIF-1α protein synthesis and stability and HIF-1-dependent metabolic reprogramming. The rate of translation of HIF-1α mRNA into protein in cancer cells is dependent upon the activity of the mammalian target of rapamycin (mTOR), which is determined by the activity of upstream tumor suppressor proteins (green ovals) and oncoproteins (orange rectangles). Arrows indicate stimulation, blocked lines indicate inhibition. HIF-1α protein stability is regulated by O2- and α-ketoglutarate-dependent prolyl hydroxylation catalyzed by PHD2. Hydroxylation is required for the binding of the von Hippel-Lindau protein (VHL), which recruits a ubiquitin ligase that targets HIF-1α for proteasomal degradation. Loss of function for any of the tumor suppressor genes encoding fumarate hydratase (FH), isocitrate dehydrogenase (IDH), or succinate dehydrogenase (SDH) inhibits PHD2 activity. HIF-1α dimerizes with HIF-1β (not shown) and activates transcription of target genes encoding proteins (yellow rectangles) that play key roles in the metabolic reprogramming of cancer cells. Abbreviations: PTKs, protein tyrosine kinases; PI3K, phosphatidylinositol-3-kinase; S6K, ribosomal protein S6 kinase; ALD, aldolase; PGK, phosphoglycerate kinase; ENO, enolase; PKM, pyruvate kinase M. Other HIF-1-regulated glycolytic enzymes that are not shown: glucosephosphate isomerase, phosphofructokinase L, triosephosphate isomerase, glyceraldehyde-3-phosphate dehydrogenase, and phosphoglyceromutase. (B) Oxidative and glycolytic metabolism of glucose. HIF-1-regulated genes (yellow ovals) play key roles in converting extracellular glucose to extracellular lactate and blocking entry of pyruvate into the tricarboxylic acid cycle. Loss of function for each of the enzymes shown in green is associated with tumor formation and HIF-1α stabilization due to PHD2 inhibition by accumulation of the enzyme substrate. Arrows indicate the direction of the cycle. CS, citrate synthase; αKGDH, α-ketoglutarate dehydrogenase; SCS, succinyl-CoA synthetase; MDH, malate dehydrogenase.
The finding that acute changes in PO2 increase mitochondrial ROS production suggests that cellular respiration is optimized at physiological PO2 to limit ROS generation and that any deviation in PO2 -- up or down -- results in increased ROS generation. If hypoxia persists, induction of HIF-1 leads to adaptive mechanisms to reduce ROS and re-establish homeostasis, as described below. Prolyl and asparaginyl hydroxylation provide a molecular mechanism by which changes in cellular oxygenation can be transduced to the nucleus as changes in HIF-1 activity. This review will focus on recent advances in our understanding of the role of HIF-1 in controlling glucose and energy metabolism, but it should be appreciated that any increase in HIF-1 activity that leads to changes in cell metabolism will also affect many other critical aspects of cancer biology [5] that will not be addressed here.
HIF-1 target genes involved in glucose and energy metabolism
HIF-1 activates the transcription of SLC2A1 and SLC2A3, which encode the glucose transporters GLUT1 and GLUT3, respectively, as well as HK1 and HK2, which encode hexokinase, the first enzyme of the Embden-Meyerhoff (glycolytic) pathway [9]. Once taken up by GLUT and phosphorylated by HK, FDG cannot be metabolized further; thus, FDG-PET signal is determined by FDG delivery to tissue (i.e. perfusion) and GLUT/HK expression/activity. Unlike FDG, glucose is further metabolized to pyruvate by the action of the glycolytic enzymes, which are all encoded by HIF-1 target genes (Figure 1A). Glycolytic intermediates are also utilized for nucleotide and lipid synthesis [10]. Lactate dehydrogenase A (LDHA), which converts pyruvate to lactate, and monocarboxylate transporter 4 (MCT4), which transports lactate out of the cell (Figure 1B), are also regulated by HIF-1 [9,11]. Remarkably, lactate produced by hypoxic cancer cells can be taken up by non-hypoxic cells and used as a respiratory substrate [12**].
