Summary
Long-term survival of persistent bacterial pathogens in mammalian hosts critically depends on their ability to avoid elimination by innate and adaptive immune responses. The persistent human pathogens that cause typhoid fever and tuberculosis exemplify alternative strategies for survival in the host: immune evasion and immune adaptation, respectively. Salmonella enterica serotype Typhi evades host innate immune responses and inflammation by expressing factors that interfere with its detection as a Gram-negative bacterium, enabling persistent colonization of an immunologically privileged niche, the gallbladder. In contrast, Mycobacterium tuberculosis has adapted to survive within phagocytic cells, which typically eliminate invading microbes, by deploying stress resistance mechanisms that counteract the harsh environment of the phagolysosome.
Introduction
Many pathogenic bacteria cause acute infections characterized by an adaptive immune response that clears the invading microbe and generates immunological memory. Some bacterial pathogens, however, maintain infections for the lifetime of their mammalian hosts without causing overt disease signs or symptoms, despite triggering a robust adaptive immune response [1,2]. These persistent bacterial pathogens depend on infected carriers for survival in the host population and transmission to naïve individuals.
Salmonella enterica serotype Typhi (S. Typhi), the bacterium that causes systemic typhoid fever, establishes persistent infection of the gallbladder in 1–4% of typhoid patients [3]. Typhoid carriers are asymptomatic but periodically shed large numbers of S. Typhi in their stools. Periodic transmission from asymptomatic carriers is essential for long-term maintenance of S. Typhi in human populations. Mary Mallone, a notorious typhoid carrier better known as “Typhoid Mary”, infected at least 57 people in New York City before she was confined to lifelong quarantine in 1907. Similarly, Mycobacterium tuberculosis typically persists as an asymptomatic latent infection of immune competent humans before reactivating to cause full-blown tuberculosis (TB) disease that can be transmitted to new hosts. Compelling molecular evidence for decades-long persistence of latent M. tuberculosis came from molecular subtyping experiments conducted on more than 2,000 clinical M. tuberculosis specimens [4]. Remarkably, isolates from a father and son whose TB diagnoses were separated by 33 years had identical DNA restriction fragment patterns indicating an epidemiological relationship. These were the only samples with a specific molecular signature, strongly suggesting direct transmission of M. tuberculosis from father to son, followed by a 33 year period of latency [5].
Although both S. Typhi and M. tuberculosis avoid elimination by the immune response, these pathogens use different strategies to persist in their hosts. S. Typhi evades host immunity by expressing factors that reduce the host inflammatory response, enabling systemic invasion and colonization of the gallbladder, a privileged anatomical site that receives little immune surveillance. In contrast, M. tuberculosis employs stress resistance mechanisms to counteract the harsh environment of the activated macrophage phagolysosome, a compartment that typically eliminates microbial invaders.
S. Typhi immune evasion - molecular masking
S. Typhi is a human-specific pathogen that is related to the non-typhoidal serotype Salmonella enterica serotype Typhimurium (S. Typhimurium), which causes gastroenteritis. Because S. Typhi is host-specific, it does not persist in animals that are used as infection models. S. Typhimurium has a broader host range that includes mice as a model to study Salmonella persistence (Box 1). Both Salmonella species initiate infection by invading the intestinal mucosa, a process that requires two type 3 secretion systems (T3SS-1 and T3SS-2), which promote uptake by intestinal epithelial cells and survival in host macrophages, respectively (Figure 1) [6,7]. Mucosal invasion by S. Typhimurium is detected by pattern recognition receptors including Toll-like receptor 4 (TLR4), which recognizes lipopolysaccharide (LPS), and TLR5, which binds flagellin. Activation of TLR signaling induces expression of inflammatory cytokines such as IL-8 and TNF-α, which recruit neutrophils to contain the infection [6]. In contrast, S. Typhi invasion of the intestinal mucosa does not trigger neutrophil influx, allowing the bacteria to disseminate to the liver, spleen, bone marrow, and gall bladder (Figure 1) [8]. S. Typhi evasion of innate immunity suggests that it can disguise its identity as a Gram-negative bacterial pathogen.
Box 1. Persistent Infection by Salmonella enterica serotype Typhimurium
A mouse model of persistent S. Typhimurium infection that recapitulates important aspects of typhoid fever has begun to shed light on Salmonella immune evasion mechanisms. Persistent infection with S. Typhimurium can be achieved in mice that express wild type Nramp1, an ion transport protein that restricts availability of divalent cations to intracellular pathogens [45]. S. Typhimurium colonizes the mesenteric lymph nodes of these mice within infected macrophages, and is occasionally found in the gallbladder, liver, and spleen [45]. Some mice (~25%) become “super-shedders” that continuously excrete S. Typhimurium in their stools and spread the infection to naïve mice [46]. Factors that contribute to S. Typhimurium persistence include components of both type 3 secretion systems (T3SS-1 and T3SS-2) [47]. The T3SS-2 effector protein SseI is required for long-term systemic infection of mice [48]. SseI interferes with the migration of infected cells by specifically binding the cell migration regulator IQGAP, thereby preventing normal dendritic cell migration to lymphoid tissues. These activities may constitute a mechanism for limiting presentation of Salmonella antigens and naïve T cell priming to inhibit adaptive immunity [48].
