Abstract
Function of the eukaryotic genome depends on efficient and accurate replication of anti-parallel DNA strands. Eukaryotic DNA polymerases have different properties adapted to perform a wide spectrum of DNA transactions. Here we focus on major players in the bulk replication, DNA polymerases of the B-family. We review the organization of the replication fork in eukaryotes in a historical perspective, analyze contemporary models and propose a new integrative model of the fork.
Keywords: replication fork, DNA polymerases, replication origins
1. The hypothesis of three DNA polymerases (Pols) at the fork
The need for a model accommodating three DNA polymerases at the fork first arose in 1990 when the list of eukaryotic DNA polymerases expanded to include the third B-family enzyme, Pol ε. Two papers important for the DNA replication field were simultaneously published that year. Three distinct DNA Pols (α, δ and ε) from HeLa cells have been characterized in Stuart Linn’s laboratory [1]. Akio Sugino’s laboratory described the POL2 gene encoding for the third DNA Pol (Pol ε) essential for DNA replication in yeast [2]. Based on the biochemical properties of the three replicative Pols known at that time, Morrison and co-authors proposed a novel model of the replication fork in eukaryotes, where each Pol had a specialized role (Fig. 1A). In this model, Pol α, the only Pol possessing the primase activity, initiates DNA synthesis at origins and primes Okazaki fragments on the lagging strand. Pol δ replicates the rest of the lagging strand, and Pol ε replicates the leading DNA strand. This assignment was suggested largely because Pol δ is conditionally processive depending on the presence of the proliferating cell nuclear antigen (PCNA), and Pol ε was believed to have a high intrinsic processivity (but see [3] and discussion below). Almost two decades later, this model was re-introduced as proven for eukaryotes (Fig. 1B)[4,5]. Indeed, it gained cumulative biochemical and, especially, genetic support, which was considered compelling. Now it is a predominant view on the eukaryotic fork. In the current review, we analyze evidence in favor of the three-Pol model of the fork and examine if all of the available experimental data could be explained by this model. We came to the conclusion that the role of Pol δ may be underestimated in the current model. In addition, a role is emerging for the fourth Pol of the B-family, Pol ζ, as an accessory polymerase in chromosomal DNA replication. We present a new version of Sugino’s fork model that takes into account both the data consistent with and the data in conflict with the original model.
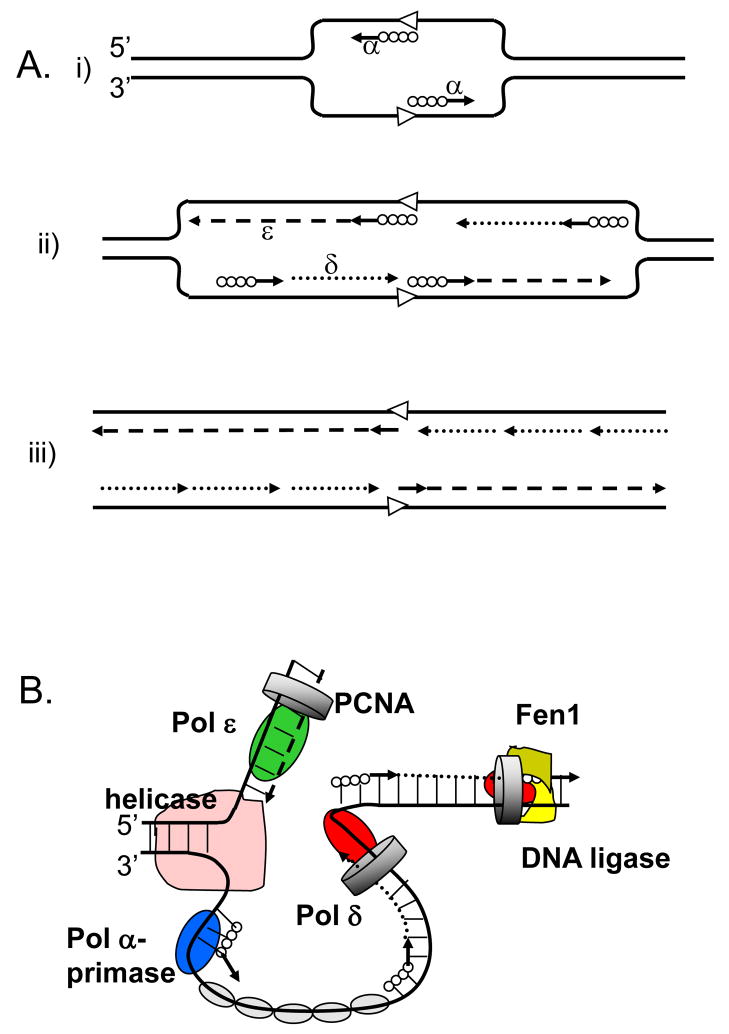
Figure 1. Three-polymerase model of replication fork in eukaryotes.
A. The model proposed in 1990 by Sugino group (adapted from [2].
(i) Pol α synthesizes short DNA segments (straight lines) primed by RNA (solid circles) at the replication origin (open arrowheads). (ii) Pol ε synthesizes the leading strand (dashed line); Pol α synthesizes short RNA-DNA stretches on the lagging strand, which are subsequently extended by Pol δ. (iii) After removal of RNA primers, Pol δ completes the lagging strand synthesis (dotted lines).
B. The currently accepted model (adapted from [85]).
The model illustrates primary roles for Pol ε (green oval) in leading DNA strand replication (dashed line) and Pol δ (red oval) in lagging strand replication (dotted line). Other proteins shown include the Pol α-primase (blue oval) synthesizing RNA-DNA hybrids (solid circles and straight lines), the MCM helicase (pink), the eukaryotic single-stranded-DNA-binding protein, replication protein A (RPA; gray ovals), the sliding clamp proliferating cell nuclear antigen (PCNA; gray ring) and the Fen1–DNA ligase complex (khaki-yellow).
2. Introduction to the structure of the Pol players at the fork
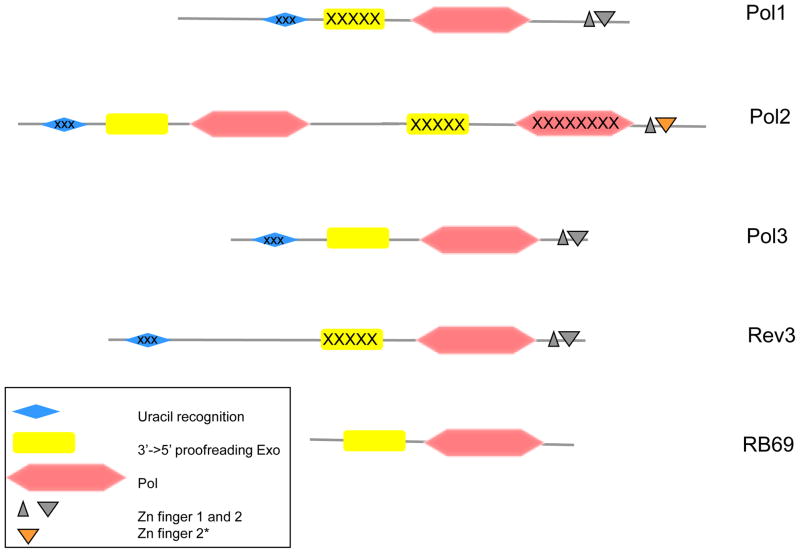
In this section, we briefly describe the four B-family Pols that are critical for the DNA replication fork in our model. DNA Pols α, ε, δ and ζ all belong to the B-family. Their catalytic subunits, called in yeast Pol1, Pol2, Pol3 and Rev3, respectively, possess the same general domain arrangement on the primary amino acid sequence (Fig 2; [6,7]). In addition to the polymerase domain, all Pols have conserved 3′→5′ exonuclease domains. Pol α and ζ, however, lack exonuclease activity, because the sequence of catalytic motifs in the Exo domains is destroyed. All Pols possess remnants of the uracil recognizing domain but do not sense uracil like their archael homologs [8]. The C-terminal end of Pols has two Zn-finger domains critical for the assembly of the holoenzymes [9–11]. The size of the catalytic subunits widely varies due to the presence of additional N-terminal (in Rev3 and Pol1) or C-terminal extensions (in Pol2 and Pol1), whose structure and roles are mainly unknown. A recent breakthrough was the discovery that the catalytic subunit of Pol ε is a fusion of two distinct, active and inactive, Pols of the B family [7]. Included in Fig. 2 for comparison is the schematic outline of phage RB69 DNA polymerase, whose crystal structure is often used to model eukaryotic DNA polymerases. It obviously lacks the complexity of the eukaryotic enzymes.
Figure 2. A schematic diagram of conserved blocks of the four eukaryotic B-family DNA polymerases in comparison with the phage RB69 DNA polymerase.
The drawing is based on the amino acid sequences of the catalytic subunits of yeast Pols and RB69 Pol and is roughly to scale. The inactivated C-terminal domains of Pol2, uracil-recognizing domains and exonuclease domains of Pol1 and Rev3 are marked by “X” symbols. Zn-finger 2* denotes the distinct version of this module in Pol2 that is highly similar to the Zn-finger of archaeal PolD [7].
