Abstract
Cardiovascular diseases are responsible for increased morbidity and mortality in people with diabetes. Diabetic macrovasculopathy is associated with structural and functional changes in large arteries, which causes endothelial dysfunction, increased arterial stiffness, or decreased arterial distensability. Diabetic complications can be controlled and avoided by strict glycemic control, maintaining normal lipid profiles, regular physical exercise, adopting a healthy lifestyle and pharmacological interventions. Treatment goals for patients with type 2 diabetes specify targets for glycemia and other cardiometabolic risk factors, for example, hypertension and dyslipidemia. In recent years, special attention has been devoted to both thiazolidindiones (TZDs) and angiotensin converting enzyme (ACE) inhibitors as clinical trials revealed that these drugs may reduce the rate of progression to diabetes or delay the onset of diabetes, regression of impaired glucose tolerance (IGT) to normoglycemia and reduces the composite of all-cause mortality, nonfatal myocardial infarction and stroke in patients with diabetes. This review focuses on the potential roles of rosiglitazone, a member of TZD class of antidiabetic agents, and ramipril, an ACE inhibitor, in preventing the preclinical macrovasculopathy in diabetes and IGT population.
Keywords: Diabetic vasculopathy, ramipril, rosiglitazone
Introduction
Diabetes is one of the most challenging health problems in the twenty-first century. It is ranked as the fifth leading cause of death and is a major risk factor for various cardiovascular diseases (CVD).[1] Cardiovascular diseases are responsible for more than 50% and up to 80% of deaths in people with diabetes as well as for very substantial morbidity and loss of quality of life[2] [Table 1]. The most important forms of CVD are coronary heart disease, cerebrovascular disease, and peripheral vascular disease. These lead to heart attacks, angina, heart failure, stroke, and gangrene or ulceration of the feet and legs requiring amputation. People with diabetes are also prone to developing CVD at a younger age and having more severe effects than people without diabetes. In addition, risk is increased even at the earlier stages of glucose intolerance.
Table 1.
Cardiovascular diseases and diabetes: Double jeopardy[2]
|
Diabetic vasculopathy
Diabetes mellitus is a multifactorial disease associated with a number of microvascular (retinopathy, neuropathy, and nephropathy) and macrovascular complications.[3,4] Diabetic macrovasculopathy is associated with structural and functional changes in large arteries that lead to increased stiffness, abnormal pulse wave travel, and systolic hypertension.[4] Structural changes mainly result from glycation of wall components and functional changes originate in endothelial dysfunction, increased arterial stiffness or decreased arterial distensibility [Figure 1]. These changes promote the development of left ventricular hypertrophy, an independent risk factor for cardiovascular (CV) mortality.[5] Apart from the above-mentioned mechanisms, metabolic [advanced glycation end production (AGE), cytokines], humoral (renin-angiotensin system, endothelin, sympathetic nervous system) and hemodynamic (arterial hypertension and mechanical strain) factors contribute to the characteristic dysfunction in diabetic vasculopathy.[6] The initiators of vasculopathy that ultimately develop into long-term diabetic complications can be controlled and avoided by strict glycemic control, maintaining normal lipid profiles, regular physical exercise, adopting a healthy lifestyle and pharmacological interventions.
Figure 1.