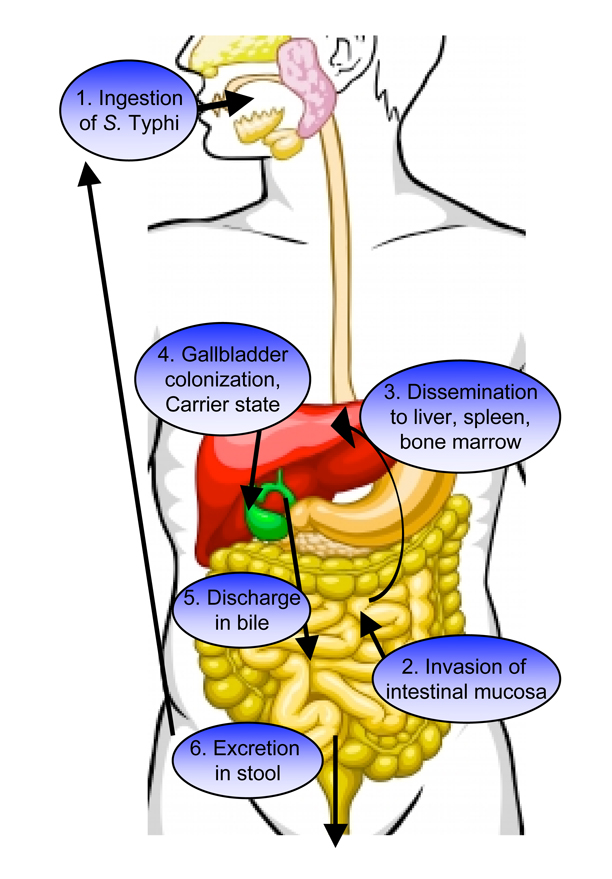
Figure 1. Pathogenesis of Salmonella Typhi infection.
Typhoid fever is acquired by ingestion of food or water contaminated with S. Typhi (1). Bacteria that survive passage through the gastric acid barrier of the stomach invade intestinal epithelial cells and migrate through them to reach the lamina propria (2). In the intestinal mucosa, S. Typhi is phagocytosed by macrophages and survives within these phagocytic cells by T3SS-2 mediated secretion of effectors that interfere with host cell function. Following invasion, S. Typhi expresses factors that inhibit detection by the host innate immune system. This “masking” enables the bacteria to disseminate systemically to colonize macrophages in the liver (shown in red), spleen, and bone marrow (3). From the liver, S. Typhi can reach the gallbladder (shown in green) in bile. Infection of the gallbladder can lead to conversion to an asymptomatic carrier state (4). S. Typhi carriers continuously discharge the typhoid bacillus from the gallbladder to the small intestine in bile (5) and excrete viable bacteria in their stools (6) that can infect naïve hosts.
Whole-genome sequencing revealed striking genetic differences between S. Typhi and other Salmonellae, including the presence of a unique 134 kbp region designated Salmonella pathogenicity island 7 (SPI-7) [9]. SPI-7 is genetically unstable and is readily lost during passage of S. Typhi in the laboratory [6]. SPI-7 deficient S. Typhi strains fail to inhibit inflammatory cytokine production in cultured human colonic epithelial cells, suggesting that this locus is important for immune evasion [10].
SPI-7 encodes functions for production and export of the Vi capsular polysaccharide antigen. The Vi capsule is expressed during human S. Typhi infection and contributes significantly to S. Typhi pathogenesis in human volunteers [6]. Ectopic expression of the Vi capsule in S. Typhimurium reduces TLR4-dependent production of the pro-inflammatory cytokine TNF-α in cultured macrophages and in mice [11•]. Although the mechanism by which Vi capsule prevents TLR4 signaling is unclear, it is possible that the capsule layer shields LPS from detection by TLR4, since strains expressing the Vi capsule are not agglutinated by antibody against the LPS O-antigen [11•].
SPI-7 also encodes TviA, a regulatory protein that controls expression of the Vi capsule, flagellar motility, and the invasion-associated T3SS-1 in response to osmolarity, in cooperation with the RcsC/RcsD/RcsB signal transduction system [12••]. Under conditions of low osmolarity, Vi capsule production is induced and genes encoding T3SS-1 and the flagellar apparatus are repressed in a TviA- and RcsB-dependent manner [12••]. Inverse osmo-regulation of flagellar motility and T3SS-1 (required for mucosal invasion) and Vi capsule (required for systemic immune evasion) has important consequences for S. Typhi pathogenesis. During invasion of the intestinal mucosa, S. Typhi encounters relatively high osmolarity in the intestinal lumen followed by low osmolarity inside host tissue. This environmental shift should promote expression of Vi capsule and repression of motility and T3SS-1 post-invasion. Analysis of Vi capsule expression in a bovine ligated ileal loop model revealed increased expression of Vi capsule by S. Typhi associated with host tissue compared to bacteria in the intestinal lumen [14]. In addition, TviA-mediated repression of flagellin expression avoids detection of S. Typhi by host TLR5. Compared to wild-type S. Typhi, a tviA mutant produced more flagellin and induced more TLR5-dependent pro-inflammatory IL-8 production by human colonic epithelial cells [13].