Functional B-family Pols in eukaryotes are multi-subunit complexes. Pol α is a four-subunit complex [12]. All four subunits are essential. The largest subunit is a catalytic polypeptide capable of accurate and robust but low-processivity DNA synthesis [13,14]. The current model, based partially on crystallography of yeast Pol α fragments, partially on low-resolution electron microscopy (EM) images, suggests that one larger domain has all the structural elements required for the DNA polymerase reaction and is connected by a flexible linker to the C-terminal Zn-finger domain responsible for interactions with the other subunits [11]. The smallest polypeptide in the four-subunit complex is the catalytic primase subunit. It is tightly associated with the larger accessory primase subunit that, in turn, interacts with p166. The second largest subunit has a unique iron-sulfur domain essential for the priming reaction in addition to the primase catalytic subunit and is also responsible for the association with the origin recognition complex [15,16].
Pol δ is a complex of four (three in budding yeast) polypeptides [17–19]. The largest catalytic subunit has DNA polymerase and 3′→5′ exonuclease active sites, as well as sites for protein-protein interactions [20,21] and a PCNA binding motif [19]. The essential second subunit serves as a stabilizer for the catalytic subunit and as a matchmaker with the third subunit. In yeast, mutations abolishing interactions between the second and third subunit phenocopy the deletion of the third subunit gene [21]. The third subunit plays several important roles. It has a conserved PCNA-binding motif and a motif that mediates interaction with Pol α [22–24]. However, the corresponding gene, POL32, is dispensable for growth in budding yeast [19]. Deletion of this gene renders yeast unable to undergo ultraviolet (UV)-induced mutagenesis, similar to deletions of REV1 and REV3 genes encoding for translesion synthesis (TLS) polymerases. This suggests a role of the Pol32 protein in the regulation of error-prone TLS [6,25,26]. Pol32 interacts with Rev1 and can recruit Pol ζ via this interaction, which could explain the role of this subunit in induced mutagenesis ([27]; see also discussion below). The role of the fourth subunit is enigmatic. The deletion does not result in noticeable phenotypes in fission yeast [28], while the experiments with human enzyme suggested that it plays a regulatory role in Pol δ response to DNA damage [29].
Pol ε is a four-subunit complex [30]. A low-resolution cryo-EM structure of the yeast complex is available [31]. The largest catalytic subunit has robust DNA polymerase and proofreading exonuclease activity. The second subunit mediates all protein-protein interactions within the holoenzyme and is essential. Mutations that weaken these interactions confer a mutator phenotype [32,33]. The third and fourth subunits are involved in the interaction with double-stranded DNA [31,34] but are not required for growth, although their absence results in an elevation of spontaneous mutagenesis [35,36]. It has also been shown that the fourth subunit is involved in chromatin remodeling [37]. Pol ε is additionally regulated by multiple accessory factors involved in origin recognition [30,38,39].
Pol ζ has been isolated from yeast in the active form as a two-subunit complex [40]. A heterodimer of the Rev3 protein and the second subunit encoded by the REV7 gene has DNA polymerase activity and is uniquely proficient in the extension of mismatched primer termini [25,26]. The genetic data suggest that the role of Pol ζ as a key player in TLS is conserved between yeast and humans [41,42]. In addition, the human homolog of the second subunit of the yeast enzyme may have an additional role in regulating cell cycle progression. The human REV7 shows similarity to the spindle checkpoint protein MAD2 and was reported to interact with MAD2 in vitro [43].
Evolutionary scenarios that led to the creation of the four polymerases are very complex [7,44]. For example, in addition to the fusion of two Pols to generate Pol as discussed previously, the second subunits (so-called B-subunits) of Pol α, Pol δ and Pol ε share some similarity and have homologs in Archaea [45,46]. In Archaea, these polypeptides have 3′->5′ exonuclease motifs and are subunits of a so-called D family of DNA polymerases [47]. Changes of critical catalytic residues in the phosphodiesterase motifs in the eukaryotic orthologs of B-subunits renders them inactive as nucleases. A crystal structure of the B subunit of human Pol δ complexed with a part of the third subunit reveals an OB-fold DNA binding domain and a surface for interaction with the other subunits [21]. The Zn-finger domain history is also non-trivial, involving duplication and the use of different ancestors for Pol2 and the other B-family Pols [7].
3. Lessons from simple organisms
The asymmetric nature of DNA poses topological problems for replication of the anti-parallel strands by a fork moving in one direction, so the replication of the two strands is inherently different [48]. In simple DNA replication systems, such as bacteriophage T4, one B-family DNA polymerase is sufficient for rapid synthesis of leading and lagging strands [49]. In the bacterium Escherichia coli, the fork is managed by a dimeric or even trimeric DNA Pol III [50,51]. Some bacterial species, like Bacillus subtilis, utilize two separate Pols for the leading and lagging DNA strands [52]. Replication of the mammalian virus SV40 requires only primase-associated Pol α and Pol δ for synthesis of both strands [53]. Even when the same Pol replicates both DNA strands, the accuracy of lagging strand synthesis is up to 10-fold higher than that of the leading DNA strand [54].
4. Early insights into the roles of Pol δ and Pol ε
The first attempt to solve the puzzle of three polymerases at the fork was undertaken in the early 1990s by Alan Morrison in Sugino’s lab at NIEHS. Morrison and co-authors generated Exo− derivatives of Pol ε and Pol δ by altering the conserved ExoI motif FDIE (mutations pol2-4 and pol3-01, respectively;[55,56]). The corresponding Pols are inaccurate because they can not proofread replication errors. The phenotypes of the corresponding yeast mutants are summarized in Table 1. The studies of the proofreading-deficient mutants provided the following information.
Table 1.
Severity of the mutator effects of mutations affecting the fidelity of yeast Pol α, δ, ε and ζ*.
| Allelle | Pol defect | Alone | With defect of Pol ε Exo | With defect of Pol δ Exo | With defect of MMR |
|---|---|---|---|---|---|
| pol1-L868M | base selection | very weak | weak, additivity | strong, synergy | very strong, synergy |
| pol2-M644G | base selection | weak | very strong, synergy | very strong, synergy | very strong, synergy |
| pol3-L612M | base selection | weak | weak, additivity | catastrophic**, synergy | very strong, synergy |
| rev3-L979F | base selection | very weak | weak, additivity | weak, additivity | weak, additivity |
| pol2-4 | proofreading | moderate | n/a | catastrophic**, synergy y | very strong, synergy |
| pol3-01 | proofreading | very strong | catastrophic**, synergy | n/a | catastrophic**, synergy nergy |
| pol3-5DV*** | proofreading | strong | catastrophic**, synergy rgy | n/a | catastrophic**, synergy nergy |
The pol2-4 and pol3-01 mutations conferred a moderate and strong mutator effect, respectively. This was consistent with the involvement of both Pols in chromosomal DNA replication. The substantially more severe mutator effect of pol3-01, however, was not expected from the “one strand – one DNA polymerase” model. Although various explanations were discussed (for example, [57–59]), the difference between the phenotypes of pol3-01 and pol2-4 mutants remains somewhat puzzling.
A combination of each of the pol3-01 and pol2-4 mutations with a DNA mismatch repair (MMR) defect (pms1 mutation) resulted in a synergistic increase in mutation rates (Table 1, [56,60]). This indicated that the proofreading activities of Pol ε and Pol δ act in series with the MMR system, providing further evidence for the involvement of these activities in chromosomal replication. In accordance with the stronger effect of the pol3-01 allele in MMR-proficient strains, the combination of pol3-01 and pms1 had more dramatic consequences than the combination of pol2-4 and pms1. The double pol3-01 pms1 mutation was lethal in haploid cells because of an extremely high level of spontaneous mutagenesis. The synergistic interaction of the two mutations was established by studying the diploid strains that can tolerate a higher mutation rate [56]. In contrast, the mutation rate in the pol2-4 pms1 strains, although quite high, was still below the level that would result in lethality in haploid cells, so the haploid double mutants survived [60]. This, again, raised a question of whether Pol ε and Pol δ copy equal portions of the genome.
A combination of pol3-01 and pol2-4 mutations resulted in a synergistic increase in the mutation rate that was, similar to the pol3-01 pms1 combination, incompatible with life in haploid cells [60]. Note that if the two Pols worked on different DNA strands, the interaction of the mutator effects of pol3-01 and pol2-4 would be expected to be simply additive. The synergistic interaction was interpreted as an indication of competition of the two exonucleases for the same pool of replication errors. An alternative explanation for the excessively high mutation rate in the pol3-01 pol2-4 strain is that MMR system becomes saturated when the two proofreading defects are added up [60]. This, however, seems unlikely, because the mutator effect of pol2-4 is small in comparison to the pol3-01 effect. If the uncorrected errors resulting from the two proofreading defects simply sum up, the effect of the double pol3-01 pol2-4 mutation would not be significantly different from the effect of pol3-01 alone, and MMR is clearly not saturated in the pol3-01 mutants [56]. As we discuss further below, the synergistic interaction of Pol ε and Pol δ proofreading defects is the observation that is most difficult to reconcile with the model wherein these two Pols replicate opposite DNA strands.