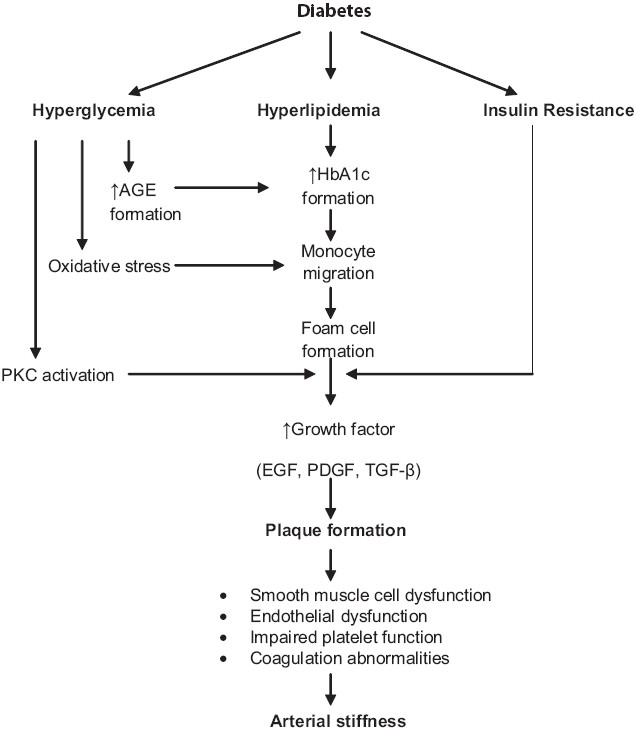
Pathogenesis and pathophysiology of diabetic macrovasculopathy
Treatment Modalities of Type 2 Diabetes
As the prevalence of type 2 diabetes continues to increase worldwide, there is an enhanced need for effective disease management. The International Diabetes Federation (IDF) has recently introduced new global guidelines for the management of diabetes.[7] Three modalities of treatment are currently available to manage diabetes: lifestyle modification including appropriate diet and exercise programs, oral anti-diabetic agents, and insulin. Patients with diabetes are insulin resistant and often have metabolic syndrome, which requires a multifactorial intervention in order to reduce the incidence of CV complications[8] [Table 2]. Treatment goals for patients with type 2 diabetes specify targets for glycemia and other cardiometabolic risk factors, for example, hypertension and dyslipidemia[7,9] [Table 3].
Table 2.
Control of cardiometabolic parameters in the management of type 2 diabetes as recommended by IDF[8]
| Cardiometabolic parameters | Target values |
|---|---|
| Glycemia | |
| Prebreakfast and premain | |
| evening-meal glucose | < 6.0 mmol/l (<110 mg/dl) |
| BP | <130/80 mmHg |
| Lipids | |
| LDL-C | < 2.5 mmol/l (95 mg/dl) |
| HDL-C | > 1.0 mmol/l (_40 mg/dl) |
| Triglycerides | < 2.3 mmol/l (<200 mg/dl) |
Table 3.
Treatment modalities of type 2 diabetes
| Cardio-metabolic abnormalities | Drugs | Mode of action |
|---|---|---|
| Hyperglycemia Insulin resistance | Biguanides | Increases liver and muscle insulin sensitivity; decreases hepatic glucose production |
| Sulphonylureas | Insulin secretogogues | |
| Alpha-glucosidase inhibitors | Delay the absorption of polysaccharides and also act to attenuate postprandial glucose excursions | |
| Sulphonylurea-like agents | Insulin secretogogues | |
| Thiazolidinediones | Insulin sensitizers that improve glucose uptake in adipose tissues and skeletal muscles | |
| Insulin | Reduces hepatic glucose output and increases peripheral glucose utilization | |
| Hypertension | ACE inhibitors | Block the formation of AT-II, increase bradykinin level. As a result reduce vasoconstriction, reduce sodium and water retension, and increase vasodilation (through bradykinin). |
| Angiotensin receptor blockers Losartan and valsartan | Competitive inhibition of AT-II receptor (Type 1). Effect more specific on AT-II action, less or none on bradykinin production or metabolism. | |
| Beta blockers | Inhibit renin release and AT-II and aldosterone production and lower peripheral resistance; may decrease adrenergic outflow from the CNS. | |
| Calcium channel blockers | Dilate peripheral arterioles and thereby reduce BP by inhibiting calcium influx into arterial SM cells. | |
| Diuretics | Lower BP by depleting body sodium stores resulting in reduction of total blood volume and cardiac output; initially peripheral vascular resistance increases but declines when CO returns to normal level (6-8 weeks) | |
| Dyslipidemia | Statins | Increase lipid profile and decrease atherogenic tendency. Lower LDL-C, improve TC:HDL-C, lower apo B. |
| Fibric acid derivatives | Increase lipid profile and decrease atherogenic tendency. Lower TGs, raise HDL-C, lower TC:HDL-C and shift LDL from smaller to larger particles. | |
| Platelet activation and | Aspirin | Antiplatelet effect |
| aggregation | Clopidogrel | Irreversible blockade of the adenosine diphosphate (ADP) receptor on platelet cell membranes |
| Ticlopidine | Interferes with platelet membrane function |