S. Typhi gallbladder persistence - bile resistance
While induction of Vi capsule and repression of flagellin contribute to S. Typhi immune evasion and systemic infection, persistent colonization of the gallbladder depends on additional factors including bile resistance. Bile - a lipid-rich, detergent-like digestive secretion with antimicrobial properties - is produced by the liver and concentrated in the gallbladder for delivery to the small intestine [15]. A genome-wide screen identified more than 150 S. Typhi genes required for bile tolerance [16]. Among the putative bile resistance genes are acrAB and tolC, encoding a bile acids efflux system, and LPS biosynthesis genes [16].
S. Typhi may also resist bile by forming biofilms on gallstones. S. Typhi biofilms, comprising microcolonies encased in an exopolysaccharide (EPS) matrix, are resistant to environmental insults and host immune mechanisms [17]. Bile induces production of an EPS O-antigen that facilitates S. Typhi biofilm formation on human gallstones [18]. Gallstone biofilms may promote S. Typhi carriage in the gallbladder by increasing bile resistance. Indeed, conversion to the chronic typhoid carrier state is strongly correlated with the presence of gallstones [3].
S. Typhi may also persist within gallbladder epithelial cells, a unique niche of S. Typhimurium replication identified in a mouse model of acute typhoid fever [19•]. Invasion of the gallbladder epithelium requires a functional T3SS-1, but induces strong inflammatory responses and neutrophil influx [19•]. It is conceivable that S. Typhi could limit this inflammatory response to persist in the gallbladder epithelium using the same immune evasion tactics that enable it to disseminate systemically.
Mycobacterium tuberculosis persistence in macrophages - stress management
Throughout infection M. tuberculosis persists within host phagocytic cells (Figure 2), which normally serve as a first line of host defense by internalizing and destroying microorganisms within phagolysosomes. The acidic pH of phagolysosome compartments suppresses microbial metabolism and activates intralumenal hydrolytic enzymes that degrade bacterial components such as proteins and lipids [20,21]. Reactive oxygen and nitrogen species (ROS and RNS) are generated in the maturing phagolysosome by the NADPH phagocyte oxidase (NOX2) and inducible nitric oxide synthase (iNOS), respectively [22]. ROS and RNS kill bacteria by damaging protein tyrosine residues, DNA bases, lipids, thiols, and metal centers [22]. Phagolysosomes also contain cationic antimicrobial peptides (CAMPs) that permeabilize bacterial cell membranes [21,23].
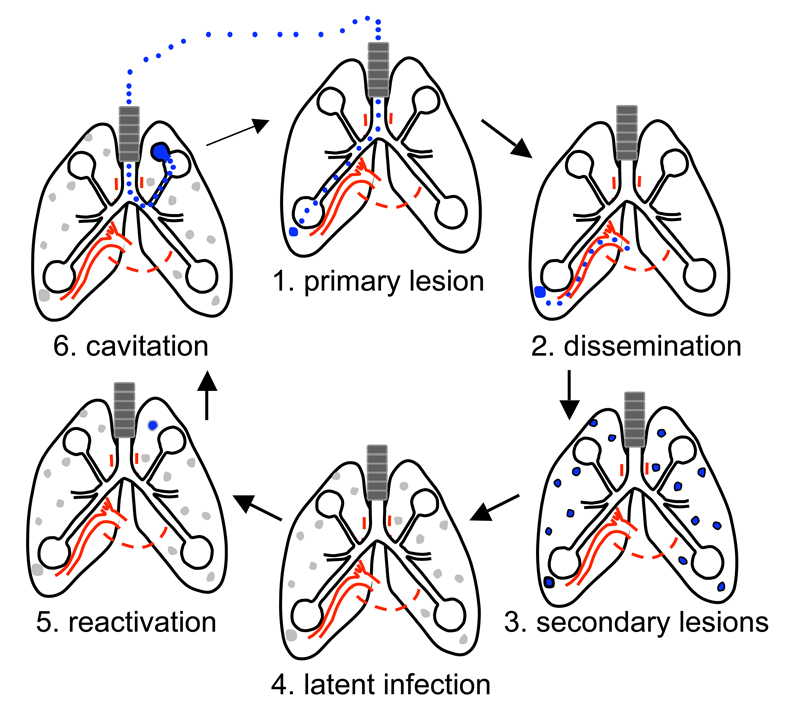
Figure 2. Pathogenesis of Mycobacterium tuberculosis infection.