To address the question of whether Pol δ and Pol ε work on the opposite strands more specifically, Morrison and Sugino analyzed the spectra of spontaneous mutations arising in the pol3-01 and pol2-4 mutants using two different orientations of the reporter gene in respect to the nearest origin of replication [60]. The authors reasoned that if Pol δ synthesized both DNA strands of this reporter gene, the spectra of mutations generated in the proofreading-deficient mutant should be similar in the two orientations. A limited number of mutants sequenced indicated that this simple prediction is not completely met and there might be some strand specificity of Pol involvement at the fork.
Although not sufficient to confirm or refute the Sugino lab model, these experiments provided the foundation for those that followed. Particularly useful was the development of the theoretical basis for the analysis of synergistic interactions of mutation rates in yeast and the use of the mutational spectra analysis to decipher the role of DNA polymerases at the fork. As described in the following sections, this approach appeared to be most instrumental in shaping our current understanding of the eukaryotic replication fork.
5. Current evidence supporting Sugino’s lab model
Substantial evidence for Sugino’s model was obtained by studying the mutational spectra in yeast strains carrying inaccurate variants of replicative Pols. This approach is based on the assumption that finding a mutation that represents the specific “signature” of a particular Pol reveals the participation of this Pol in the copying of this region of the genome. The main obstacle in the analysis of spontaneous mutational spectrum in vivo is the difficulty of assigning the strand where the mistake was made. For example, an A•T to G•C transition could result from an initial C incorporation opposite A or a T incorporation opposite G in the complementary DNA strand. One approach to solve this problem was the use of a purine base analog. The strand, into which such an analog is incorporated at each particular site, is unambiguously defined by the orientation of the purine•pyrimidine pair in the DNA. When the base analog-induced mutagenesis at specific base pairs was analyzed, the pol3-01 and pol2-4 mutations elevated the mutation frequency in different orientations of the reporter gene, which placed the proofreading activities of Pol δ and Pol ε on opposite DNA strands [61]. Similar conclusions were later drawn for plasmid DNA replication from the analysis of spontaneous mutation spectra in proofreading-deficient strains [62], which used the logic originally proposed by Morrison and Sugino [60].
The use of proofreading-defective mutants had limitations, because the Exo of one Pol can correct errors made by another Pol [63,64]. It would have been better to use mutations affecting the base selectivity of the Pol that would specifically allow tracing of the DNA synthetic activity of each Pol. Such mutants were not available until recently. Most mutator variants of replicative Pols were compromised in catalytic efficiency and were unable to compete with wild-type Pols (e.g.,[65,66]). Finally and fortunately, in the last several years, mutations in the conserved Pol region II (S/AL/MYPS/NI) were found to lead to loss of fidelity without loss of Pol activity. Mutation of this motif was proven to be universally useful for Pol α [67,68], Pol ε [69,70], Pol δ [71,72] and Pol ζ [73,74]. Substantiating common knowledge that “everything new is just forgotten old”, we acknowledge the seminal research of Linda Reha-Krantz who described mutator mutations in this motif in phage T4 a decade before its usefulness was appreciated [75,76].
The use of the Pol fidelity mutants was very fruitful. Using the Pol α fidelity mutant, it was established that Exo of Pol δ, but not of Pol ε, corrects errors made by Pol α [77]. This suggested that the Pols are regulated in such a manner that only Pol δ has access to the primer termini generated by Pol α on the lagging DNA strand. This is consistent with the clear role of Pol δ in Okazaki fragment maturation [58,78]. These studies, taken together with the demonstration that Exos of Pol ε and Pol δ operate on different strands, led to the inevitable conclusion that Pol ε is involved in the leading DNA strand replication [19]. Subsequently, analysis of error specificity of the inaccurate Pol δ and Pol ε variants in vitro revealed the types of errors that could be used to distinguish between the two DNA strands in vivo. For example, the inaccurate variant of Pol δ generated T•dGTP errors ~30 times more frequently than the A•dCTP pair, implying that the increase in A•T to C•G transitions in vivo can be attributed largely to errors during the copying of the T-containing strand. Elegant and scrupulous study of the mutational spectrum in Pol δ fidelity mutants confirmed the role of Pol δ in lagging strand replication and suggested its lesser charge for the errors in the leading DNA strand [79].
In an attempt to provide direct evidence for the involvement of Pol ε in the leading DNA strand replication, Pursell and co-authors employed an analogous Pol ε motif II mutant [69]. As in the case of Pol δ, the inaccurate variant of Pol ε showed asymmetric error rates for several mispairs in vitro. Quite strikingly, the spectrum of spontaneous mutations in the corresponding Pol ε mutant in vivo, was comprised almost exclusively of only one type of base substitution, A•T to T•A transversion. Moreover, the mutations were largely confined to two hotspots. The difference in the rate of these mutations in opposite orientations of the reporter gene was consistent with Pol ε generating errors during leading and not lagging strand DNA synthesis. However, the absence of other types of mutations, despite the capability of this Pol ε variant to produce a wide spectrum of errors, is an argument against the role of Pol ε in copying extensive stretches of DNA. Another caveat is that the analysis was performed in an MMR -proficient yeast strain, and, therefore took into account only those in vivo errors that escaped MMR. While there could be several explanations for why these particular errors escaped repair, one possibility is that these errors were not generated in the context of the moving replication fork. Finally, the efficiency of MMR in yeast is known to be unequal on the leading and lagging strands [80], which could contribute to the mutational bias observed by Pursell and co-authors. Further studies using MMR-deficient strains and a reporter gene lacking strong mutation hotspots would help to better understand the role of Pol ε at the fork.
Despite these uncertainties, these results, along with the ample evidence for the role of Pol δ in lagging strand replication, were considered to provide final proof of Sugino’s lab model of the replication fork (Fig 1A, B)[4,5]. Indeed, when Pols are tracked genetically, the results are always consistent with the model of Pol α and Pol δ synthesizing the lagging DNA strand and Pol ε the leading strand. A limitation of the genetic mutational approach, however, is that it registers rare events happening once in 10,000 – 1,000,000 cells. The results of such studies need to be interpreted with caution, as these rare events may not necessarily reflect the way replication typically occurs.
6. What is not consistent with the “one strand – one DNA polymerase” model?
Several observations are difficult to reconcile with the strict division of the synthetic activity of Pol ε and Pol δ between two different DNA strands.
Strikingly, the deletion of the first part of the POL2 gene, encoding for the N-terminal, active Pol (Fig. 2) is not lethal in yeast [81–83]. Thus, yeast can survive without the DNA polymerase activity of Pol ε. However, the mutants display severe growth and replication defects [84], and mutants with single amino acid substitutions in the active site of Pol ε are inviable [65,82]. These observations suggest that Pol ε is normally a component of the replication machinery, but in the absence of Pol ε, Pol δ can be a substitute for it, comprising an “alternative” fork [4,85]. In contrast, deletion of the catalytic subunit of Pol δ is lethal, so, apparently, Pol ε can not compensate for the absence of Pol δ.
The second observation not easily reconciled with the current fork model is that inactivation of the proofreading activity of Pol δ has a stronger effect on the genome-wide mutation rate than an analogous defect in Pol ε (Table 1),[56,86]. Consequently, a combination of the proofreading defect of Pol δ with a MMR defect is lethal in haploids, while strains simultaneously lacking the proofreading activity of Pol ε and MMR survive [56,60]. There are also examples of amino acid substitutions in the DNA polymerase domain of Pol δ having more severe consequences for growth and mutagenesis in comparison to analogous mutations in Pol ε (Table 1;[65]).
The third conflicting observation is the competition of the exonucleases of Pol δ and Pol ε for the same pool of replication mistakes (Table 1), which does not quite agree with these Pols working on separate DNA strands. If the latter were true, the interaction of the mutator effects of Exo− mutations would have been additive.