In recent years, special attention has been devoted to both thiazolidinediones (TZDs) and angiotensin converting enzyme (ACE) inhibitors when TRIPOD study[10] demonstrated that troglitazone may reduce the rate of progression to diabetes in women diagnosed with gestational diabetes and HOPE Study[11] showed that ramipril may delay the onset of diabetes. The landmark study ProActive (PROspective pioglitAzone Clinical Trial In macroVascular Events) demonstrated that pioglitazone reduces the composite of all-cause mortality, nonfatal myocardial infarction, and stroke in patients with T2DM who have a high risk of macrovascular events.[12] Recently, published landmark DREAM study demonstrated that rosiglitazone has a substantial benefit on prevention of diabetes and regression to normoglycemia and ramipril has a modest benefit on regression to normoglycemia.[13,14]
The TZDs are new oral antidiabetic agents providing a novel means to reduce hyperglycemia by improving insulin sensitivity. Moreover, TZDs have vasculoprotective properties beyond glycemic control.[15] These drugs have potentially favorable effects on other components of the insulin resistance syndrome. As insulin sensitizers, they may modify CV risk factors and reduce CV mortality in T2DM and insulin resistance subjects.[16] The ACE inhibitors therapy reduces both microvascular and macrovascular complications in diabetes and appears to improve insulin sensitivity and glucose metabolism.[17] This review focuses on the potential roles of rosiglitazone, a member of TZD class of antidiabetic agents, and ramipril, an ACE inhibitor, in preventing the preclinical macrovasculopathy in diabetes and IGT population.
Rosiglitazone is a highly selective and potent agonist for the peroxisome proliferator-activated receptor-gamma (PPARγ). These proliferator-activated receptors are found in key target tissues for insulin action such as adipose tissue, skeletal muscle, and liver. Activation of PPARγ nuclear receptors regulates the transcription of insulin responsive genes involved in the control of glucose production, transport, and utilization. In addition, PPARγ-responsive genes also participate in the regulation of fatty acid metabolism. Ramipril has direct effects on the renin-angiotensin-kallikrein system and may prevent diabetes through effects on the beta cell and by vascular and metabolic effects on muscle that may amplify the effects of insulin.[18] The ACE inhibitors increase islet blood flow and pancreatic beta-cell perfusion by reducing angiotensin II-mediated vasoconstriction in the pancreas,[19] which may potentially slow down or reverse the decline in beta-cell function. The ACE inhibitors may also increase the insulin-mediated glucose disposal, thereby decreasing the need for pancreatic insulin secretion and may reduce insulin resistance in skeletal muscle.[20] This may be due to increased bradykinin-mediated nitric oxide production.[21] The ACE inhibitors may also reduce insulin resistance at the liver and fat cell, which reduce hepatic glucose production and lower free fatty acid level.[22]
Randomized Controlled Trials with Rosiglitazone and Ramipril: Effects on Diabetes
A number of clinical trials were conducted to investigate the efficacy of rosiglitazone in improving the glycemic status in type 2 diabetes and IGT patients [Table 4]. As monotherapy, three 8-12 weeks dose-finding placebo-controlled randomized trials were found to reduce fasting plasma glucose (FPG) and HbA1c levels with rosiglitazone 4-12 mg/day.[23–25] In two 26-week placebo-controlled studies,[26,27] significant reductions in FPG and HbA1c were seen with rosiglitazone. A 52-week randomized, double-blind trials showed that FPG and HbA1c levels fell significantly with rosiglitazone in comparison to glibenclamide.[28] A recently published study demonstrated that rosiglitazone therapy reduced plasma insulin, proinsulin, split proinsulin, and free fatty acid level compared with glibenclamide therapy.[29]
Table 4.