Infection with M. tuberculosis is initiated by inhalation of aerosols containing the bacterium (1). In the lung, M. tuberculosis is rapidly phagocytosed by resident alveolar macrophages. Actively replicating bacilli (shown in blue) induce inflammatory responses that recruit blood monocyte-derived macrophages. During growth of the primary lesion, some infected cells disseminate systemically to seed secondary lesions elsewhere in the lung (2, 3). In the majority of individuals, M. tuberculosis enters a latent state characterized by bacteria that are relatively quiescent (shown in grey) (4). When the immune system is compromised, for example by old age or HIV infection, M. tuberculosis resumes active growth (usually in secondary lesions) and elicits overt signs and symptoms of disease (5). Replication of M. tuberculosis leads to growth of the lesion and tissue destruction, which releases infectious bacteria into the lung airways (6). The characteristic cough of tuberculosis generates aerosols containing bacteria that can be inhaled by a naïve host to initiate a new round of infection.
M. tuberculosis produces lipid and protein factors that block phagosome maturation and phago-lysosome fusion in non-activated macrophages [24]. Although IFN-γ-activated macrophages deliver M. tuberculosis to a mature phagolysosome, the bacteria are nonetheless capable of surviving in this harsh environment [25]. Consistent with these observations, M. tuberculosis mutants that fail to block phagosome-lysosome fusion in resting macrophages are not impaired for intracellular survival [26,27]. A growing body of evidence (see below) indicates that persistence of M. tuberculosis in mature phagolysosomes is due to specific mechanisms that counteract the stresses inflicted by activated macrophages. Thus, in contrast to S. Typhi, which persists by evading the host immune response and colonizing a niche outside immune surveillance, M. tuberculosis persists by counteracting host immune mechanisms and colonizing a niche within the immune system. This “counter-immune” strategy is shared by some but not all mycobacterial pathogens. For example, the related pathogen Mycobacterium ulcerans evades host immunity by secreting mycolactone, a cytotoxic compound with immunomodulatory properties (Box 2).
Box 2. Immune Suppression by Mycobacterium ulcerans
Mycobacterium ulcerans is closely related phylogenetically to M. tuberculosis, but causes a markedly different disease, Buruli ulcer (BU), which is characterized by painless necrotic skin lesions [49•]. Replication and persistence of M. ulcerans in BU lesions is profoundly influenced by production of mycolactone, a macrolide cytotoxin that has immunosuppressive properties. Although the precise cellular targets of mycolactone remain mysterious, it induces apoptosis of infected host cells [49•], inhibits production of the pro-inflammatory cytokine TNF-α by macrophages [50], and suppresses dendritic cell priming of T cells [51]. Mycolactone synthesized by M. ulcerans can diffuse from infected skin tissue to lymphoid organs within mononuclear cells, where it may exert some of these immune suppressive functions [52]. Mycolactone also damages nerve cells, which contributes to the painlessness of BU [53]. The genes required for mycolactone synthesis are carried on a virulence plasmid, pMUM001, that was apparently acquired by horizontal gene transfer from an unknown source [49•]. Perhaps because mycolactone came to dominate the interaction of M. ulcerans with its human host, following acquisition of pMUM001 other mycobacterial virulence factors were apparently lost by reductive evolution [49•].
M. tuberculosis counteracts the toxic ROS and RNS encountered in the phagolysosome by three general strategies: enzymatic detoxification, scavenging, and damage repair (reviewed in [28]). M. tuberculosis also requires a protein degradation complex, the proteasome, to counteract damage caused by RNS. Mutations in genes encoding two putative accessory factors of the proteasome, mpa and pafA, confer sensitivity to RNS [29]. Attenuation of Mpa-deficient bacteria in wild-type mice is partially reversed in iNOS−/− mice, suggesting that proteasome-mediated protein degradation is required to counteract RNS and other stresses that M. tuberculosis encounters in the host [29]. Depletion of the proteasomal core subunits PrcBA, which have proteolytic activity against a broad range of peptide substrates, also renders M. tuberculosis more sensitive to RNS and impairs persistence in the lungs of mice [30•].
Mpa is the proteasomal ATPase that interacts with the core proteasome in the presence of ATP and probably unfolds and translocates proteins into the protease core for degradation [31]. Mpa specifically recognizes proteins that are targeted for degradation by a short peptide tag, the prokaryotic ubiquitin-like protein Pup [32••,33]. The Pup tag is coupled to target proteins in two steps: deamidation of the Pup C-terminal glutamine by Dop and subsequent conjugation to target proteins by PafA [34]. Proteasomal degradation of Pup-tagged proteins, mediated by Mpa and PafA, might be required for survival of nitrosative stress because it removes proteins that are irreversibly damaged by RNS, removes specific RNS-damaged proteins that are toxic, or upregulates transcription of genes encoding anti-oxidants by removing a transcriptional repressor [35].
M. tuberculosis factors that contribute to acid resistance and pH homeostasis were identified by screening for acid-sensitive mutants. Most of the identified mutations affect genes required for biogenesis of the mycobacterial cell wall, a complex lipid-rich structure that functions as a permeability barrier [36]. These mutations also confer hypersensitivity to other stressors, including lipophilic antibiotics and detergents, suggesting increased permeability of the cell wall to these compounds as well as to protons [36]. Apparently the cell wall permeability barrier also contributes to survival of M. tuberculosis in acidified phagolysosomes, since cell wall deficient mutants are attenuated in IFN-γ-activated macrophages and in mice [36].