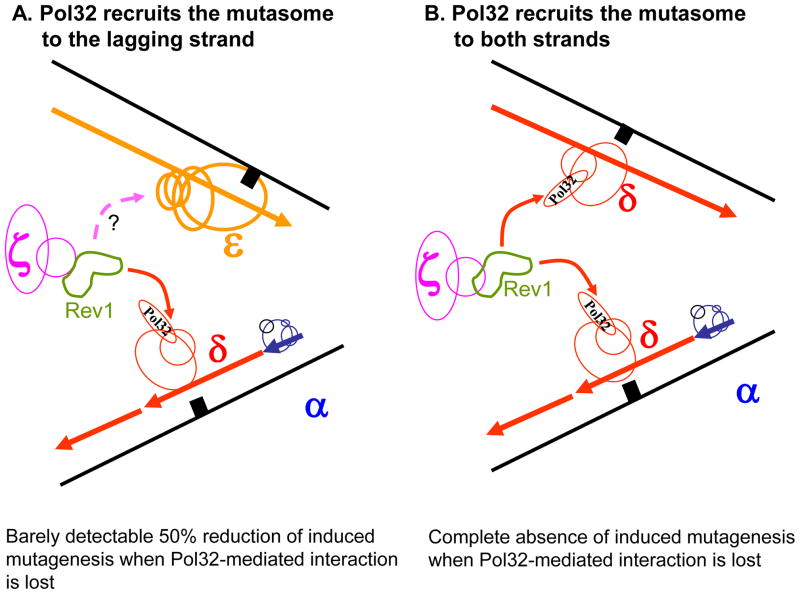
The fourth argument against the strict division of labor comes from the studies of DNA damage-induced mutagenesis. Lesions in the template stall replicative Pols, which signals for a switch to a specialized TLS polymerase. The TLS Pols bypass the lesion, often in a way that generates mutations. All induced mutagenesis in yeast vanishes with the deletion of REV1 or REV3 genes, encoding for TLS Pols. Strikingly, damage-induced mutagenesis also disappears when the third subunit of Pol δ, Pol32, is absent [23,87,88]; or when the interaction between Pol32 and the second subunit of Pol δ, Pol31, is abolished [21,23]. The Pol32 subunit was recently shown to interact with Rev1 and subsequently Pol ζ [27]. These observations suggest that Pol δ may be responsible for the recruitment of TLS Pols, as proposed by Chris Lawrence (see discussion in [6], regardless of the DNA strand where the lesion resides. Indeed, if Pol δ operated only on the lagging strand (Fig. 3A) the absence of Pol32 would eliminate only half of the induced mutations. In contrast, if Pol δ worked on both strands (Fig. 3B), the suppression of induced mutagenesis would be complete. This is exactly what is observed. Of course, Pol δ and its Pol32 subunit could be required for TLS at a step subsequent to the initial DNA polymerase switch at the lesion site. This unidentified step could proceed similarly on both strands irrespective of what Pol synthesized this strand before the encounter with a lesion. In this case, the absence of induced mutagenesis in pol32 mutants could not be considered an argument against the “one strand – one DNA polymerase” model.
Figure 3. Models of Pol δ-mediated induced mutagenesis.
Pol32 mediates the recruitment of the mutasome including Rev1 and Pol ζ to the lagging strand (A) or both strands (B).
Pol holoenzymes are drawn in blue (Pol α), red (Pol δ), yellow (Pol ε), purple (Pol ζ) and green (Rev1). A solid arrow acknowledges the proven interaction of Rev1 with Pol32. A broken arrow with a question mark indicates the hypothetical interaction of mutasome with Pol ε. A black square represents damaged DNA.
Finally, recent careful comparison of the in vitro activities of yeast Pol ε and Pol δ has shown that Pol ε is a less efficient enzyme than Pol δ [3]. Although not directly contradictory to the current replication fork model, this fact does not agree well with the proposed role of Pol ε as a major leading strand replicase.
7. The integrated new model
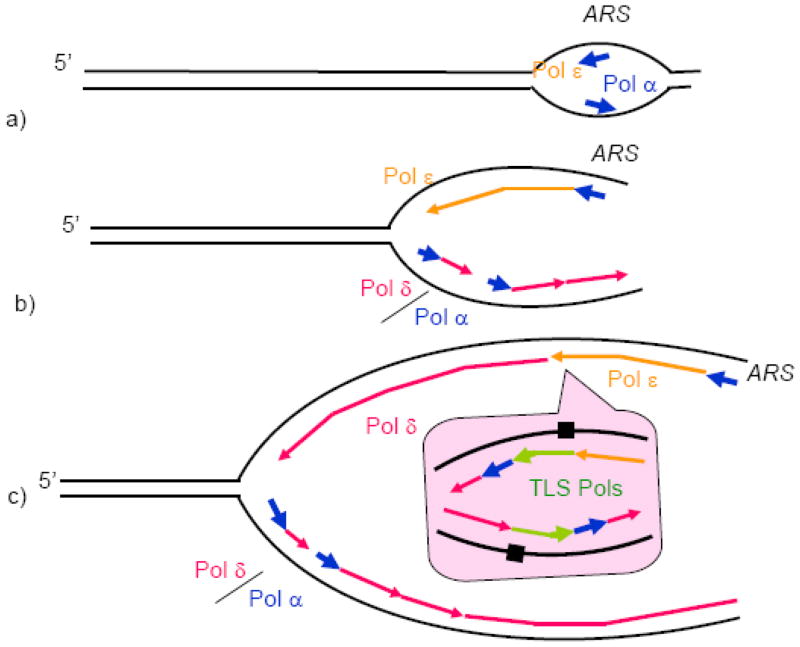
The model we are proposing was inspired by the original Sugino’s lab model. It, however, takes into account recent studies of the properties of the replicative Pols, as well as earlier data that the original model does not easily explain. Our model is based on the view that all three enzymes, Pol α, Pol δ, and Pol ε, contribute to the chromosomal DNA replication, for which there is compelling evidence [4,6,19,50]. Similar to the currently accepted model, Pol α synthesizes short RNA-DNA fragments at origins and on the lagging strand, and Pol δ extends these fragments on the lagging strand (Fig. 4). We discussed the extensive evidence for this assignment in the previous sections. The principal novel feature of our model is in the mechanism of leading strand synthesis. We postulate that Pol ε is responsible for the initiation of leading strand synthesis (Fig. 4a), as well as elongation of the leading strand in the vicinity of the origin (Fig. 4b). It dissociates from the primer terminus with an increasing probability as the distance from the origin increases, and Pol δ takes over the leading strand synthesis. As a result, the majority of the genome replication involves copying of both DNA strands by Pol δ (Fig. 4c). This DNA polymerase arrangement was previously described as an “alternative” replication fork that could be assembled if functional Pol ε is not available or upon replication restart after the standard fork stalls [4]. We propose that this “alternative” fork is a rule rather than exception, whereas the strict assignment of Pol ε and Pol δ to the leading and lagging strands, respectively, is only observed in the vicinity of the origin. We summarize the arguments in favor of the leading strand synthesis scenario shown in Fig. 4 below.
Figure 4. Model of replication fork.
a) Pol ε and Pol α are recruited to the origins (ARS, standing for Autonomous Replication Sequence according to the yeast nomenclature) by the replication initiation machinery and start leading DNA strand synthesis.
b) Pol δ is recruited to the lagging DNA strand, and replication of both strands proceeds further (only one fork moving to the left is shown for simplicity).
c) At a random site away from the origin, Pol ε encounters an obstacle and dissociates. Its recruitment back is not possible due to the absence of origin-specific factors. Pol δ rapidly takes over the leading DNA strand synthesis.
c, inset) Obstacles that can be overcome neither by Pol ε, nor by Pol δ, result in the recruitment of TLS polymerases. In the example shown, the replication block results in a replication restart downstream of the block, which requires Pol α and Pol δ. The remaining gaps are filled postreplicatively, as postulated by one of the current TLS models [98]. Replication block is shown as damaged DNA for simplicity. In reality, an elevated level of dissociation of the main replicative DNA polymerases due to genetic or physiological perturbations can results in the recruitment of TLS Pols to the fork.
There is substantial evidence for the involvement of Pol ε in early chromosomal replication at chromosomal origins, mediated by interactions with GINS [84,89–92]. This is formally consistent with the possibility that, once initiated at the origin, the Pol ε-dependent leading strand synthesis continues until the entire replicon is copied. However, evidence has been accumulated that the leading strand DNA synthesis is not truly continuous. Multiple in vivo studies reported that the products of both leading and lagging strand replication first appear as short fragments, suggesting discontinuous synthesis of both strands [93,94]. As we discuss below, multiple circumstances could lead to the untimely pausing or termination of the leading strand synthesis. This suggests that re-initiation of DNA synthesis must occur on the leading DNA strand at sites other than the replication origins. While the initiation of DNA synthesis at origins is tightly regulated and is allowed only once per cell cycle, the replication restart away from the origin has to be regulated in a cell cycle-independent manner. Accordingly, while the cell cycle-regulated Pol ε activity might be an excellent candidate for the initiation of the leading strand synthesis at origins, it might not be able to re-bind to the primer terminus at other genome sites. This idea is supported, although indirectly, by the fact that Pol ε does not participate in the replication initiated at the cell cycle-independent SV40 origin [95]. In contrast, Pol δ, the major lagging strand replicase, is clearly capable of rebinding to the primer terminus multiple times during a cell cycle. Thus, it is possible that a restart of replication on the leading DNA strand requires the activities of Pol α-primase and Pol δ rather than Pol ε. The idea of the limited involvement of Pol ε in the replication of regions distant from the origins is supported by the observation that less Pol ε is detected at such sites by chromatin immunoprecipitation [89].
The exact mechanism of the switching from Pol ε to Pol δ could depend on the particular circumstances that led to the termination of DNA synthesis by Pol ε. Two possible scenarios are shown in Fig. 4. Pol ε could potentially pause due to the incorporation of an incorrect nucleotide, which generates a primer terminus that is difficult to extend. The pausing could subsequently lead to the dissociation of Pol ε. The abandoned primer terminus could then be bound by Pol δ that would correct the mismatch through its proofreading activity and continue the leading strand synthesis (Fig. 4). The existence of this mechanism is supported by our new experimental data described below. This way of polymerase switching could be more frequent in cells carrying inaccurate variants of Pol ε due to the higher rate of mismatch generation.