Monotherapy clinical trials on rosiglitazone in T2DM patients
| Researchers | Study population | Methodology | Results/comments |
|---|---|---|---|
| Nolan et al.[23] | T2DM; n=380 | Rosiglitazone 4, 8, or 12 mg q.i.d.; duration: 8 weeks | All doses lowered FPG significantly |
| Patel et al.[24] | T2DM; n=380 | Rosiglitazone 0.05, 0.25, 1, 2 mg twice daily; duration: 12 weeks | FPG was reduced significantly by rosiglitazone 1 and 2 mg b.i.d. Only 2 mg b.i.d. produced a significant reduction HbA1c |
| Raskin et al.[25] | T2DM; n=30 | Rosiglitazone 2, 4, 6 mg b.i.d.; duration: 38 weeks | Significantly reduced FPG and postprandial glucose, C-peptide and insulin with rosiglitazone 4 mg b.i.d. |
| Phillips et al.[26] | T2DM; n= 959 | Rosiglitazone 4 mg o.d.,2 mg b.i.d, 4 mg b.i.d.,8 mg o.d.; duration: 26 weeks | Produced drug-dependent reduction in HbA1c |
| Lebovitz et al.[27] | T2DM; n= 493 | Rosiglitazone 2 or 4 mg b.i.d.; duration: 26 weeks | Rosiglitazone 2 and 4 mg b.i.d decreased mean HbA1cand FPG |
| Charbonnel et al.[28] | T2DM; n=587 | Rosiglitazone 2, 4 mg b.i.d and glibenclamide (15 mg/day); duration: 52 weeks | At week 52, significant decrease in mean HbA1cand FPG |
| Hanefeld et al.[29] | T2DM; n=598 | Rosiglitazone 4, 8mg/day or glibenclamide 15mg/day; duration: 52 weeks | Rosiglitazone therapy reduced plasma insulin, proinsulin, split proinsulin and free fatty acid level compared with glibenclamide |
A recent publication of a meta-analysis of data from 42 clinical trials suggesting an increased risk of myocardial infarction (MI) and cardiovascular death with rosiglitazone led to a media furor and widespread patient panic.[30] The pooled data showed a 43% increase in relative risk of MI among T2DM treated with rosiglitazone. However, interim findings from ongoing RECORD (rosiglitazone evaluated for cardiac outcomes and regulation of glycemia in diabetes) study were inconclusive regarding the effect of rosiglitazone on the overall risk of hospitalization or death from cardiovascular causes.[31] The study found no evidence of any increased mortality, either from any cause or from cardiovascular causes. Further research is needed to determine the long-term cardiovascular effects of rosiglitazone.
Diabetes-preventive benefits have also been claimed by a number of previous studies with angiotensin-converting enzyme (ACE) inhibitors. The HOPE study was the first to explore that ACE inhibitor ramipril prevents the development of diabetes.[11] The possibility that reduce the number of new cases of diabetes was also supported by a number of other studies.[32–36] As monotherapy, two trials have evaluated the use of ramipril in diabetic patients [Table 5]. Trevisan and Tiengo[37] showed that low dose ACE inhibition with ramipril could arrest the progressive rise in albuminuria in diabetic patients with persistent microalbuminuria. The beneficial effects of this therapy were accompanied by relatively few adverse events and none of them was directly related to treatment. Another study conducted by Nielsen et al,[38] demonstrated that ramipril induces regression of left ventricular hypertrophy in normotensive, nonalbuminuric NIDDM patients, independent of reduction in systemic blood pressure.