One of the factors required for acid resistance is Rv3671c, a membrane-localized serine protease that is critical for maintenance of M. tuberculosis pH homeostasis. Unlike wild-type M. tuberculosis, the Rv3671c mutant failed to maintain neutral cytoplasmic pH either in vitro in acidified medium or in vivo in the phagolysosomes of IFN-γ-activated macrophages [37••]. Although its precise function is unknown, the Rv3671c protease might modify the mycobacterial cell wall or activate stress-response signaling pathways to maintain intrabacterial pH and promote survival in acidified phagolysosomes [38].
Within mature phagolysosomes M. tuberculosis also encounters CAMPs including cathelicidin, hepcidin, and ubiquitin-derived peptides that kill by disrupting the bacterial cell wall [39–41]. Other bacteria avoid CAMP-mediated cytolysis by reducing their surface negative charge, thus reducing their affinity for the positively charged CAMPs [42]. M. tuberculosis also resists CAMPs by cell surface modification. The LysX lysine transferase is required for linkage of positively charged lysine moieties to phosphatidyl glycerol (PG), a lipid component of the M. tuberculosis membrane [43•]. LysX-deficient bacteria are more sensitive to HNP-1, a CAMP produced by neutrophils, as well as the cationic antibiotics vancomycin and polymyxin-B [43•]. Replication of lysX mutant bacteria is impaired in animal infection models, indicating that PG lysinylation contributes to M. tuberculosis survival in the host [43•].
An unusually impermeable cell wall also protects M. tuberculosis from certain CAMPs. Mycobacterium smegmatis, a non-pathogenic relative of M. tuberculosis, is sensitive to ubiquitin-derived peptides within the mature phagolysosome [41]. Mutations in mspA, encoding a porin protein that forms aqueous channels in the M. smegmatis cell wall, confer resistance to ubiquitin-derived peptides [44]. Although the M. tuberculosis genome does not encode an MspA ortholog, expression of M. smegmatis mspA in M. tuberculosis increases membrane permeability and sensitivity to ubiquitin-derived peptides, and impairs survival in autophagic macrophages that deliver ubiquitin-derived peptides to the maturing phagolysosome [44]. Thus, the relative impermeability of the M. tuberculosis cell wall may have evolved as a mechanism to increase resistance to acidic pH and CAMPs encountered within mature phagolysosomes.
Conclusions
Although S. Typhi and M. tuberculosis use contrasting strategies to persist in their human hosts, both effectively thwart the immune system. Whereas S. Typhi evades host immunity in order to invade and colonize an immunologically privileged niche (the gallbladder), M. tuberculosis counteracts host immunity in order to establish a niche within the cell-mediated immune system (the macrophage). Recent work has begun to identify the gene products that allow M. tuberculosis to counteract the stresses encountered in macrophage phagolysosomes, but the underlying mechanisms of stress resistance remain ill defined. For example, although the proteasome is clearly important for resistance to RNS and persistence in mice, it is unclear whether the proteasome’s critical role is general turnover of proteins that have incurred irreparable damage, or degradation of specific proteins that control the nitrosative stress response. Similarly, it is still largely unknown which mycobacterial cell wall components confer impermeability to protons and CAMPs. In the case of S. Typhi, although examination of genetic elements unique to this species has revealed novel mechanisms of immune evasion, the mechanisms that contribute to S. Typhi persistence in chronic carriers remain largely a black box. Despite the impressive recent advances reviewed here, for persistent investigators in both fields the most exciting discoveries undoubtedly still lie ahead.
Acknowledgements
A.D.T. is supported by an Irvington Institute Post-doctoral Fellowship of the Cancer Research Institute. J.D.M. acknowledges support from National Institutes of Health Grants AI046392 and HL088906.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anna D. Tischler, Global Health Institute, Swiss Federal Institute of Technology (EPFL), EPFL/SV/GHI/UPKIN, Station 19, CH-1015 Lausanne, SWITZERLAND, anna.tischler@epfl.ch
John D. McKinney, Global Health Institute, Swiss Federal Institute of Technology (EPFL), EPFL/SV/GHI/UPKIN, Station 19, CH-1015 Lausanne, SWITZERLAND, john.mckinney@epfl.ch
References and recommended reading
- 1.Young D, Hussell T, Dougan G. Chronic bacterial infections: living with unwanted guests. Nat. Immunol. 2002;3:1026–1032. doi: 10.1038/ni1102-1026. [DOI] [PubMed] [Google Scholar]
- 2.Monack DM, Mueller A, Falkow S. Persistent bacterial infections: the interface of the pathogen and the host immune system. Nat. Rev. Microbiol. 2004;2:747–765. doi: 10.1038/nrmicro955. [DOI] [PubMed] [Google Scholar]