Alternatively, Pol ε could stall due to the presence of a lesion in the template (Fig. 4, insert). Cellular DNA is continuously damaged by endogenously generated agents, such as oxygen radicals, and lesions are routinely encountered by the replication fork. According to one of the current models of TLS [96], replication stalling at a lesion site results in a quick restart of replication downstream of the lesion, which leaves a gap between the site of the lesion and the site of the restart. TLS polymerases then bypass the lesion and, possibly, fill the remaining gap. As discussed above, Pol δ may be a more likely candidate for accomplishing the replication restart than Pol ε, and, thus would be in a position to continue the leading strand synthesis. Note that, in this scenario, TLS polymerases assist the leading strand replication by accomplishing the bypass of the lesion that triggered the polymerase switch (Fig. 4, insert). Obviously the TLS polymerases participate in the bypass of lesions on the lagging strand template as well, although the mechanism of the replication restart might not differ significantly from the normal Okazaki fragment initiation process.
In addition, a recent study suggested that a collision of the E. coli replisome with RNA polymerase could be a source of discontinuities in the leading strand synthesis [97]. If similar events take place in eukaryotic cells, this could potentially lead to the dissociation of Pol ε and the Pol δ-mediated restart downstream of the RNA polymerase. Finally, as mentioned previously, Pol ε is substantially less efficient and somewhat less processive than Pol δ while replicating circular ssDNA in vitro [3]. Pausing and/or dissociation of Pol ε for reasons other than template damage, the generation of mismatched primer termini or the collision with RNA polymerase could also lead to switching to the more mobile and robust Pol δ.
This model allows us to explain a number of observations that could not easily be reconciled with the strict assignment of Pol δ and Pol ε to the lagging and leading DNA strands, respectively. First, it would explain the fact that mutations affecting Pol δ typically have a much stronger effect on strain growth and the genome stability than analogous defects in Pol ε (Table 1; see previous section). Second, the model in Fig. 4 satisfactorily explains the competition of the exonucleases of Pol δ and Pol ε for the same pool of replication errors suggesting that the two Pols can correct each other’s errors [60]. In our unpublished experiments, we further characterized this competition. We combined mutations affecting the fidelity of polymerization by one of the two Pols, Pol δ and Pol ε, with mutations inactivating the exonuclease of the other Pol. The strain with the polymerization fidelity defect of Pol δ and the exonuclease defect of Pol ε, showed an additive increase in the mutation rate (Table 1). This implies that Pol ε does not proofread errors generated by Pol δ. In a reciprocal experiment, when a mutation affecting the polymerization fidelity of Pol ε was combined with an exonuclease defect of Pol δ, a synergistic increase in the mutation rate was seen for several reporter genes. This is consistent with the idea that mistakes made by the inaccurate variant of Pol ε are corrected by the proofreading activity of Pol δ. Accordingly, the model in Fig. 4 predicts that Pol δ takes over the primer terminus generated by Pol ε during the leading strand synthesis, but Pol ε does not access the primer termini generated by Pol δ. We propose that the reporters that detect the synergistic interaction of the Pol ε nucleotide selectivity and Pol δ proofreading defects are in the regions, where the switching from Pol ε to Pol δ is most probable.
The model shown in Fig. 4 is also consistent with the earlier genetics data demonstrating the primary roles for Pol δ and Pol ε in the copying of the opposite DNA strands [61,62,69,79]. All these experiments monitored DNA replication at a reporter gene located close to a replication origin. Interestingly, and in agreement with our model, we were able to detect some proofreading activity of Pol δ on the strand that is preferentially proofread by Pol ε even at the reporter placed close to the origin (~4.4 kb; [61]). In general, however, the genetic studies confirm the idea that the fork arrangement proposed by the Sugino lab, exists at the vicinity of the origin. Future studies will help establish if this arrangement could also be seen further away from the origin or, as shown in Fig. 4, Pol δ takes over the synthesis of both strands as the fork progresses. We observed previously that the leading and lagging DNA strands have unequal susceptibility to base analog-induced replication errors, and the strand bias is maintained over the entire replicon studied [98]. One explanation for the bias is the involvement of different Pols in the copying of the two strands [4,98]. In the case of 8-oxoguanine-generated errors, however, the bias was shown to result from differential MMR efficiency on the leading and lagging strands [80]. The example of E. coli, where the two strands are copied by the same Pol III holoenzyme with unequal fidelity [54], indicates that there could be inherent differences in the error rate on the leading and lagging strands. Apparently, this bias could reflect the fundamental differences in the mechanism of replication of the two strands rather than the identity of Pol that does the copying.
The last comment we would like to make relates to the possible role of Pol ζ, the fourth eukaryotic B family polymerase, in the chromosomal DNA replication. Fig. 4 illustrates that TLS polymerase activity is an integral part of the genome replication. TLS polymerases, including Pol ζ, as well as the Y family DNA polymerases, help to overcome replication barriers created by lesions [26,96]. Our model proposes that, in addition to other genome sites, the TLS activity could be specifically observed in areas where the switching from Pol ε to Pol δ occurs on the leading DNA strand. In addition, our earlier and more recent studies suggested that Pol ζ may be recruited to restart DNA synthesis at stalled or slowly progressing replication forks regardless of the presence of DNA damage. A Pol ζ-dependent increase in spontaneous mutagenesis is observed in strains with defects in normal replicative DNA polymerases [36,65,99]. Genetic studies suggested that this mutagenesis results from error-prone copying of undamaged DNA (M. R. Northam, H. A. Robinson and P. V. Shcherbakova, unpublished observations). Moreover, some fraction of the errors introduced by Pol ζ is corrected by MMR, similar to the regular DNA replication errors [65]. It is, therefore, possible that Pol ζ assists the main trio during the genome replication. The role of Pol ζ in the copying of undamaged DNA likely reflects its ability to extend terminally mismatched primers and other aberrant substrates that are poorly extended by the replicative DNA polymerases[26]. However, Pol ζ is dispensable for normal growth and replication in yeast. We propose that Pol ζ is the fourth Pol at the fork, whose involvement is of a limited extent and in a highly controlled fashion.
Table 2.
Nomenclature (yeast)
| Gene | Holoenzyme | Catalytic subunit |
|---|---|---|
| POL1 | Pol α | Pol1 |
| POL2 | Pol ε | Pol2 |
| POL3 | Pol δ | Pol3 |
| REV3 | Pol ζ | Rev3 |
Acknowledgments
We thank Peter Burgers for reading the manuscript and providing constructive comments despite the difference in the views on the prevailing replication mechanisms. We thank Tahir Tahirov, Tom Kunkel, Dmitry Gordenin, Erik Johansson, and Farid Kadyrov for valuable discussions of the topics related to the current review. We thank Victoria Liston for expert technical assistance in experiments. The work in the authors’ laboratories is supported in part by the NIH grant CA129925, Eppley Institute Pilot grant and NE DHHS 2008 grant LB506 (to Y.I.P.) and the NIH grant ES015869 (to P.V.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Syvaoja J, Suomensaari S, Nishida C, Goldsmith JS, Chui GS, Jain S, Linn S. DNA polymerases alpha, delta, and epsilon: three distinct enzymes from HeLa cells. Proc Natl Acad Sci U S A. 1990;87:6664–6668. doi: 10.1073/pnas.87.17.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrison A, Araki H, Clark AB, Hamatake RK, Sugino A. A third essential DNA polymerase in S. cerevisiae. Cell. 1990;62:1143–1151. doi: 10.1016/0092-8674(90)90391-q. [DOI] [PubMed] [Google Scholar]
