Abstract
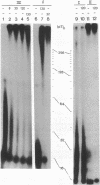
DNA polymerase III from Saccharomyces cerevisiae is analogous to the mammalian DNA polymerase delta by several criteria, including an increased synthetic activity on poly(dA).oligo(dT) (40:1 nucleotide ratio) in the presence of calf thymus proliferating-cell nuclear antigen (PCNA), or cyclin. This stimulation assay has been used to purify the yeast analog of PCNA/cyclin (yPCNA) to homogeneity. yPCNA is a trimer or tetramer (Mr approximately 82,000) of identical subunits with a denatured Mr of 26,000. On a molar basis yPCNA and calf thymus PCNA/cyclin are equally active in stimulating DNA synthesis by DNA polymerase III. About 10 times more yPCNA than calf thymus PCNA/cyclin is needed, however, to stimulate calf thymus DNA polymerase delta, and the degree of stimulation obtained at saturating levels of yPCNA is a factor of 2-3 less than with calf thymus PCNA/cyclin. Both stimulatory proteins exert their effect in an identical fashion, i.e., by increasing the processivity of the DNA polymerase. Yeast DNA polymerases I and II and calf thymus DNA polymerase alpha are not stimulated by yPCNA. Treatment of logarithmic-phase cells with hydroxyurea blocks them in the S phase and produces a 4- to 5-fold increase in yPCNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Huebsch D., Blundell P. A., Macdonald-Bravo H., Bravo R. Cloning and sequence of the human nuclear protein cyclin: homology with DNA-binding proteins. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1575–1579. doi: 10.1073/pnas.84.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer G. A., Heller H. M., Burgers P. M. DNA polymerase III from Saccharomyces cerevisiae. I. Purification and characterization. J Biol Chem. 1988 Jan 15;263(2):917–924. [PubMed] [Google Scholar]
- Bravo R., Celis J. E. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Biol. 1980 Mar;84(3):795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H. Changes in the nuclear distribution of cyclin (PCNA) but not its synthesis depend on DNA replication. EMBO J. 1985 Mar;4(3):655–661. doi: 10.1002/j.1460-2075.1985.tb03679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers P. M., Bauer G. A. DNA polymerase III from Saccharomyces cerevisiae. II. Inhibitor studies and comparison with DNA polymerases I and II. J Biol Chem. 1988 Jan 15;263(2):925–930. [PubMed] [Google Scholar]
- Burgers P. M. Mammalian cyclin/PCNA (DNA polymerase delta auxiliary protein) stimulates processive DNA synthesis by yeast DNA polymerase III. Nucleic Acids Res. 1988 Jul 25;16(14A):6297–6307. doi: 10.1093/nar/16.14.6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes J. J., Downey K. M., Black V. L., So A. G. A new mammalian DNA polymerase with 3' to 5' exonuclease activity: DNA polymerase delta. Biochemistry. 1976 Jun 29;15(13):2817–2823. doi: 10.1021/bi00658a018. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Celis A. Cell cycle-dependent variations in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cells: subdivision of S phase. Proc Natl Acad Sci U S A. 1985 May;82(10):3262–3266. doi: 10.1073/pnas.82.10.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis J. E., Madsen P., Celis A., Nielsen H. V., Gesser B. Cyclin (PCNA, auxiliary protein of DNA polymerase delta) is a central component of the pathway(s) leading to DNA replication and cell division. FEBS Lett. 1987 Aug 10;220(1):1–7. doi: 10.1016/0014-5793(87)80865-7. [DOI] [PubMed] [Google Scholar]
- Crute J. J., Wahl A. F., Bambara R. A. Purification and characterization of two new high molecular weight forms of DNA polymerase delta. Biochemistry. 1986 Jan 14;25(1):26–36. doi: 10.1021/bi00349a005. [DOI] [PubMed] [Google Scholar]