- 3.Parry CM, Hien TT, Dougan G, White N, Farrar JJ. Typhoid fever. N. Engl. J. Med. 2002;347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 4.Lillebaek T, Dirksen A, Vynnycky E, Baess I, Thomsen VØ, Andersen AB. Stability of DNA patterns and evidence of Mycobacterium tuberculosis reactivation occurring decades after the initial infection. J. Infect. Dis. 2003;188:1032–1039. doi: 10.1086/378240. [DOI] [PubMed] [Google Scholar]
- 5.Lillebaek T, Dirksen A, Baess I, Strunge B, Thomsen VØ, Andersen AB. Molecular evidence of endogenous reactivation of Mycobacterium tuberculosis after 33 years of latent infection. J. Infect. Dis. 2002;185:401–404. doi: 10.1086/338342. [DOI] [PubMed] [Google Scholar]
- 6.Raffatellu M, Chesa D, Wilson RP, Tükel C, Akcelik M, Bäumler A. Capsule-mediated immune evasion: a new hypothesis explaining aspects of typhoid fever pathogenesis. Infect. Immun. 2006;74:19–27. doi: 10.1128/IAI.74.1.19-27.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibara JA, Steele-Mortimer O. Salmonella - the ultimate insider: Salmonella virulence factors that modulate intracellular survival. Cell. Microbiol. 2009;11:1579–1586. doi: 10.1111/j.1462-5822.2009.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsolis RM, Young GM, Solnick JV, Bäumler AJ. From bench to bedside: stealth of enteroinvasive pathogens. Nat. Rev. Microbiol. 2008;6:883–892. doi: 10.1038/nrmicro2012. [DOI] [PubMed] [Google Scholar]
- 9.Baker S, Dougan G. The genome of Salmonella enterica serovar Typhi. Clin. Infect. Dis. 2007;45:S29–S33. doi: 10.1086/518143. [DOI] [PubMed] [Google Scholar]
- 10.Raffatellu M, Chessa D, Wilson RP, Dusold R, Rubino S, Bäumler AJ. The Vi-capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent IL-8 expression in the intestinal mucosa. Infect. Immun. 2005;73:3367–3374. doi: 10.1128/IAI.73.6.3367-3374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilson RP, Raffatellu M, Chessa D, Winter SE, Tükel C, Bäumler AJ. The Vi-capsule prevents Toll-like receptor 4 recognition of Salmonella. Cell. Microbiol. 2008;10:876–890. doi: 10.1111/j.1462-5822.2007.01090.x. This paper demonstrated for the first time that ectopic expression of the Vi capsular polysaccharide by S. Typhimurium is sufficient to reduce the TLR4-dependent inflammatory response in murine infection models by "masking" detection of lipopolysaccharide.
- 12. Winter SE, Winter MG, Thiennimitr P, Gerriets VA, Nuccio S-P, Rüssmann H, Bäumler AJ. The TviA auxiliary protein renders the Salmonella enterica serotype Typhi RcsB regulon responsive to changes in osmolarity. Mol. Microbiol. 2009;74:175–193. doi: 10.1111/j.1365-2958.2009.06859.x. This study revealed that osmolarity, an environmental signal that changes upon S. Typhi invasion of the intestinal mucosa, is integrated by the S. Typhi-specific transcription factor TviA to control expression of virulence factors. The authors demonstrate that TviA mediates the inverse regulation of the Vi capsule with flagellar motility and the invasion-associated T3SS in response to changes in osmolarity, and suggest that this inverse regulation is essential for appropriate timing of virulence factor expression.
- 13.Winter SE, Raffatellu M, Wilson RP, Rüssmann H, Bäumler AJ. The Salmonella enterica serotype Typhi regulator TviA reduces interleukin-8 production in intestinal epithelial cells by repressing flagellin secretion. Cell. Microbiol. 2008;10:247–261. doi: 10.1111/j.1462-5822.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- 14.Tran QT, Gomez G, Khare S, Lawhon SD, Raffatellu M, Bäumler AJ, Ajithdoss D, Dhavala S, Adams LG. The Salmonella enterica serotype Typhi Vi capsular antigen is expressed after the bacterium enters the ileal mucosa. Infect. Immun. 2010;78 doi: 10.1128/IAI.00972-09. doi:10.1128/IAI00972-00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Begley M, Gahan CGM, Hill C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005;29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Langridge GC, Phan M-D, Turner DJ, Perkins TT, Parts L, Haase J, Charles I, Maskell DJ, Peters SE, Dougan G, et al. Simultaneous assay of every Salmonella Typhi gene using one million transposon mutants. Genome Res. 2009 doi: 10.1101/gr.097097.109. doi:10.1101/gr/097097.097109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell. Microbiol. 2009;11:1034–1043. doi: 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 18.Crawford RW, Gibson DL, Kay WW, Gunn JS. Identification of a bile-induced exopolysaccharide required for Salmonella biofilm formation on gallstone surfaces. Infect. Immun. 2008;76:5341–5349. doi: 10.1128/IAI.00786-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Menendez A, Arena ET, Guttman JA, Thorson L, Vallance BA, Vogl W, Finlay BB. Salmonella infection of gallbladder epithelial cells drives local inflammation and injury in a model of acute typhoid fever. J. Infect. Dis. 2009;200:1703–1013. doi: 10.1086/646608. Using a mouse model of S. Typhimurium infection, the authors demonstrate for the first time that Salmonella can invade and replicate within gallbladder epithelial cells, a potential site of persistence in chronic typhoid carriers.