- 3.Chilkova O, Stenlund P, Isoz I, Stith CM, Grabowski P, Lundstrom EB, Burgers PM, Johansson E. The eukaryotic leading and lagging strand DNA polymerases are loaded onto primer-ends via separate mechanisms but have comparable processivity in the presence of PCNANucleic. Acids Res. 2007;35:6588–6597. doi: 10.1093/nar/gkm741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunkel TA, Burgers PM. Dividing the workload at a eukaryotic replication fork. Trends Cell Biol. 2008;18:521–527. doi: 10.1016/j.tcb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stillman B. DNA polymerases at the replication fork in eukaryotes. Mol Cell. 2008;30:259–260. doi: 10.1016/j.molcel.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavlov YI, Shcherbakova PV, Rogozin IB. Roles of DNA polymerases in replication, repair, and recombination in eukaryotes. Int Rev Cytol. 2006;255:41–132. doi: 10.1016/S0074-7696(06)55002-8. [DOI] [PubMed] [Google Scholar]
- 7.Tahirov TH, Makarova KS, Rogozin IB, Pavlov YI, Koonin EV. Evolution of DNA polymerases: an inactivated polymerase-exonuclease module in Pol epsilon and a chimeric origin of eukaryotic polymerases from two classes of archaeal ancestors. Biol Direct. 2009;4:11. doi: 10.1186/1745-6150-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wardle J, Burgers PM, Cann IK, Darley K, Heslop P, Johansson E, Lin LJ, McGlynn P, Sanvoisin J, Stith CM, Connolly BA. Uracil recognition by replicative DNA polymerases is limited to the archaea, not occurring with bacteria and eukarya. Nucleic Acids Res. 2008;36:705–711. doi: 10.1093/nar/gkm1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dua R, Levy DL, Campbell JL. Role of the putative zinc finger domain of Saccharomyces cerevisiae DNA polymerase in DNA replication and the S/M checkpoint pathway. J Biol Chem. 1998;273:30046–30055. doi: 10.1074/jbc.273.45.30046. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez Garcia J, Ciufo LF, Yang X, Kearsey SE, MacNeill SA. The C-terminal zinc finger of the catalytic subunit of DNA polymerase delta is responsible for direct interaction with the B-subunit. Nucleic Acids Res. 2004;32:3005–3016. doi: 10.1093/nar/gkh623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klinge S, Nunez-Ramirez R, Llorca O, Pellegrini L. 3D architecture of DNA Pol alpha reveals the functional core of multi-subunit replicative polymerases. EMBO J. 2009;28:1978–87. doi: 10.1038/emboj.2009.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muzi-Falconi M, Giannattasio M, Foiani M, Plevani P. The DNA polymerase α-primase complex: multiple functions and interactions. Scientific World Journal. 2003;3:21–33. doi: 10.1100/tsw.2003.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Copeland WC, Wang TS. Catalytic subunit of human DNA polymerase α overproduced from baculovirus-infected insect cells. Structural and enzymological characterization. J Biol Chem. 1991;266:22739–22748. [PubMed] [Google Scholar]
- 14.Takada-Takayama R, Suzuki M, Enomoto T, Hanaoka F, Ui M. Purification and characterization of mouse DNA polymerase devoid of primase activity. FEBS Lett. 1990;273:27–30. doi: 10.1016/0014-5793(90)81043-n. [DOI] [PubMed] [Google Scholar]
- 15.Uchiyama M, Wang TS. The B-subunit of DNA polymerase α-primase associates with the origin recognition complex for initiation of DNA replication. Mol Cell Biol. 2004;24:7419–7434. doi: 10.1128/MCB.24.17.7419-7434.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klinge S, Hirst J, Maman JD, Krude T, Pellegrini L. An iron-sulfur domain of the eukaryotic primase is essential for RNA primer synthesis. Nat Struct Mol Biol. 2007;14:875–877. doi: 10.1038/nsmb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacNeill SA, Baldacci G, Burgers PM, Hubscher U. A unified nomenclature for the subunits of eukaryotic DNA polymerase δ. Trends Biochem Sci. 2001;26:16–17. doi: 10.1016/s0968-0004(00)01709-6. [DOI] [PubMed] [Google Scholar]
- 18.McHenry CS. Chromosomal replicases as asymmetric dimers: studies of subunit arrangement and functional consequences. Mol Microbiol. 2003;49:1157–1165. doi: 10.1046/j.1365-2958.2003.03645.x. [DOI] [PubMed] [Google Scholar]
- 19.Garg P, Burgers PM. DNA polymerases that propagate the eukaryotic DNA replication fork. Crit Rev Biochem Mol Biol. 2005;40:115–128. doi: 10.1080/10409230590935433. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez Garcia J, Ciufo LF, Yang X, Kearsey SE, MacNeill SA. The C-terminal zinc finger of the catalytic subunit of DNA polymerase δ is responsible for direct interaction with the B-subunit. Nucleic Acids Res. 2004;32:3005–3016. doi: 10.1093/nar/gkh623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baranovskiy AG, Babayeva ND, Liston VG, Rogozin IB, Koonin EV, Pavlov YI, Vassylyev DG, Tahirov TH. X-ray structure of the complex of regulatory subunits of human DNA polymerase delta. Cell Cycle. 2008;7:3026–3036. doi: 10.4161/cc.7.19.6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomes XV, Burgers PM. Two modes of FEN1 binding to PCNA regulated by DNA. EMBO J. 2000;19:3811–3821. doi: 10.1093/emboj/19.14.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson E, Garg P, Burgers PM. The Pol32 subunit of DNA polymerase δ contains separable domains for processive replication and proliferating cell nuclear antigen (PCNA) binding. J Biol Chem. 2004;279:1907–1915. doi: 10.1074/jbc.M310362200. [DOI] [PubMed] [Google Scholar]
- 24.Gray FC, Pohler JR, Warbrick E, MacNeill SA. Mapping and mutation of the conserved DNA polymerase interaction motif (DPIM) located in the C-terminal domain of fission yeast DNA polymerase δ subunit Cdc27. BMC Mol Biol. 2004;5:21. doi: 10.1186/1471-2199-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawrence CW. Cellular roles of DNA polymerase ζ and Rev1 protein. DNA Repair (Amst) 2002;1:425–435. doi: 10.1016/s1568-7864(02)00038-1. [DOI] [PubMed] [Google Scholar]
- 26.Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu Rev Biochem. 2005;74:317–353. doi: 10.1146/annurev.biochem.74.082803.133250. [DOI] [PubMed] [Google Scholar]
- 27.Acharya N, Johnson RE, Pages V, Prakash L, Prakash S. Yeast Rev1 protein promotes complex formation of DNA polymerase zeta with Pol32 subunit of DNA polymerase delta. Proc Natl Acad Sci U S A. 2009;106:9631–9636. doi: 10.1073/pnas.0902175106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds N, Watt A, Fantes PA, MacNeill SA. Cdm1, the smallest subunit of DNA polymerase d in the fission yeast Schizosaccharomyces pombe, is non-essential for growth and division. Curr Genet. 1998;34:250–258. doi: 10.1007/s002940050394. [DOI] [PubMed] [Google Scholar]
- 29.Zhang S, Zhou Y, Trusa S, Meng X, Lee EY, Lee MY. A novel DNA damage response: rapid degradation of the p12 subunit of dna polymerase delta. J Biol Chem. 2007;282:15330–15340. doi: 10.1074/jbc.M610356200. [DOI] [PubMed] [Google Scholar]
- 30.Pospiech H, Syvaoja JE. DNA polymerase ε-more than a polymerase. Scientific World Journal. 2003;3:87–104. doi: 10.1100/tsw.2003.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asturias FJ, Cheung IK, Sabouri N, Chilkova O, Wepplo D, Johansson E. Structure of Saccharomyces cerevisiae DNA polymerase ε by cryo-electron microscopy. Nat Struct Mol Biol. 2006;13:35–43. doi: 10.1038/nsmb1040. [DOI] [PubMed] [Google Scholar]
- 32.Jaszczur M, Rudzka J, Kraszewska J, Flis K, Polaczek P, Campbell JL, Fijalkowska IJ, Jonczyk P. Defective interaction between Pol2p and Dpb2p, subunits of DNA polymerase epsilon, contributes to a mutator phenotype in Saccharomyces cerevisiae. Mutat Res. 2009 doi: 10.1016/j.mrfmmm.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaszczur M, Flis K, Rudzka J, Kraszewska J, Budd ME, Polaczek P, Campbell JL, Jonczyk P, Fijalkowska IJ. Dpb2p, a noncatalytic subunit of DNA polymerase epsilon, contributes to the fidelity of DNA replication in Saccharomyces cerevisiae. Genetics. 2008;178:633–647. doi: 10.1534/genetics.107.082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsubota T, Maki S, Kubota H, Sugino A, Maki H. Double-stranded DNA binding properties of Saccharomyces cerevisiae DNA polymerase ε and of the Dpb3p-Dpb4p subassembly. Genes Cells. 2003;8:873–888. doi: 10.1046/j.1365-2443.2003.00683.x. [DOI] [PubMed] [Google Scholar]
