Abstract
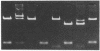
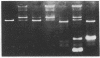
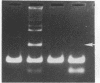
We have partially purified a DNA strand-exchange activity (recombinase) from nuclear extracts of Drosophila melanogaster embryos. The protein fraction forms a joint molecule between a circular single-strand DNA and a homologous linear duplex DNA that is resolved from the substrates by agarose gel electrophoresis. A strand-exchange activity can be obtained from nuclear extracts from embryos as old as 24 hr. The activity is similar to that partially purified from human cells [Hsieh, P., Meyn, S.M. & Camerini-Otero, R.D. (1986) Cell 44, 885-894]. It is homology-dependent, requires Mg2+, appears to be directional in that it prefers to displace the 3' end of the noncomplementary strand, and does not require exogenous ATP. Forty nanograms of protein in the partially purified DNA strand-exchange fraction from D. melanogaster embryos can completely convert 50 ng of substrate single-strand DNA into joint molecules in 10 min. In the electron microscope, joint molecules are seen to consist of a circular single-strand DNA molecule attached to only one end of a linear duplex DNA molecule; a displaced strand is also seen. The region of heteroduplex formation can be as long as 600 base pairs. The demonstration of a strand-exchange activity from wild-type D. melanogaster embryos invites analysis of recombination-defective mutants to explore the role of DNA strand exchange in homologous recombination.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., Yancopoulos G. D. Development of the primary antibody repertoire. Science. 1987 Nov 20;238(4830):1079–1087. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- Baker B. S., Carpenter A. T., Esposito M. S., Esposito R. E., Sandler L. The genetic control of meiosis. Annu Rev Genet. 1976;10:53–134. doi: 10.1146/annurev.ge.10.120176.000413. [DOI] [PubMed] [Google Scholar]
- Borst P., Greaves D. R. Programmed gene rearrangements altering gene expression. Science. 1987 Feb 6;235(4789):658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- Cassuto E., Lightfoot L. A., Howard-Flanders P. Partial purification of an activity from human cells that promotes homologous pairing and the formation of heteroduplex DNA in the presence of ATP. Mol Gen Genet. 1987 Jun;208(1-2):10–14. doi: 10.1007/BF00330415. [DOI] [PubMed] [Google Scholar]
- Cox M. M., Lehman I. R. Enzymes of general recombination. Annu Rev Biochem. 1987;56:229–262. doi: 10.1146/annurev.bi.56.070187.001305. [DOI] [PubMed] [Google Scholar]
- Fishel R. A., Detmer K., Rich A. Identification of homologous pairing and strand-exchange activity from a human tumor cell line based on Z-DNA affinity chromatography. Proc Natl Acad Sci U S A. 1988 Jan;85(1):36–40. doi: 10.1073/pnas.85.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganea D., Moore P., Chekuri L., Kucherlapati R. Characterization of an ATP-dependent DNA strand transferase from human cells. Mol Cell Biol. 1987 Sep;7(9):3124–3130. doi: 10.1128/mcb.7.9.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda M. A. Molecular genetics and structure of the human immunodeficiency virus. J Electron Microsc Tech. 1988 Jan;8(1):17–40. doi: 10.1002/jemt.1060080104. [DOI] [PubMed] [Google Scholar]
- Hotta Y., Tabata S., Bouchard R. A., Piñon R., Stern H. General recombination mechanisms in extracts of meiotic cells. Chromosoma. 1985;93(2):140–151. doi: 10.1007/BF00293161. [DOI] [PubMed] [Google Scholar]
- Hsieh P., Meyn M. S., Camerini-Otero R. D. Partial purification and characterization of a recombinase from human cells. Cell. 1986 Mar 28;44(6):885–894. doi: 10.1016/0092-8674(86)90011-5. [DOI] [PubMed] [Google Scholar]
- Kenne K., Ljungquist S. A DNA-recombinogenic activity in human cells. Nucleic Acids Res. 1984 Apr 11;12(7):3057–3068. doi: 10.1093/nar/12.7.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiec E., Holloman W. K. Homologous pairing of DNA molecules promoted by a protein from Ustilago. Cell. 1982 Jun;29(2):367–374. doi: 10.1016/0092-8674(82)90153-2. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Evans D. H., Morrison P. T. Purification and characterization of an activity from Saccharomyces cerevisiae that catalyzes homologous pairing and strand exchange. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5560–5564. doi: 10.1073/pnas.84.16.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez B., Rousset S., Coppey J. Homologous recombination intermediates between two duplex DNA catalysed by human cell extracts. Nucleic Acids Res. 1987 Jul 24;15(14):5643–5655. doi: 10.1093/nar/15.14.5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radding C. M. Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet. 1982;16:405–437. doi: 10.1146/annurev.ge.16.120182.002201. [DOI] [PubMed] [Google Scholar]
- Riddles P. W., Lehman I. R. The formation of paranemic and plectonemic joints between DNA molecules by the recA and single-stranded DNA-binding proteins of Escherichia coli. J Biol Chem. 1985 Jan 10;260(1):165–169. [PubMed] [Google Scholar]
- Riddles P. W., Lehman I. R. The formation of plectonemic joints by the recA protein of Escherichia coli. Requirement for ATP hydrolysis. J Biol Chem. 1985 Jan 10;260(1):170–173. [PubMed] [Google Scholar]
- Varmus H. E. The molecular genetics of cellular oncogenes. Annu Rev Genet. 1984;18:553–612. doi: 10.1146/annurev.ge.18.120184.003005. [DOI] [PubMed] [Google Scholar]
- Walker G. C., Marsh L., Dodson L. A. Genetic analyses of DNA repair: inference and extrapolation. Annu Rev Genet. 1985;19:103–126. doi: 10.1146/annurev.ge.19.120185.000535. [DOI] [PubMed] [Google Scholar]
- von Wettstein D., Rasmussen S. W., Holm P. B. The synaptonemal complex in genetic segregation. Annu Rev Genet. 1984;18:331–413. doi: 10.1146/annurev.ge.18.120184.001555. [DOI] [PubMed] [Google Scholar]