- 20.Huynh KK, Grinstein S. Regulation of vacuolar pH and its modulation by some microbial species. Microbiol. Mol. Biol. Rev. 2007;71:452–462. doi: 10.1128/MMBR.00003-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flannagan RS, Cosîo G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat. Rev. Microbiol. 2009;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- 22.Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nar. Rev. Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 23.Purdy GE. Lysosomal ubiquitin and the demise of Mycobacterium tuberculosis. Cell. Microbiol. 2007;9:2768–2774. doi: 10.1111/j.1462-5822.2007.01039.x. [DOI] [PubMed] [Google Scholar]
- 24.Philips JA. Mycobacterial manipulation of vacuolar sorting. Cell. Microbiol. 2008;10:2408–2415. doi: 10.1111/j.1462-5822.2008.01239.x. [DOI] [PubMed] [Google Scholar]
- 25.Via Le, Fratti RA, McFalone M, Pagán-Ramos E, Deretic D, Deretic V. Effects of cytokines on mycobacterial phagosome maturation. J. Cell Sci. 1998;111:897–905. doi: 10.1242/jcs.111.7.897. [DOI] [PubMed] [Google Scholar]
- 26.Pethe K, Swenson DL, Alonso S, Anderson J, Wang C, Russell DG. Isolation of Mycobacterium tuberculosis mutations defective in the arrest of phagosome maturation. Proc. Natl. Acad. Sci. USA. 2004;101:13642–13647. doi: 10.1073/pnas.0401657101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacGurn JA, Cox JS. A genetic screen for Mycobacterium tuberculosis mutants defective for phagosome maturation arrest identifies components of the ESX-1 secretion system. Infect. Immun. 2007;75:2668–2678. doi: 10.1128/IAI.01872-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehrt S, Schnappinger D. Mycobacterial survival strategies in the phagosome: defense against host stresses. Cell. Microbiol. 2009;11:1170–1178. doi: 10.1111/j.1462-5822.2009.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darwin KH, Ehrt S, Gutierrez-Ramos JC, Weich N, Nathan CF. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science. 2003;302:1963–1966. doi: 10.1126/science.1091176. [DOI] [PubMed] [Google Scholar]
- 30. Gandotra S, Schnappinger D, Monteleone M, Hillen W, Ehrt S. In vivo gene silencing identifies the Mycobacterium tuberculosis proteasome as essential for the bacteria to persist in mice. Nat. Med. 2007;13:1515–1520. doi: 10.1038/nm1683. The authors use a conditional gene silencing technique to demonstrate that the core proteasome subunits, which are required for optimal growth of M. tuberculosis in vitro, are also required for persistence in the lungs of mice. This was the first time that conditional gene expression was successfully used to analyze the contribution of an M. tuberculosis gene to persistence in vivo.
- 31.Wang T, Li H, Lin G, Tang C, Li D, Nathan C, Darwin KH, Li H. Structural insights on the Mycobacterium tuberculosis proteasome ATPase Mpa. Structure. 2009;17:1377–1385. doi: 10.1016/j.str.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pearce MJ, Mintseris J, Ferreyra J, Gygi SP, Darwin KH. Ubiquitin-like protein involved in the proteasome pathway of Mycobacterium tuberculosis. Science. 2008;322:1104–1107. doi: 10.1126/science.1163885. Using bacterial two-hybrid systems, the authors identified a prokaryotic ubiquitin-like protein (Pup) that modifies substrates of the mycobacterial proteasome. This was the first demonstration that a proteasome-containing prokaryote uses post-translational protein modification to target specific protein substrates for degradation.
- 33.Sutter M, Striebel F, Damberger FF, Allain FH, Weber-Ban E. A distinct structural region of the prokaryotic ubiquitin-like protein (Pup) is recognized by the N-terminal domain of the proteasomal ATPase Mpa. FEBS Lett. 2009;583:3151–3157. doi: 10.1016/j.febslet.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Striebel F, Imkamp F, Sutter M, Steiner M, Mamedov A, Weber-Ban E. Bacterial ubiquitin-like modifier Pup is deamidated and conjugated to substrates by distinct but homologous enzymes. Nat. Struct. Mol. Biol. 2009;16:647–651. doi: 10.1038/nsmb.1597. [DOI] [PubMed] [Google Scholar]
- 35.Darwin KH. Prokaryotic ubiquitin-like protein (Pup), proteasomes and pathogenesis. Nat. Rev. Microbiol. 2009;7:485–491. doi: 10.1038/nrmicro2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vandal OH, Roberts JA, Odaira T, Schnappinger D, Nathan CF, Ehrt S. Acid-susceptible mutants of Mycobacterium tuberculosis share hypersusceptibility to cell wall and oxidative stress and to the host environment. J. Bacteriol. 2009;191:625–631. doi: 10.1128/JB.00932-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vandal OH, Pierini LM, Schnappinger D, Nathan CF, Erht S. A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat. Med. 2008;8:849–854. doi: 10.1038/nmXXXX. The authors used a pH-sensitive GFP and fluorescence microscopy to make non-invasive measurements of intra-bacterial pH on live M. tuberculosis. They demonstrate for the first time that a membrane-localized protease, Rv3671c, is required for maintenance of neutral cytoplasmic pH in both acidified growth medium and in the acidified phagosomes of activated macrophages.
