Abstract
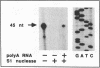
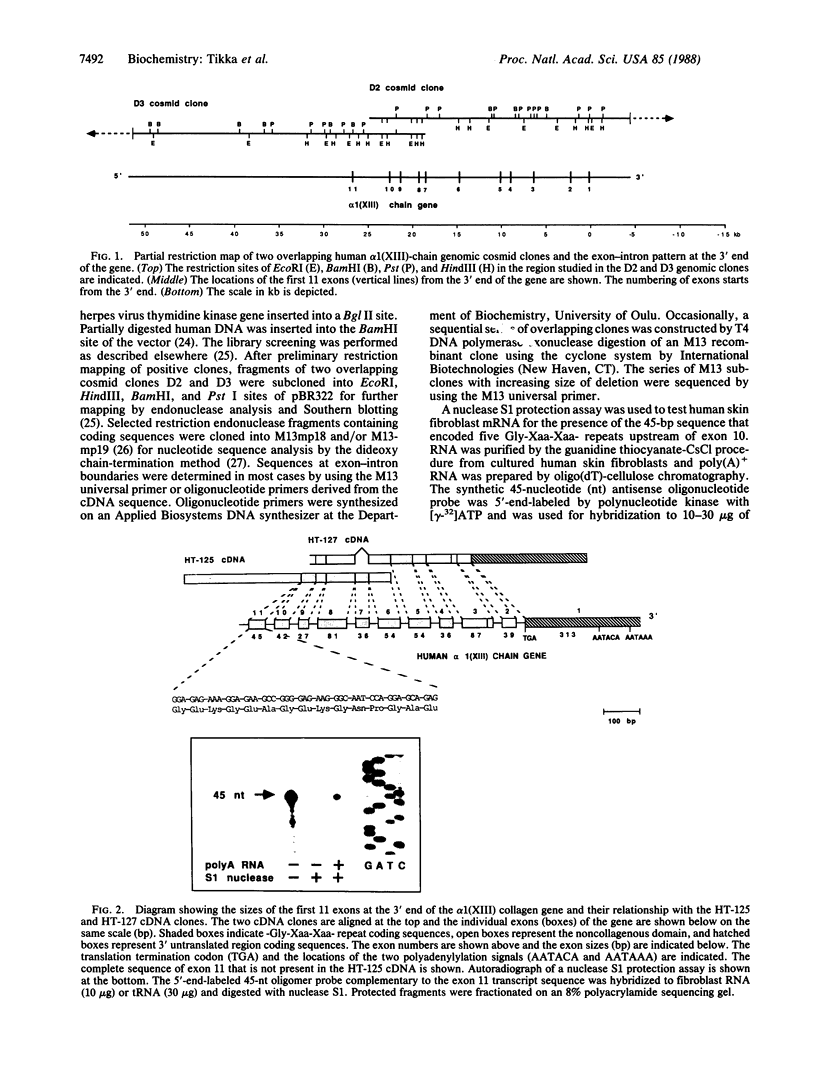
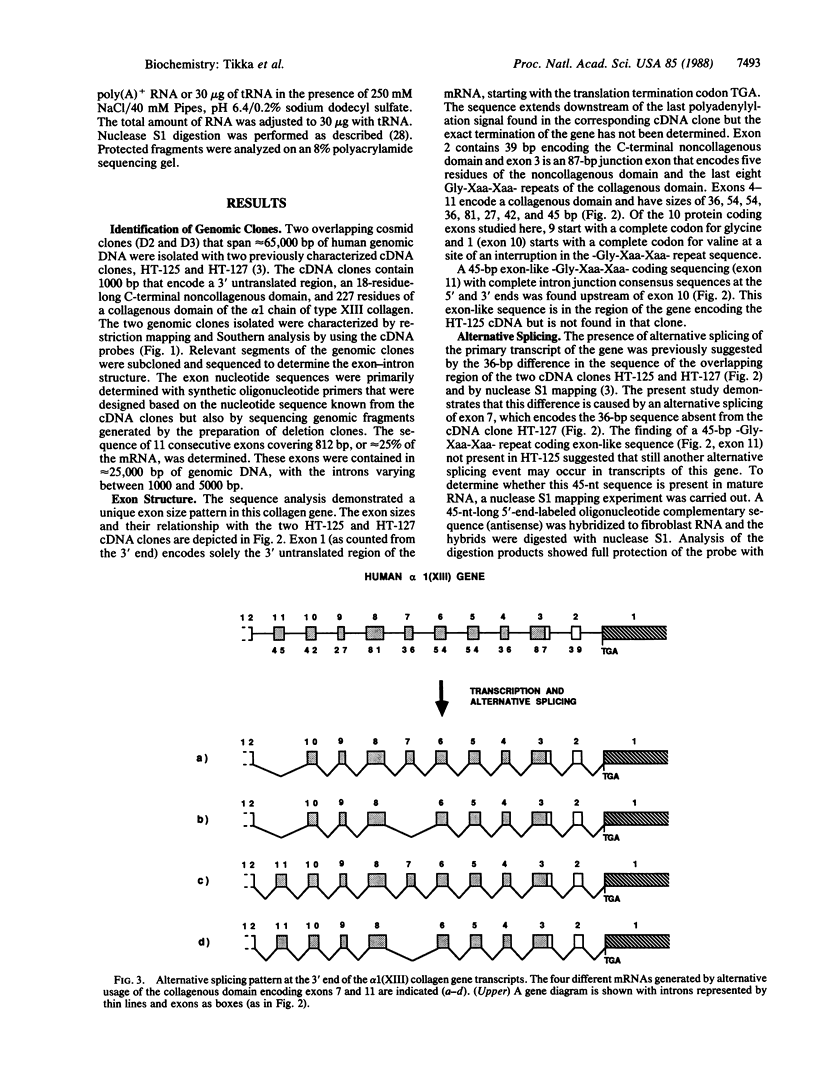
Two overlapping human genomic clones that encode a short-chain collagen, designated alpha 1(XIII), were isolated by using recently described cDNA clones. Characterization of the cosmid clones that span approximately equal to 65,000 base pairs (bp) of the 3' end of the gene established several unusual features of this collagen gene. The last exon encodes solely the 3' untranslated region and it begins with a complete stop codon. The 10 adjacent exons vary in size from 27 to 87 bp and two of them are 54 bp. Therefore, the alpha 1-chain gene of type XIII collagen has some features found in genes for fibrillar collagens but other features that are distinctly different. Previous analysis of overlapping cDNA clones and nuclease S1 mapping of mRNAs indicated one alternative splicing site causing a deletion of 36 bp from the mature mRNA. The present study showed that the 36 bp is contained within the gene as a single exon and also that the gene has a 45-bp -Gly-Xaa-Xaa- repeat coding exon not found in the cDNA clones previously characterized. Nuclease S1 mapping experiments indicated that this 45-bp exon is found in normal human skin fibroblast mRNAs. Accordingly, the data demonstrate that there is alternative splicing of at least two exons of the type alpha 1(XIII)-chain gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Boedtker H., Finer M., Aho S. The structure of the chicken alpha 2 collagen gene. Ann N Y Acad Sci. 1985;460:85–116. doi: 10.1111/j.1749-6632.1985.tb51159.x. [DOI] [PubMed] [Google Scholar]
- Breitbart R. E., Andreadis A., Nadal-Ginard B. Alternative splicing: a ubiquitous mechanism for the generation of multiple protein isoforms from single genes. Annu Rev Biochem. 1987;56:467–495. doi: 10.1146/annurev.bi.56.070187.002343. [DOI] [PubMed] [Google Scholar]
- Gordon M. K., Gerecke D. R., Olsen B. R. Type XII collagen: distinct extracellular matrix component discovered by cDNA cloning. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6040–6044. doi: 10.1073/pnas.84.17.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostikka S. L., Tryggvason K. Extensive structural differences between genes for the alpha 1 and alpha 2 chains of type IV collagen despite conservation of coding sequences. FEBS Lett. 1987 Nov 30;224(2):297–305. doi: 10.1016/0014-5793(87)80473-8. [DOI] [PubMed] [Google Scholar]