- Focher F., Spadari S., Ginelli B., Hottiger M., Gassmann M., Hübscher U. Calf thymus DNA polymerase delta: purification, biochemical and functional properties of the enzyme after its separation from DNA polymerase alpha, a DNA dependent ATPase and proliferating cell nuclear antigen. Nucleic Acids Res. 1988 Jul 25;16(14A):6279–6295. doi: 10.1093/nar/16.14.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goscin L. P., Byrnes J. J. DNA polymerase delta: one polypeptide, two activities. Biochemistry. 1982 May 11;21(10):2513–2518. doi: 10.1021/bi00539a034. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Sequential function of gene products relative to DNA synthesis in the yeast cell cycle. J Mol Biol. 1976 Jul 15;104(4):803–817. doi: 10.1016/0022-2836(76)90183-2. [DOI] [PubMed] [Google Scholar]
- Lee M. Y., Tan C. K., Downey K. M., So A. G. Further studies on calf thymus DNA polymerase delta purified to homogeneity by a new procedure. Biochemistry. 1984 Apr 24;23(9):1906–1913. doi: 10.1021/bi00304a003. [DOI] [PubMed] [Google Scholar]
- Lee M. Y., Toomey N. L. Human placental DNA polymerase delta: identification of a 170-kilodalton polypeptide by activity staining and immunoblotting. Biochemistry. 1987 Feb 24;26(4):1076–1085. doi: 10.1021/bi00378a014. [DOI] [PubMed] [Google Scholar]
- Ludueńa R. F., Shooter E. M., Wilson L. Structure of the tubulin dimer. J Biol Chem. 1977 Oct 25;252(20):7006–7014. [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M., Franza B. R., Jr, Garrels J. I. Identity of the proliferating cell nuclear antigen and cyclin. Nature. 1984 May 24;309(5966):374–376. doi: 10.1038/309374a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Moriuchi T., Koji T., Nakane P. K. Molecular cloning of cDNA coding for rat proliferating cell nuclear antigen (PCNA)/cyclin. EMBO J. 1987 Mar;6(3):637–642. doi: 10.1002/j.1460-2075.1987.tb04802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyachi K., Fritzler M. J., Tan E. M. Autoantibody to a nuclear antigen in proliferating cells. J Immunol. 1978 Dec;121(6):2228–2234. [PubMed] [Google Scholar]
- Nishida C., Reinhard P., Linn S. DNA repair synthesis in human fibroblasts requires DNA polymerase delta. J Biol Chem. 1988 Jan 5;263(1):501–510. [PubMed] [Google Scholar]
- Ogata K., Kurki P., Celis J. E., Nakamura R. M., Tan E. M. Monoclonal antibodies to a nuclear protein (PCNA/cyclin) associated with DNA replication. Exp Cell Res. 1987 Feb;168(2):475–486. doi: 10.1016/0014-4827(87)90020-6. [DOI] [PubMed] [Google Scholar]
- Prelich G., Kostura M., Marshak D. R., Mathews M. B., Stillman B. The cell-cycle regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro. Nature. 1987 Apr 2;326(6112):471–475. doi: 10.1038/326471a0. [DOI] [PubMed] [Google Scholar]
- Prelich G., Stillman B. Coordinated leading and lagging strand synthesis during SV40 DNA replication in vitro requires PCNA. Cell. 1988 Apr 8;53(1):117–126. doi: 10.1016/0092-8674(88)90493-x. [DOI] [PubMed] [Google Scholar]
- Prelich G., Tan C. K., Kostura M., Mathews M. B., So A. G., Downey K. M., Stillman B. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987 Apr 2;326(6112):517–520. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- Sabatino R. D., Myers T. W., Bambara R. A., Kwon-Shin O., Marraccino R. L., Frickey P. H. Calf thymus DNA polymerases alpha and delta are capable of highly processive DNA synthesis. Biochemistry. 1988 Apr 19;27(8):2998–3004. doi: 10.1021/bi00408a050. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Slater M. L. Effect of reversible inhibition of deoxyribonucleic acid synthesis on the yeast cell cycle. J Bacteriol. 1973 Jan;113(1):263–270. doi: 10.1128/jb.113.1.263-270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C. K., Castillo C., So A. G., Downey K. M. An auxiliary protein for DNA polymerase-delta from fetal calf thymus. J Biol Chem. 1986 Sep 15;261(26):12310–12316. [PubMed] [Google Scholar]
- Tan C. K., Sullivan K., Li X. Y., Tan E. M., Downey K. M., So A. G. Autoantibody to the proliferating cell nuclear antigen neutralizes the activity of the auxiliary protein for DNA polymerase delta. Nucleic Acids Res. 1987 Nov 25;15(22):9299–9308. doi: 10.1093/nar/15.22.9299. [DOI] [PMC free article] [PubMed] [Google Scholar]