- 35.Araki H, Hamatake RK, Morrison A, Johnson AL, Johnston LH, Sugino A. Cloning DPB3, the gene encoding the third subunit of DNA polymerase II of Saccharomyces cerevisiae. Nucleic Acids Res. 1991;19:4867–4872. doi: 10.1093/nar/19.18.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Northam MR, Garg P, Baitin DM, Burgers PM, Shcherbakova PV. A novel function of DNA polymerase zeta regulated by PCNA. EMBO J. 2006;25:4316–4325. doi: 10.1038/sj.emboj.7601320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iida T, Araki H. Noncompetitive counteractions of DNA polymerase epsilon and ISW2/yCHRAC for epigenetic inheritance of telomere position effect in Saccharomyces cerevisiae. Mol Cell Biol. 2004;24:217–227. doi: 10.1128/MCB.24.1.217-227.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takayama Y, Kamimura Y, Okawa M, Muramatsu S, Sugino A, Araki H. GINS, a novel multiprotein complex required for chromosomal DNA replication in budding yeast. Genes Dev. 2003;17:1153–1165. doi: 10.1101/gad.1065903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pursell ZF, Kunkel TA. DNA polymerase epsilon: a polymerase of unusual size (and complexity) Prog Nucleic Acid Res Mol Biol. 2008;82:101–145. doi: 10.1016/S0079-6603(08)00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson JR, Lawrence CW, Hinkle DC. Thymine-thymine dimer bypass by yeast DNA polymerase ζ. Science. 1996;272:1646–1649. doi: 10.1126/science.272.5268.1646. [DOI] [PubMed] [Google Scholar]
- 41.Li Z, Zhang H, McManus TP, McCormick JJ, Lawrence CW, Maher VM. hREV3 is essential for error-prone translesion synthesis past UV or benzo[a]pyrene diol epoxide-induced DNA lesions in human fibroblasts. Mutat Res. 2002;510:71–80. doi: 10.1016/s0027-5107(02)00253-1. [DOI] [PubMed] [Google Scholar]
- 42.Gan GN, Wittschieben JP, Wittschieben BO, Wood RD. DNA polymerase zeta (pol zeta) in higher eukaryotes. Cell Res. 2008;18:174–183. doi: 10.1038/cr.2007.117. [DOI] [PubMed] [Google Scholar]
- 43.Murakumo Y, Roth T, Ishii H, Rasio D, Numata S, Croce CM, Fishel R. A human REV7 homolog that interacts with the polymerase ζ catalytic subunit hREV3 and the spindle assembly checkpoint protein hMAD2. J Biol Chem. 2000;275:4391–4397. doi: 10.1074/jbc.275.6.4391. [DOI] [PubMed] [Google Scholar]
- 44.Koonin EV. Temporal order of evolution of DNA replication systems inferred by comparison of cellular and viral DNA polymerases. Biol Direct. 2006;1:39. doi: 10.1186/1745-6150-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aravind L, Koonin EV. Phosphoesterase domains associated with DNA polymerases of diverse origins. Nucleic Acids Res. 1998;26:3746–3752. doi: 10.1093/nar/26.16.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Makiniemi M, Pospiech H, Kilpelainen S, Jokela M, Vihinen M, Syvaoja JE. A novel family of DNA-polymerase-associated B subunits. Trends Biochem Sci. 1999;24:14–16. doi: 10.1016/s0968-0004(98)01327-9. [DOI] [PubMed] [Google Scholar]
- 47.Jokela M, Raki M, Heikkinen K, Sepponen K, Eskelinen A, Syvaoja JE. The screening of expression and purification conditions for replicative DNA polymerase associated B-subunits, assignment of the exonuclease activity to the C-terminus of archaeal pol D DP1 subunit. Protein Expr Purif. 2005;43:73–84. doi: 10.1016/j.pep.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Kornberg A, Baker T. DNA Replication. W.H. Freeman & Co; 1991. [Google Scholar]
- 49.Trakselis MA, Mayer MU, Ishmael FT, Roccasecca RM, Benkovic SJ. Dynamic protein interactions in the bacteriophage T4 replisome. Trends Biochem Sci. 2001;26:566–572. doi: 10.1016/s0968-0004(01)01929-6. [DOI] [PubMed] [Google Scholar]
- 50.Johnson A, O'Donnell M. Cellular DNA replicases: components and dynamics at the replication fork. Annu Rev Biochem. 2005;74:283–315. doi: 10.1146/annurev.biochem.73.011303.073859. [DOI] [PubMed] [Google Scholar]
- 51.McInerney P, Johnson A, Katz F, O'Donnell M. Characterization of a triple DNA polymerase replisome. Mol Cell. 2007;27:527–538. doi: 10.1016/j.molcel.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 52.Dervyn E, Suski C, Daniel R, Bruand C, Chapuis J, Errington J, Janniere L, Ehrlich SD. Two essential DNA polymerases at the bacterial replication fork. Science. 2001;294:1716–1719. doi: 10.1126/science.1066351. [DOI] [PubMed] [Google Scholar]
- 53.Waga S, Stillman B. Anatomy of a DNA replication fork revealed by reconstitution of SV40 DNA replication in vitro. Nature. 1994;369:207–212. doi: 10.1038/369207a0. [DOI] [PubMed] [Google Scholar]
- 54.Fijalkowska IJ, Jonczyk P, Tkaczyk MM, Bialoskorska M, Schaaper RM. Unequal fidelity of leading strand and lagging strand DNA replication on the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1998;95:10020–10025. doi: 10.1073/pnas.95.17.10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morrison A, Bell JB, Kunkel TA, Sugino A. Eukaryotic. DNA polymerase amino acid sequence required for 3′->5′ exonuclease activity. Proc Natl Acad Sci U S A. 1991;88:9473–9477. doi: 10.1073/pnas.88.21.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morrison A, Johnson AL, Johnston LH, Sugino A. Pathway correcting DNA replication errors in Saccharomyces cerevisiae. EMBO J. 1993;12:1467–1473. doi: 10.1002/j.1460-2075.1993.tb05790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Datta A, Schmeits JL, Amin NS, Lau PJ, Myung K, Kolodner RD. Checkpoint-dependent activation of mutagenic repair in Saccharomyces cerevisiae pol3–01 mutants. Mol Cell. 2000;6:593–603. doi: 10.1016/s1097-2765(00)00058-7. [DOI] [PubMed] [Google Scholar]
- 58.Jin YH, Obert R, Burgers PM, Kunkel TA, Resnick MA, Gordenin DA. The 3′-->5′ exonuclease of DNA polymerase δ can substitute for the 5′ flap endonuclease Rad27/Fen1 in processing Okazaki fragments and preventing genome instability. Proc Natl Acad Sci U S A. 2001;98:5122–5127. doi: 10.1073/pnas.091095198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kadyrov FA, Genschel J, Fang Y, Penland E, Edelmann W, Modrich P. A possible mechanism for exonuclease 1-independent eukaryotic mismatch repair. Proc Natl Acad Sci U S A. 2009;106:8495–8500. doi: 10.1073/pnas.0903654106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morrison A, Sugino A. The 3′-->5′ exonucleases of both DNA polymerases δ and ε participate in correcting errors of DNA replication in Saccharomyces cerevisiae. Mol Gen Genet. 1994;242:289–296. doi: 10.1007/BF00280418. [DOI] [PubMed] [Google Scholar]
- 61.Shcherbakova PV, Pavlov YI. 3′-->5′ exonucleases of DNA polymerases ε and δ correct base analog induced DNA replication errors on opposite DNA strands in Saccharomyces cerevisiae. Genetics. 1996;142:717–726. doi: 10.1093/genetics/142.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karthikeyan R, Vonarx EJ, Straffon AF, Simon M, Faye G, Kunz BA. Evidence from mutational specificity studies that yeast DNA polymerases δ and ε replicate different DNA strands at an intracellular replication fork. J Mol Biol. 2000;299:405–419. doi: 10.1006/jmbi.2000.3744. [DOI] [PubMed] [Google Scholar]
- 63.McCulloch SD, Kokoska RJ, Chilkova O, Welch CM, Johansson E, Burgers PM, Kunkel TA. Enzymatic switching for efficient and accurate translesion DNA replication. Nucleic Acids Res. 2004;32:4665–4675. doi: 10.1093/nar/gkh777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nick McElhinny SA, Pavlov YI, Kunkel TA. Evidence for extrinsic exonucleolytic proofreading. Cell Cycle. 2006;5:958–962. doi: 10.4161/cc.5.9.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pavlov YI, Shcherbakova PV, Kunkel TA. In vivo consequences of putative active site missense mutations in yeast replicative DNA polymerases α, ε, δand ζ. Genetics. 2001;159:47–64. doi: 10.1093/genetics/159.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pavlov YI, Maki S, Maki H, Kunkel TA. Evidence for interplay among yeast replicative DNA polymerases α, δ and ε from studies of exonuclease and polymerase active site mutations. BMC Biol. 2004;2:11. doi: 10.1186/1741-7007-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Niimi A, Limsirichaikul S, Yoshida S, Iwai S, Masutani C, Hanaoka F, Kool ET, Nishiyama Y, Suzuki M. Palm mutants in DNA polymerases α and η alter DNA replication fidelity and translesion activity. Mol Cell Biol. 2004;24:2734–2746. doi: 10.1128/MCB.24.7.2734-2746.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki M, Niimi A, Limsirichaikul S, Tomida S, Huang QM, Izuta S, Usukura J, Itoh Y, Hishida T, Akashi T, Nakagawa Y, Kikuchi A, Pavlov Y, Murate T, Takahashi T. PCNA Mono-ubiquitination and Activation of Translesion DNA Polymerases by DNA Polymerase alpha. J Biochem. 2009;146:13–21. doi: 10.1093/jb/mvp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pursell ZF, Isoz I, Lundstrom EB, Johansson E, Kunkel TA. Yeast DNA polymerase epsilon participates in leading-strand DNA replication. Science. 2007;317:127–130. doi: 10.1126/science.1144067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pursell ZF, Isoz I, Lundstrom EB, Johansson E, Kunkel TA. Regulation of B family DNA polymerase fidelity by a conserved active site residue: characterization of M644W, M644L and M644F mutants of yeast DNA polymerase epsilon. Nucleic Acids Res. 2007;35:3076–3086. doi: 10.1093/nar/gkm132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li L, Murphy KM, Kanevets U, Reha-Krantz LJ. Sensitivity to phosphonoacetic acid: a new phenotype to probe DNA polymerase δ in Saccharomyces cerevisiae. Genetics. 2005;170:569–580. doi: 10.1534/genetics.104.040295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Venkatesan RN, Hsu JJ, Lawrence NA, Preston BD, Loeb LA. Mutator phenotypes caused by substitution at a conserved motif A residue in eukaryotic DNA polymerase δ. J Biol Chem. 2006;281:4486–4494. doi: 10.1074/jbc.M510245200. [DOI] [PubMed] [Google Scholar]