- Indik Z., Yeh H., Ornstein-Goldstein N., Sheppard P., Anderson N., Rosenbloom J. C., Peltonen L., Rosenbloom J. Alternative splicing of human elastin mRNA indicated by sequence analysis of cloned genomic and complementary DNA. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5680–5684. doi: 10.1073/pnas.84.16.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Umezawa K., Vibe-Pedersen K., Baralle F. E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985 Jul;4(7):1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkinen M., Bernard M. P., Barlow D. P., Chow L. T. Characterization of 64-, 123- and 182-base-pair exons in the mouse alpha 2(IV) collagen gene. Nature. 1985 Sep 12;317(6033):177–179. doi: 10.1038/317177a0. [DOI] [PubMed] [Google Scholar]
- Lau Y. F., Kan Y. W. Versatile cosmid vectors for the isolation, expression, and rescue of gene sequences: studies with the human alpha-globin gene cluster. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5225–5229. doi: 10.1073/pnas.80.17.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano G., Ninomiya Y., Thompson H., Olsen B. R. A distinct class of vertebrate collagen genes encodes chicken type IX collagen polypeptides. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4050–4054. doi: 10.1073/pnas.82.12.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Gay S. The collagens: an overview and update. Methods Enzymol. 1987;144:3–41. doi: 10.1016/0076-6879(87)44170-0. [DOI] [PubMed] [Google Scholar]
- Morris N. P., Bächinger H. P. Type XI collagen is a heterotrimer with the composition (1 alpha, 2 alpha, 3 alpha) retaining non-triple-helical domains. J Biol Chem. 1987 Aug 15;262(23):11345–11350. [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya Y., Gordon M., van der Rest M., Schmid T., Linsenmayer T., Olsen B. R. The developmentally regulated type X collagen gene contains a long open reading frame without introns. J Biol Chem. 1986 Apr 15;261(11):5041–5050. [PubMed] [Google Scholar]
- Ninomiya Y., Olsen B. R. Synthesis and characterization of cDNA encoding a cartilage-specific short collagen. Proc Natl Acad Sci U S A. 1984 May;81(10):3014–3018. doi: 10.1073/pnas.81.10.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. R., Ninomiya Y., Lozano G., Konomi H., Gordon M., Green G., Parsons J., Seyer J., Thompson H., Vasios G. Short-chain collagen genes and their expression in cartilage. Ann N Y Acad Sci. 1985;460:141–153. doi: 10.1111/j.1749-6632.1985.tb51162.x. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T., Myllylä R., Seyer J., Kurkinen M., Prockop D. J. Partial characterization of a low molecular weight human collagen that undergoes alternative splicing. Proc Natl Acad Sci U S A. 1987 Feb;84(4):940–944. doi: 10.1073/pnas.84.4.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Ramirez F., Bernard M., Chu M. L., Dickson L., Sangiorgi F., Weil D., De Wet W., Junien C., Sobel M. Isolation and characterization of the human fibrillar collagen genes. Ann N Y Acad Sci. 1985;460:117–129. doi: 10.1111/j.1749-6632.1985.tb51160.x. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Krainer A. R., Maniatis T., Green M. R. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984 Aug;38(1):317–331. doi: 10.1016/0092-8674(84)90553-1. [DOI] [PubMed] [Google Scholar]
- Sakurai Y., Sullivan M., Yamada Y. Alpha 1 type IV collagen gene evolved differently from fibrillar collagen genes. J Biol Chem. 1986 May 25;261(15):6654–6657. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Soininen R., Chow L., Kurkinen M., Tryggvason K., Prockop D. J. The gene for the alpha 1(IV) chain of human type IV procollagen: the exon structures do not coincide with the two structural subdomains in the globular carboxy-terminus of the protein. EMBO J. 1986 Nov;5(11):2821–2823. doi: 10.1002/j.1460-2075.1986.tb04574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soininen R., Tikka L., Chow L., Pihlajaniemi T., Kurkinen M., Prockop D. J., Boyd C. D., Tryggvason K. Large introns in the 3' end of the gene for the pro alpha 1 (IV) chain of human basement membrane collagen. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1568–1572. doi: 10.1073/pnas.83.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Dziadek M. Structure, development, and molecular pathology of basement membranes. Int Rev Exp Pathol. 1986;29:1–112. [PubMed] [Google Scholar]
- Weil D., Bernard M., Gargano S., Ramirez F. The pro alpha 2(V) collagen gene is evolutionarily related to the major fibrillar-forming collagens. Nucleic Acids Res. 1987 Jan 12;15(1):181–198. doi: 10.1093/nar/15.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crombrugghe B., Schmidt A., Liau G., Setoyama C., Mudryj M., Yamada Y., McKeon C. Structural and functional analysis of the genes for alpha 2(I) and alpha 1(III) collagens. Ann N Y Acad Sci. 1985;460:154–162. doi: 10.1111/j.1749-6632.1985.tb51163.x. [DOI] [PubMed] [Google Scholar]