Pyruvate represents a critical metabolic control point, as it can be converted to acetyl coenzyme A (AcCoA) by pyruvate dehydrogenase (PDH) for entry into the tricarboxylic acid (TCA) cycle or it can be converted to lactate by LDHA (Figure 1B). Pyruvate dehydrogenase kinase (PDK), which phosphorylates and inactivates the catalytic domain of PDH, is encoded by four genes and PDK1 is activated by HIF-1 [13,14]. (Further studies are required to determine whether PDK2, PDK3, or PDK4 is regulated by HIF-1.) As a result of PDK1 activation, pyruvate is actively shunted away from the mitochondria, which reduces flux through the TCA cycle, thereby reducing delivery of NADH and FADH2 to the electron transport chain. This is a critical adaptive response to hypoxia, because in HIF-1α–null mouse embryo fibroblasts (MEFs), PDK1 expression is not induced by hypoxia and the cells die due to excess ROS production, which can be ameliorated by forced expression of PDK1 [13]. MYC, which is activated in ~40% of human cancers, cooperates with HIF-1 to activate transcription of PDK1, thereby amplifying the hypoxic response [15]. Pharmacological inhibition of HIF-1 or PDK1 activity increases O2 consumption by cancer cells and increases the efficacy of a hypoxia-specific cytotoxin [16].
HIF-1 also activates transcription of the gene encoding the BH3 domain protein BNIP3 (Figure 1A), which induces selective mitochondrial autophagy by competing with Beclin 1 for binding to Bcl2, thereby freeing Beclin 1 to trigger autophagy [17**]. BNIP3-induced autophagy was originally associated with hypoxic cell death [18], but studies of HIF-1α-null MEFs have revealed that mitochondrial autophagy is an adaptive response that maintains cell viability under conditions of prolonged hypoxia [17**]. MEFs exposed to 1% O2 reduce their mitochondrial mass by >50% within 48 hours. Hypoxic HIF-1α-null MEFs, which died due to excess ROS production, were rescued by treatment with superoxide scavenger or by forced expression of BNIP3 or PDK1 [17**]. Hypoxia induced mitochondrial autophagy in wild type but not in HIF-1α-null MEFs and knockdown of BNIP3, Beclin 1, or Atg5 expression also resulted in ROS-induced cell death, demonstrating that autophagy triggered by HIF-1-dependent BNIP3 expression is required for cell survival under conditions of prolonged hypoxia [13,17**].
HIF-1α-null MEFs exposed to 1% O2 for 48 hours have higher ATP levels than do wild type MEFs cultured at 20% O2, indicating that O2 is not limiting for ATP production under these conditions [17**]. Maintenance of respiration in hypoxic HIF-1α-null MEFs is associated with the production of toxic levels of ROS. HIF-1 appears to play a critical homeostatic role in managing O2 consumption to balance ATP and ROS production. This is illustrated by the recent finding that HIF-1 orchestrates a subunit switch in cytochrome c oxidase (complex IV) that optimizes the efficiency of respiration based on the cellular O2 concentration [20].
Hypoxia also induces mitochondrial autophagy in many human cancer cell lines through HIF-1-dependent expression of BNIP3 and a related BH3 domain protein, BNIP3L [19**]. Autocrine signaling through the platelet-derived growth factor receptor in cancer cells increases HIF-1 activity and thereby increases autophagy and cell survival under hypoxic conditions [21]. Autophagy may also occur in a HIF-1-independent manner in response to other physiological stimuli that are associated with hypoxic conditions, such as a decrease in the cellular ATP:AMP ratio, which activates AMP kinase signaling [22].
In clear cell renal carcinoma, VHL loss of function (LoF) results in constitutive HIF-1 activation, which is associated with impaired mitochondrial biogenesis that results from HIF-1-dependent expression of MXI1, which blocks MYC-dependent expression of PGC-1β, a coactivator that is required for mitochondrial biogenesis [23]. Inhibition of wild type MYC activity in renal cell carcinoma contrasts with the synergistic effect of HIF-1 and oncogenic MYC in activating PDK1 transcription [24].
