Abstract
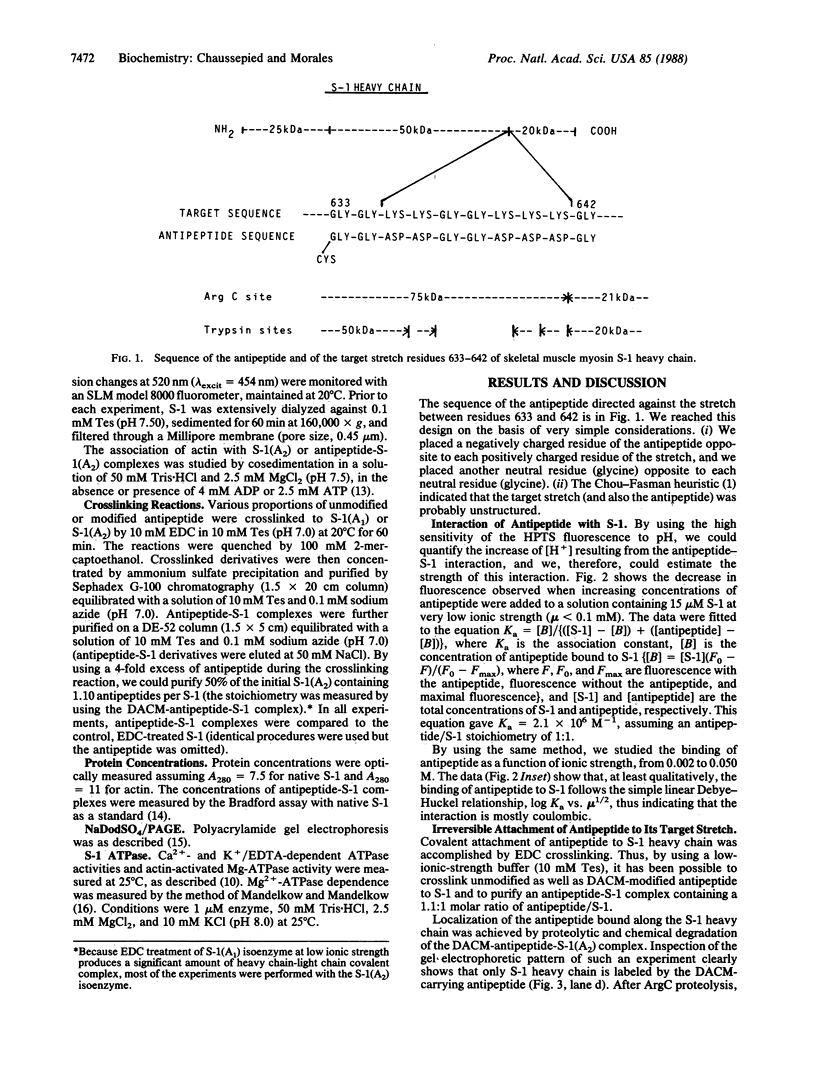
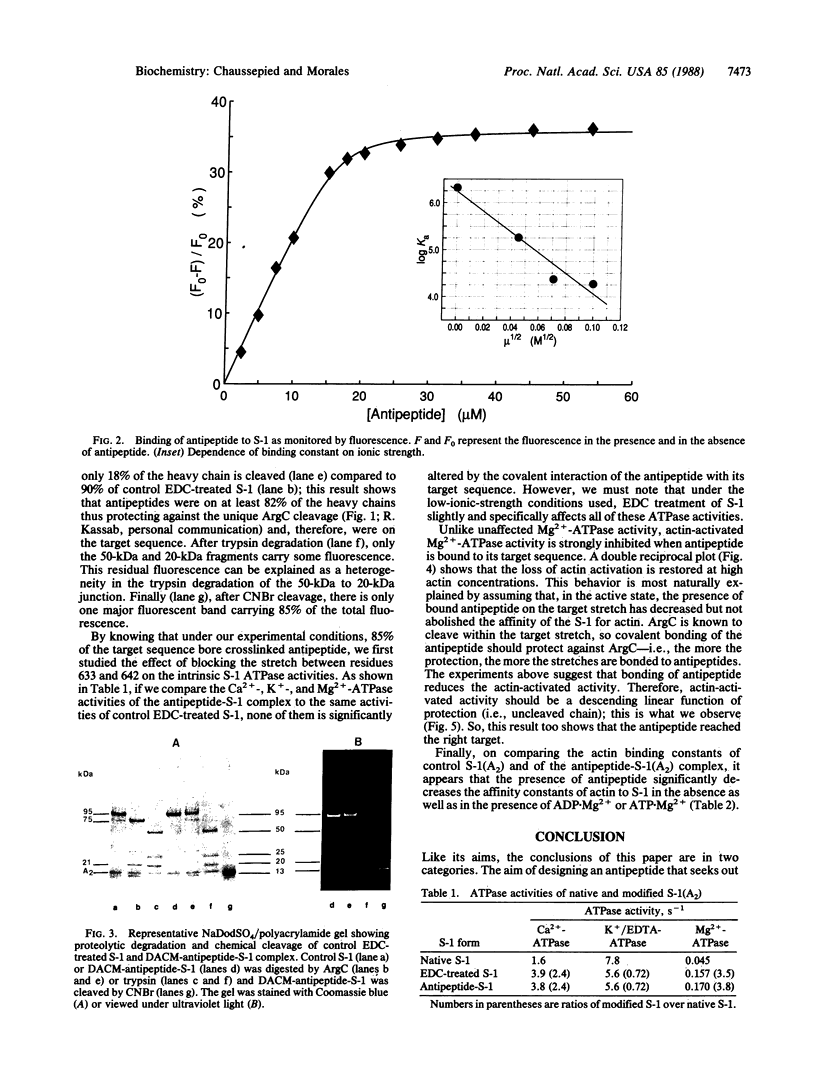
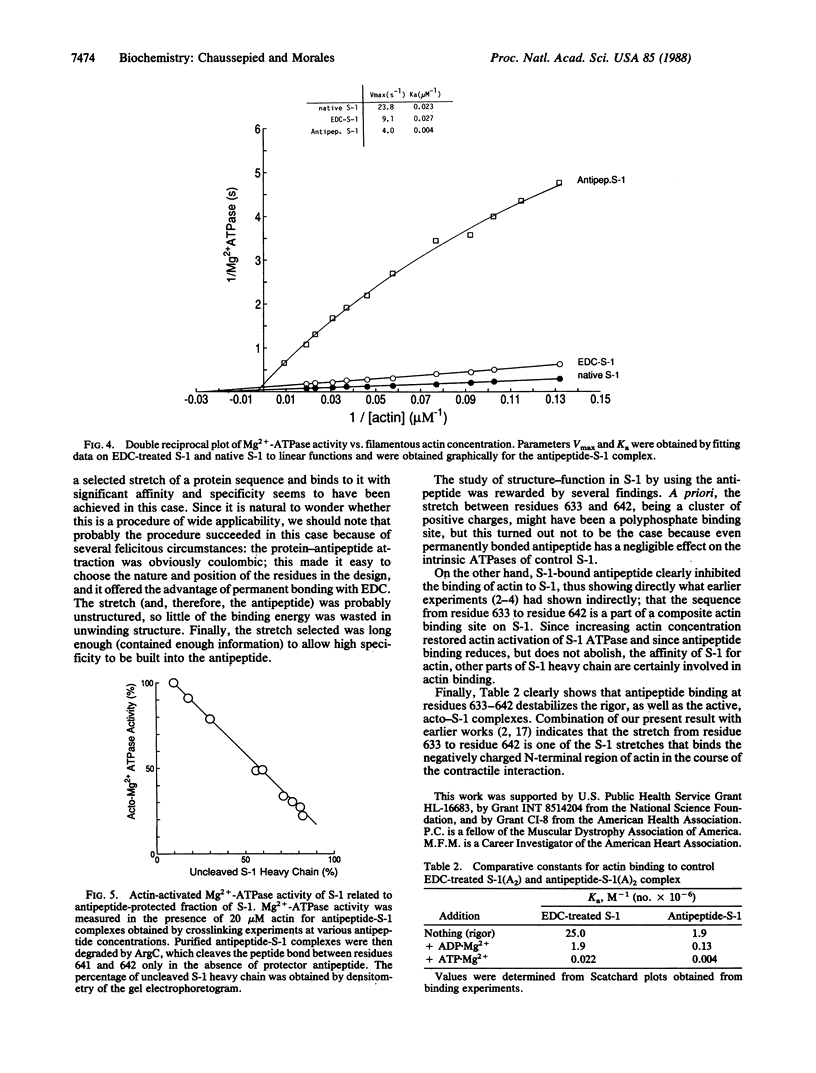
We have designed an "antipeptide" capable of firmly and specifically interacting with a preselected stretch of myosin S-1 heavy chain. Covalent attachment of this antipeptide to its target stretch, residues 633-642, does not affect the intrinsic ATPase activities of the protein but significantly reduces the actin-binding capabilities of the myosin head.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertrand R., Chaussepied P., Kassab R., Boyer M., Roustan C., Benyamin Y. Cross-linking of the skeletal myosin subfragment 1 heavy chain to the N-terminal actin segment of residues 40-113. Biochemistry. 1988 Jul 26;27(15):5728–5736. doi: 10.1021/bi00415a050. [DOI] [PubMed] [Google Scholar]
- Botts J., Muhlrad A., Takashi R., Morales M. F. Effects of tryptic digestion on myosin subfragment 1 and its actin-activated adenosinetriphosphatase. Biochemistry. 1982 Dec 21;21(26):6903–6905. doi: 10.1021/bi00269a043. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chaussepied P., Morales M. F., Kassab R. The myosin SH2-50-kilodalton fragment cross-link: location and consequences. Biochemistry. 1988 Mar 8;27(5):1778–1785. doi: 10.1021/bi00405a059. [DOI] [PubMed] [Google Scholar]