- 38.Vandal OH, Nathan CF, Ehrt S. Acid resistance in Mycobacterium tuberculosis. J. Bacteriol. 2009;191:4714–4721. doi: 10.1128/JB.00305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: Vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 2007;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 40.Sow FB, Florence WC, Satoskar AR, Schlesinger LS, Zwilling BS, Lafuse WP. Expression and localization of hepcidin in macrophages: a role in host defense against tuberculosis. J. Leukoc. Biol. 2007;82:934–945. doi: 10.1189/jlb.0407216. [DOI] [PubMed] [Google Scholar]
- 41.Alonso S, Pethe K, Russell DG, Purdy GE. Lysosomal killing of Mycobacterium tuberculosis mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc. Natl. Acad. Sci, USA. 2007;104:6031–6036. doi: 10.1073/pnas.0700036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peschel A, Sahl H-G. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 2006;4:529–536. doi: 10.1038/nrmicro1441. [DOI] [PubMed] [Google Scholar]
- 43. Maloney E, Stankowska D, Zhang J, Fol M, cheng Q-J, Lun S, Bishai WR, Rajagopalan M, Chatterjee D, Madiraju MV. The two-domain LysX protein of Mycobacterium tuberculosis is required for production of lysinylated phosphatidylglycerol and resistance to cationic antimicrobial peptides. PLoS Pathog. 2009;5:e1000534. doi: 10.1371/journal.ppat.1000534. The authors identify one component of the mycobacterial cell wall that is critical for resistance to cationic antimicrobial peptides and antibiotics and replication in animal models of infection. They demonstrate that the LysX enzyme modifies phosphatidylglycerol with positively charged lysine moieties, thereby increasing the net positive charge of the M. tuberculosis membrane.
- 44.Purdy GE, Niederweis M, Russell DG. Decreased outer membrane permeability protects mycobacteria from killing by ubiquitin-derived peptides. Mol. Microbiol. 2009;73:844–857. doi: 10.1111/j.1365-2958.2009.06801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monack DM, Bouley DM, Falkow S. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNγ neutralization. J. Exp. Med. 2004;199:231–241. doi: 10.1084/jem.20031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lawley TD, Bouley DM, Hoy YE, Gerke C, Relman DA, Monack DM. Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infect. Immun. 2008;76:403–416. doi: 10.1128/IAI.01189-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lawley TD, Chan K, Thompson LJ, Kim CC, Govoni GR, Monack DM. Genome-wide screen for Salmonella genes required for long-term systemic infection of the mouse. PLoS Pathog. 2006;2:e11. doi: 10.1371/journal.ppat.0020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLaughlin LM, Govoni GR, Gerke C, Gopinath S, Peng K, Laidlaw G, Chien Y-H, Jeong H-W, Li Z, Brown MD, et al. The Salmonella SPI2 effector SseI mediates long-term systemic infection by modulating host cell migration. PLoS. Pathog. 2009;5:e1000671. doi: 10.1371/journal.ppat.1000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Demangel C, Stinear TP, Cole ST. Buruli ulcer: reductive evolution enhances pathogenicity of Mycobacterium ulcerans. Nat. Rev. Microbiol. 2009;10:50–60. doi: 10.1038/nrmicro2077.An excellent review on M. ulcerans pathogenesis highlighting its evolution from related mycobacteria and the immune suppressive properties of mycolactone.
- 50.Torrado E, Adusumilli S, Fraga AG, Small PL, Castro AG, Pedrosa J. Mycolactone-mediated inhibition of tumor necrosis factor production by macrophages infected with Mycobacterium ulcerans has implications for the control of infection. Infect. Immun. 2007;75:3979–3988. doi: 10.1128/IAI.00290-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coutanceau E, Decalf J, Martino A, Babon A, Winter N, Cole ST, Albert ML, Demangel C. Selective immune suppression of dendritic cell functions by Mycobacterium ulcerans toxin mycolactone. J. Exp. Med. 2007;204:1395–1403. doi: 10.1084/jem.20070234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong H, Coutanceau E, Leclerc M, Caleechurn L, Leadlay PF, Demangel C. Mycolactone diffuses from Mycobacterium ulcerans-infected tissues and targets mononuclear cells in peripheral blood and lymphoid organs. PLoS Negl. Trop. Dis. 2008;2:e325. doi: 10.1371/journal.pntd.0000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.En J, Goto M, Nakanaga K, Higashi M, Ishil N, Saito H, Yonezawa S, Hamada H, Small PL. Mycolactone is responsible for the painlessness of Mycobacterium ulcerans infection (buruli ulcer) in a murine study. Infect. Immun. 2008;76:2002–2007. doi: 10.1128/IAI.01588-07. [DOI] [PMC free article] [PubMed] [Google Scholar]