Genetic and metabolic activators of HIF-1
Hypoxia plays a critical role in cancer progression [2,5] but not all cancer cells are hypoxic and a growing number of O2-independent mechanisms have been identified by which HIF-1 is induced [5]. Several mechanisms that are particularly relevant to cancer metabolism are described below.
Activation of mTOR
LoF for any of five different tumor suppressors -- LKB1 [25**], PML [26], PTEN [27,28], and TSC1/TSC2 [29]-- induces HIF-1 expression by disregulation of the mammalian target of rapamycin (mTOR), which can also be activated by gain of function affecting protein tyrosine kinases (e.g. epidermal growth factor receptor [27], HER2neu [30]), Ras, and/or the downstream phosphotidylinositol-3-kinase/AKT pathway (Figure 1A). mTOR is a serine-threonine protein kinase that phosphorylates ribosomal protein S6 kinase and eIF-4E binding protein 1, which increases the rate of translation of HIF-1α mRNA into protein [30]. LKB1, which is mutated in the Peutz-Jeghers intestinal hamartoma syndrome, is the upstream activator of AMP kinase, which activates TSC2 and inactivates Raptor, thereby inhibiting mTOR activity by two mechanisms (Figure 1A). Analysis of LKB1-deficient mice revealed a dramatic upregulation of HIF-1, GLUT1, and HK2 in gastrointestinal polyps, which were strongly positive by FDG-PET imaging; all of these findings were abolished after treatment with rapamycin [25**]. Expression of constitutively active AKT in prostatic epithelium also induced neoplasia and the HIF-1 metabolic transcriptome in a mTOR-dependent manner [31].
Alterations in mitochondrial metabolism
Recent studies have revealed that that tumor suppressor genes mutated in hereditary paraganglioma, hereditary leiomyomatosis/renal carcinoma, and secondary glioblastoma encode enzymes of the mitochondrial TCA cycle: succinate dehydrogenase (SDH), fumarate hydratase (FH), and isocitrate dehydrogenase (IDH) (Figure 1A). SDH and FH LoF lead to increased levels of the metabolic substrate of the enzyme – succinate and fumarate, respectively (Figure 1B), which inhibit PHD2 by competing with α-ketoglutarate for binding to the catalytic center, thereby reducing hydroxylation, ubiquitination, and proteasomal degradation of HIF-1α [32–35]. In contrast, IDH LoF leads to decreased levels of the reaction product, a-ketoglutarate (Figure 1B), which is a necessary substrate for PHD2 [36**].
Mutations in the gene encoding the ND2 subunit of respiratory complex I have been reported to increase ROS levels and thereby increase HIF-1α levels in head and neck squamous cell carcinoma [37]. Administration of antioxidants, such as ascorbate or N-acetylcysteine, has been shown to dramatically reduce tumor xenograft growth by inhibiting HIF-1α levels [38]. In ND2-deficient cells, expression of PDK2 was increased and administration of the PDK inhibitor dichloroacetate decreased HIF-1α levels. This result is consistent with previous studies demonstrating that both pyruvate and lactate (whose levels increase in the presence of PDK activity) induce HIF-1α expression [39**]. These results suggest the existence of a feed-forward mechanism, in which induction of HIF-1 leads to PDK activity, elevated pyruvate/lactate, and further increases in HIF-1 activity.