- Chaussepied P., Mornet D., Barman T. E., Travers F., Kassab R. Alteration of the ATP hydrolysis and actin binding properties of thrombin-cut myosin subfragment 1. Biochemistry. 1986 Mar 11;25(5):1141–1149. doi: 10.1021/bi00353a029. [DOI] [PubMed] [Google Scholar]
- Chaussepied P., Mornet D., Kassab R. Identification of polyphosphate recognition sites communicating with actin sites on the skeletal myosin subfragment 1 heavy chain. Biochemistry. 1986 Oct 21;25(21):6426–6432. doi: 10.1021/bi00369a013. [DOI] [PubMed] [Google Scholar]
- Chaussepied P., Mornet D., Kassab R. Nucleotide trapping at the ATPase site of myosin subfragment 1 by a new interthiol crosslinking. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2037–2041. doi: 10.1073/pnas.83.7.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Kielley W. W. Troponin-tropomyosin complex. Column chromatographic separation and activity of the three, active troponin components with and without tropomyosin present. J Biol Chem. 1974 Aug 10;249(15):4742–4748. [PubMed] [Google Scholar]
- Mandelkow E. M., Mandelkow E. Fluorimetric studies on the influence of metal ions and chelators on the interaction between myosin and ATP. FEBS Lett. 1973 Jul 1;33(2):161–166. doi: 10.1016/0014-5793(73)80183-8. [DOI] [PubMed] [Google Scholar]
- Mornet D., Pantel P., Audemard E., Kassab R. Involvement of an arginyl residue in the catalytic activity of myosin heads. Eur J Biochem. 1979 Oct 15;100(2):421–431. doi: 10.1111/j.1432-1033.1979.tb04185.x. [DOI] [PubMed] [Google Scholar]
- Mornet D., Pantel P., Audemard E., Kassab R. The limited tryptic cleavage of chymotryptic S-1: an approach to the characterization of the actin site in myosin heads. Biochem Biophys Res Commun. 1979 Aug 13;89(3):925–932. doi: 10.1016/0006-291x(79)91867-9. [DOI] [PubMed] [Google Scholar]
- Offer G., Moos C., Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and characterization. J Mol Biol. 1973 Mar 15;74(4):653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- Sutoh K. Identification of myosin-binding sites on the actin sequence. Biochemistry. 1982 Jul 20;21(15):3654–3661. doi: 10.1021/bi00258a020. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Sekine T. Interaction of myosin subfragment-1 with actin. I. Effect of actin binding on the susceptibility of subfragment-1 to trypsin. J Biochem. 1979 Dec;86(6):1855–1862. doi: 10.1093/oxfordjournals.jbchem.a132708. [DOI] [PubMed] [Google Scholar]