- 73.Sakamoto AN, Stone JE, Kissling GE, McCulloch SD, Pavlov YI, Kunkel TA. Mutator alleles of yeast DNA polymerase zeta. DNA Repair (Amst) 2007;6:1829–1838. doi: 10.1016/j.dnarep.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stone JE, Kissling GE, Lujan SA, Rogozin IB, Stith CM, Burgers PM, Kunkel TA. Low-fidelity DNA synthesis by the L979F mutator derivative of Saccharomyces cerevisiae DNA polymerase zeta. Nucleic Acids Res. 2009;37:3774–3787. doi: 10.1093/nar/gkp238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reha-Krantz LJ, Nonay RL. Motif A of bacteriophage T4 DNA polymerase: role in primer extension and DNA replication fidelity. Isolation of new antimutator and mutator DNA polymerases. J Biol Chem. 1994;269:5635–5643. [PubMed] [Google Scholar]
- 76.Stocki SA, Nonay RL, Reha-Krantz LJ. Dynamics of bacteriophage T4 DNA polymerase function: identification of amino acid residues that affect switching between polymerase and 3′ --> 5′ exonuclease activities. J Mol Biol. 1995;254:15–28. doi: 10.1006/jmbi.1995.0595. [DOI] [PubMed] [Google Scholar]
- 77.Pavlov YI, Frahm C, McElhinny SA, Niimi A, Suzuki M, Kunkel TA. Evidence that errors made by DNA polymerase are corrected by DNA polymerase δ. Curr Biol. 2006;16:202–207. doi: 10.1016/j.cub.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 78.Garg P, Stith CM, Sabouri N, Johansson E, Burgers PM. Idling by DNA polymerase δ maintains a ligatable nick during lagging-strand DNA replication. Genes Dev. 2004;18:2764–2773. doi: 10.1101/gad.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nick McElhinny SA, Gordenin DA, Stith CM, Burgers PM, Kunkel TA. Division of labor at the eukaryotic replication fork. Mol Cell. 2008;30:137–144. doi: 10.1016/j.molcel.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pavlov YI, Mian IM, Kunkel TA. Evidence for preferential mismatch repair of lagging strand DNA replication errors in yeast. Curr Biol. 2003;13:744–748. doi: 10.1016/s0960-9822(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 81.Kesti T, Flick K, Keranen S, Syvaoja JE, Wittenberg C. DNA polymerase ε catalytic domains are dispensable for DNA replication, DNA repair, and cell viability. Mol Cell. 1999;3:679–685. doi: 10.1016/s1097-2765(00)80361-5. [DOI] [PubMed] [Google Scholar]
- 82.Dua R, Levy DL, Campbell JL. Analysis of the essential functions of the C-terminal protein/protein interaction domain of Saccharomyces cerevisiae pol ε and its unexpected ability to support growth in the absence of the DNA polymerase domain. J Biol Chem. 1999;274:22283–22288. doi: 10.1074/jbc.274.32.22283. [DOI] [PubMed] [Google Scholar]
- 83.Feng W, D’Urso G. Schizosaccharomyces pombe cells lacking the amino-terminal catalytic domains of DNA polymerase epsilon are viable but require the DNA damage checkpoint control. Mol Cell Biol. 2001;21:4495–4504. doi: 10.1128/MCB.21.14.4495-4504.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohya T, Kawasaki Y, Hiraga S, Kanbara S, Nakajo K, Nakashima N, Suzuki A, Sugino A. The DNA polymerase domain of pol(ε) is required for rapid, efficient, and highly accurate chromosomal DNA replication, telomere length maintenance, and normal cell senescence in Saccharomyces cerevisiae. J Biol Chem. 2002;277:28099–28108. doi: 10.1074/jbc.M111573200. [DOI] [PubMed] [Google Scholar]
- 85.Burgers PM. Polymerase dynamics at the eukaryotic DNA replication fork. J Biol Chem. 2009;284:4041–4045. doi: 10.1074/jbc.R800062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simon M, Giot L, Faye G. The 3′ to 5′ exonuclease activity located in the DNA polymerase delta subunit of Saccharomyces cerevisiae is required for accurate replication. EMBO J. 1991;10:2165–2170. doi: 10.1002/j.1460-2075.1991.tb07751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gerik KJ, Li X, Pautz A, Burgers PM. Characterization of the two small subunits of Saccharomyces cerevisiae DNA polymerase δ. J Biol Chem. 1998;273:19747–19755. doi: 10.1074/jbc.273.31.19747. [DOI] [PubMed] [Google Scholar]
- 88.Huang ME, de Calignon A, Nicolas A, Galibert F. Pol32, a subunit of the Saccharomyces cerevisiae DNA polymerase δ, defines a link between DNA replication and the mutagenic bypass repair pathway. Curr Genet. 2000;38:178–187. doi: 10.1007/s002940000149. [DOI] [PubMed] [Google Scholar]
- 89.Aparicio OM, Weinstein DM, Bell SP. Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell. 1997;91:59–69. doi: 10.1016/s0092-8674(01)80009-x. [DOI] [PubMed] [Google Scholar]
- 90.Masumoto H, Sugino A, Araki H. Dpb11 controls the association between DNA polymerases α and ε and the autonomously replicating sequence region of budding yeast. Mol Cell Biol. 2000;20:2809–2817. doi: 10.1128/mcb.20.8.2809-2817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hiraga S, Hagihara-Hayashi A, Ohya T, Sugino A. DNA polymerases α, δ, and ε localize and function together at replication forks in Saccharomyces cerevisiae. Genes Cells. 2005;10:297–309. doi: 10.1111/j.1365-2443.2005.00843.x. [DOI] [PubMed] [Google Scholar]
- 92.Pai CC, Garcia I, Wang SW, Cotterill S, Macneill SA, Kearsey SE. GINS inactivation phenotypes reveal two pathways for chromatin association of replicative alpha and epsilon DNA polymerases in fission yeast. Mol Biol Cell. 2009;20:1213–1222. doi: 10.1091/mbc.E08-04-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ogawa T, Okazaki T. Discontinuous DNA replication. Annu Rev Biochem. 1980;49:421–457. doi: 10.1146/annurev.bi.49.070180.002225. [DOI] [PubMed] [Google Scholar]
- 94.Wang TC. Discontinuous or semi-discontinuous DNA replication in Escherichia coli? Bioessays. 2005;27:633–636. doi: 10.1002/bies.20233. [DOI] [PubMed] [Google Scholar]
- 95.Zlotkin T, Kaufmann G, Jiang Y, Lee MY, Uitto L, Syvaoja J, Dornreiter I, Fanning E, Nethanel T. DNA polymerase epsilon may be dispensable for SV40- but not cellular-DNA replication. EMBO J. 1996;15:2298–2305. [PMC free article] [PubMed] [Google Scholar]
- 96.Waters LS, Minesinger BK, Wiltrout ME, D’Souza S, Woodruff RV, Walker GC. Eukaryotic translesion polymerases and their roles and regulation in DNA damage tolerance. Microbiol Mol Biol Rev. 2009;73:134–154. doi: 10.1128/MMBR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pomerantz RT, O'Donnell M. The replisome uses mRNA as a primer after colliding with RNA polymerase. Nature. 2008;456:762–766. doi: 10.1038/nature07527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pavlov YI, Newlon CS, Kunkel TA. Yeast origins establish a strand bias for replicational mutagenesis. Mol Cell. 2002;10:207–213. doi: 10.1016/s1097-2765(02)00567-1. [DOI] [PubMed] [Google Scholar]
- 99.Shcherbakova PV, Noskov VN, Pshenichnov MR, Pavlov YI. Base analog 6-N-hydroxylaminopurine mutagenesis in the yeast Saccharomyces cerevisiae is controlled by replicative DNA polymerases. Mutat Res. 1996;369:33–44. doi: 10.1016/s0165-1218(96)90045-2. [DOI] [PubMed] [Google Scholar]
- 100.Nick McElhinny SA, Stith CM, Burgers PM, Kunkel TA. Inefficient proofreading and biased error rates during inaccurate DNA synthesis by a mutant derivative of Saccharomyces cerevisiae DNA polymerase delta. J Biol Chem. 2007;282:2324–2332. doi: 10.1074/jbc.M609591200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stith CM, Sterling J, Resnick MA, Gordenin DA, Burgers PM. Flexibility of eukaryotic Okazaki fragment maturation through regulated strand displacement synthesis. J Biol Chem. 2008;283:34129–34140. doi: 10.1074/jbc.M806668200. [DOI] [PMC free article] [PubMed] [Google Scholar]