NAD+ levels
In addition to HIF-1α, the PHD2-VHL pathway also regulates HIF-2α, which dimerizes with HIF-1β and activates target gene expression [40]. Increased levels of both HIF-1α and HIF-2α are observed in many human cancers and associated with increased patient mortality [5]. Studies in mice suggest that HIF-2α may regulate SOD2 and other genes encoding antioxidant proteins [41]. Thus, whereas HIF-1α inhibits oxidant generation, HIF-2α promotes antioxidant generation. It is therefore of interest that the NAD+-dependent deacetylase sirtuin 1 (SIRT1) was found to bind to, deacetylate, and increase transcriptional activation by HIF-2α but not HIF-1α [42**]. Another NAD+-dependent enzyme is poly(ADP-ribose) polymerase 1 (PARP1), which was recently shown to bind to HIF-1α and promote transactivation through a mechanism that required the enzymatic activity of PARP1 [43]. Thus, transactivation mediated by both HIF-1α and HIF-2α can be modulated according to NAD+ levels. Further studies are required to understand the significance of these novel findings in the context of cancer cell metabolism.
Nitric oxide
Increased expression of nitric oxide (NO) synthase isoforms and increased levels of NO have been shown to increase HIF-1α protein stability in human oral squamous cell carcinoma [44]. In prostate cancer, nuclear co-localization of endothelial NO synthase, estrogen receptor β, HIF-1α, and HIF-2α was associated with aggressive disease and the proteins were found to form chromatin complexes on the promoter of TERT gene encoding telomerase [45**]. The NOS2 gene encoding inducible NO synthase is HIF-1 regulated [5], suggesting another possible feed-forward mechanism.
Acknowledgments
Work in the author’s laboratory is supported by grants from the National Cancer Institute, National Heart, Lung, and Blood Institute, National Institute of General Medical Sciences, and the Johns Hopkins Institute for Cell Engineering. G.L.S. is the C. Michael Armstrong Professor at The Johns Hopkins University School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 2.Vaupel P, Mayer A, Hockel M. Tumor hypoxia and malignant progression. Methods Enzymol. 2004;381:335–354. doi: 10.1016/S0076-6879(04)81023-1. [DOI] [PubMed] [Google Scholar]
- 3*.Mole DR, Blancher C, Copley RR, Pollard PJ, Gleadle JM, Ragoussis J, Ratcliffe PJ. Genome-wide association of hypoxia-inducible factor (HIF)-1α and HIF-2α DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem. 2009;284:16767–16775. doi: 10.1074/jbc.M901790200. The authors of this paper and ref. [4] performed ChIP-chip (chromatin immunoprecipitation and mRNA microarray) analysis to identify direct target genes of HIF-1 and HIF-2. The majority of hypoxia-induced genes contained HIF-1 binding sites and expression was dependent on HIF-1α expression. HIF-2α bound to many of the same genes, but knockdown of HIF-2α levels did not affect gene expression. Hypoxia-repressed gene expression was also HIF-1α-dependent but did not involve direct HIF-1α binding to target genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4*.Xia X, Lemieux ME, Li W, Carroll JS, Brown M, Liu XS, Kung AL. Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Proc Natl Acad Sci USA. 2009;106:4260–4265. doi: 10.1073/pnas.0810067106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. doi: 10.1038/onc.2009.441. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 9.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 α. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1α-dependent mechanism. J Biol Chem. 2006;281:9030–9037. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 12**.Sonveaux P, Végran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. Tumors contain highly oxygenated (aerobic) and poorly oxygenated (hypoxic) regions, which were thought to utilize glucose for oxidative and glycolytic metabolism, respectively. Sonveaux et al. show that human cancer cells cultured under hypoxic conditions convert glucose to lactate and extrude it (via MCT4), whereas aerobic cancer cells take up lactate (via MCT1) and utilize it for oxidative phosphorylation. When MCT1 is inhibited, aerobic cancer cells take up glucose rather than lactate, and hypoxic cancer cells die due to glucose deprivation. Treatment of tumor-bearing mice with an inhibitor of MCT1 retarded tumor growth. MCT1 expression was detected exclusively in non-hypoxic regions of human cancer biopsy samples, and in combination, these data suggest that MCT1 inhibition holds potential as a novel cancer therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Kim JW, Gao P, Liu YC, Semenza GL, Dang CV. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol. 2007;27:7381–7393. doi: 10.1128/MCB.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cairns RA, Papandreou I, Sutphin PD, Denko NC. Metabolic targeting of hypoxia and HIF1 in solid tumors can enhance cytotoxic chemotherapy. Proc Natl Acad Sci USA. 2007;104:9445–9450. doi: 10.1073/pnas.0611662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. HIF-1 induces BNIP3, which triggers mitochondrial-selective autophagy as a means of reducing ROS generation under hypoxic conditions. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, Macleod KF. BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol. 2007;27:6229–6242. doi: 10.1128/MCB.02246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouysségur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. The authors show in several cancer cell lines HIF-1 that induces autophagy via expression of both BNIP3 and BNIP3L and, in these cells, knockdown of both proteins was required to block hypoxia-induced autophagy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson S, O’Prey J, Fricker M, Ryan KM. Hypoxia-selective macroautophagy and cell survival signaled by autocrine PDGFR activity. Genes Dev. 2009;23:1283–1288. doi: 10.1101/gad.521709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papandreou I, Lim AL, Laderoute K, Denko NC. Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF-1, BNIP3, and BNIP3L. Cell Death Differ. 2008;15:1572–1581. doi: 10.1038/cdd.2008.84. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Dang CV, Kim JW, Gao P, Yustein J. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8:51–56. doi: 10.1038/nrc2274. [DOI] [PubMed] [Google Scholar]
- 25**.Shackelford DB, Vasquez DS, Corbeil J, Wu S, Leblanc M, Wu CL, Vera DR, Shaw RJ. mTOR and HIF-1α-mediated tumor metabolism in an LKB1 mouse model of Peutz-Jeghers syndrome. Proc Natl Acad Sci USA. 2009;106:11137–11142. doi: 10.1073/pnas.0900465106. The authors connect LKBI loss of function with mTOR and HIF-1 gain of function, glucose uptake and gastrointestinal neoplasia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernardi R, Guernah I, Jin D, Grisendi S, Alimonti A, Teruya-Feldstein J, Cordon-Cardo C, Simon MC, Rafii S, Pandolfi PP. PML inhibits HIF-1α translation and neoangiogenesis through repression of mTOR. Nature. 2006;442:779–785. doi: 10.1038/nature05029. [DOI] [PubMed] [Google Scholar]
- 27.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1 expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 28.Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, Gottschalk AR, Ryan HE, Johnson RS, Jefferson AB, et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000;14:391–396. [PMC free article] [PubMed] [Google Scholar]
- 29.Brugarolas J, Kaelin WG., Jr Dysregulation of HIF and VEGF is a unifying feature of the familial hamartoma syndromes. Cancer Cell. 2004;6:7–10. doi: 10.1016/j.ccr.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 30.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1α (HIF-1α) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 32.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 33.Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, Merino M, Trepel J, Zbar B, Toro J, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–153. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Koivunen P, Hirsilä M, Remes AM, Hassinen IE, Kivirikko KI, Myllyharju J. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J Biol Chem. 2007;282:4524–4532. doi: 10.1074/jbc.M610415200. [DOI] [PubMed] [Google Scholar]
- 35.Hewitson KS, Liénard BM, McDonough MA, Clifton IJ, Butler D, Soares AS, Oldham NJ, McNeill LA, Schofield CJ. Structural and mechanistic studies on the inhibition of the hypoxia-inducible transcription factor hydroxylases by tricarboxylic acid cycle intermediates. J Biol Chem. 2007;282:3293–3301. doi: 10.1074/jbc.M608337200. [DOI] [PubMed] [Google Scholar]
- 36**.Zhao S, Lin Y, Xu W, Jiang W, Zha Z, Wang P, Yu W, Li Z, Gong L, Peng Y, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1α. Science. 2009;324:261–265. doi: 10.1126/science.1170944. Missense mutations in the IDH1 gene occur in secondary glioblastoma and impair the enzyme’s affinity for its substrate. Forced expression of mutant IDH1 in cultured cells reduces formation of the enzyme product, α-ketoglutarate, and thereby increases HIF-1α levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun W, Zhou S, Chang SS, McFate T, Verma A, Califano JA. Mitochondrial mutations contribute to HIF-1α accumulation via increased reactive oxygen species and up-regulated pyruvate dehydrogenase kinase 2 in head and neck squamous cell carcinoma. Clin Cancer Res. 2009;15:476–484. doi: 10.1158/1078-0432.CCR-08-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, Bhujwalla ZM, Felsher DW, Cheng L, Pevsner J, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12:230–238. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.McFate T, Mohyeldin A, Lu H, Thakar J, Henriques J, Halim ND, Wu H, Schell MJ, Tsang TM, Teahan O, et al. Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J Biol Chem. 2008;283:22700–22708. doi: 10.1074/jbc.M801765200. The authors provide evidence of a feed-forward mechanism in which increased HIF-1 activity induces expression of PDK1, which alters levels of glycolytic metabolites, which in turn further increase HIF-1 activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qing G, Simon MC. Hypoxia inducible factor-2α: a critical mediator of aggressive tumor phenotypes. Curr Opin Genet Dev. 2009;19:60–66. doi: 10.1016/j.gde.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet. 2003;35:331–340. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 42**.Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. ** Regulation of hypoxia-inducible factor 2α signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. The authors show that SIRT1 is a positive regulator of HIF-2α. It appears that SIRT1 can have oncogenic or tumor suppressor effects, depending upon the particular context [46], as is also true for HIF-2α [47] [DOI] [PubMed] [Google Scholar]
- 43.Elser M, Borsig L, Hassa PO, Erener S, Messner S, Valovka T, Keller S, Gassmann M, Hottiger MO. Poly(ADP-ribose) polymerase 1 promotes tumor cell survival by coactivating hypoxia-inducible factor-1-dependent gene expression. Mol Cancer Res. 2008;6:282–290. doi: 10.1158/1541-7786.MCR-07-0377. [DOI] [PubMed] [Google Scholar]
- 44.Quintero M, Brennan PA, Thomas GJ, Moncada S. Nitric oxide is a factor in the stabilization of hypoxia-inducible factor-1 in cancer: role of free radical formation. Cancer Res. 2006;66:770–774. doi: 10.1158/0008-5472.CAN-05-0333. [DOI] [PubMed] [Google Scholar]
- 45**.Nanni S, Benvenuti V, Grasselli A, Priolo C, Aiello A, Mattiussi S, Colussi C, Lirangi V, Illi B, D’Eletto M, et al. Endothelial NOS, estrogen receptor β, and HIFs cooperate in the activation of a prognostic transcriptional pattern in aggressive human prostate cancer. J Clin Invest. 2009;119:1093–1108. doi: 10.1172/JCI35079. The authors demonstrate that overexpressed and nuclear-localized endothelial NOS promotes the transcriptional activity of ER-β, HIF-1α, and HIF-2α, which cooperate to activate transcription of genes associated with poor prognosis in prostate cancer. The study is an elegant blend of clinical and molecular approaches. The immunohistochemical and gene expression analyses described in this study could be used to identify prostate cancer patients who might be particularly likely to benefit from treatment with NOS and/or HIF-1 inhibitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boily G, He XH, Pearce B, Jardine K, McBurney MW. SirT1-null mice develop tumors at normal rates but are poorly protected by resveratrol. Oncogene. 2009;28:2882–2893. doi: 10.1038/onc.2009.147. [DOI] [PubMed] [Google Scholar]
- 47.Imamura T, Kikuchi H, Herraiz MT, Park DY, Mizukami Y, Mino-Kenduson M, Lynch MP, Rueda BR, Benita Y, Xavier RJ, et al. HIF-1α and HIF-2α have divergent roles in colon cancer. Int J Cancer. 2009;124:763–771. doi: 10.1002/ijc.24032. [DOI] [PMC free article] [PubMed] [Google Scholar]