Table 5.
Monotherapy clinical trials on Ramipril in T2DM patients
| Researchers | Study population | Methodology | Results/Comment |
|---|---|---|---|
| Trevisan and Tiengo[37] | T2DM; n=122 | Ramipril 1.25 mg/day; duration: 6 months | Low-dose ACE inhibition with ramipril could arrest the progressive rise in albuminuria in T2DM patients with persistent microalbuminuria. |
| Nielsen et al.[38] | T2DM, n= 16 | Ramipril (5mg)/day; duration: 6 month | Beneficial impact of ramipril on left ventricular hypertrophy in normotensive nonalbuminuric T2DM patients |
| MICROHOPE Substudy[39] | T2DM; n=3577 | Ramipril 10 mg/day vs. placebo and Vitamin E; duration: 4.5 years | Lowered the risk of the combined primary outcome by 25%, myocardial infarction by 22%, stroke by 33%, CV death by 37%, total mortality by 24%, revascularization by 17%, and overt nephropathy by 24%. |
| Ramipril was beneficial for CV events and overt nephropathy in T2DM patients |
T2DM= Type 2 Diabetes Mellitus
Rosiglitazone and Preclinical Vasculopathy
A number of clinical studies involving patients with T2DM demonstrated the antiatherogenic effect of TZDs involving troglitazone,[40–42] pioglitazone[43–46] and rosiglitazone.[47–49] The clinical studies on vasculopathy with rosiglitazone are summarized in Table 6.
Table 6.
Studies investigating the effect of rosiglitazone and ramipril on arterial stiffness
| Researchers | Study population | Methodology | Comments |
|---|---|---|---|
| Kim et al.[47] | Prediabetes (n=50) or nondiabetic metabolic syndrome (n=49) | Rosiglitazone 4 mg/day; Duration: 12 weeks. brachial-ankle PWV and adiponectin levels; volume plethymographic apparatus | PWV was significantly decreased in the rosiglitazone group in comparison to baseline |
| Shargorodsky et al.[48] | T2DM; n= 52 | Rosiglitazone of 4 mg/day; duration: 6 months; large and small artery elasticity; pulse wave contour analysis | Significant change was observed in small artery elasticity but no difference in large artery elasticity |
| Pistroch et al.[49] | T2DM; n=12 | Rosiglitazone (4 mg b.i.d) with nateglinide; duration: 12 weeks; endothelial dysfunction; venous occlusion plethysmography | Rosiglitazone had therapeutic effects on endothelial dysfunction in T2DM patients |
| Lonn et al.[53] | T2DM; n=732 | Ramipril 2.5 mg/d or 10 mg/d and vitamin E or their matching placebo; duration: 4.5 years; intima-media thickness (IMT); B-mode carotid ultrasound | Ramipril 10 mg significantly reduced progression of carotid artery wall thickness |
| Rahman et al.[51] | Newly diagnosed, never treated T2DM (n=33) and IGT (n=33) | Rosiglitazone 4mg/day or Ramipril 5 mg/d or placebo; duration: 1 year; PWV and AI; Sphygmocor | Rosiglitazone significantly decreased PWV and AI and ramipril significantly reduced AI in IGT patients. |
A study by Kim et al.[47] evaluated the effect of rosiglitazone in subjects with prediabetes or nondiabetic metabolic syndrome and demonstrated significant decrease in arterial stiffness (PWV) in the rosiglitazone group in comparison to untreated control group. The observed PWV change might have resulted from additional effects of rosiglitazone beyond metabolic control. Other studies also showed that rosiglitazone in healthy subjects[50] and in T2DM patients[49] significantly improved vascular endothelial function without changes in blood glucose level. A possible explanation of reduced arterial stiffness might be that rosiglitazone directly affects PPAR-γ activation in the vascular wall.[47] Another study by Shargorodsky et al.[48] demonstrated significant improvement of the small artery elasticity with rosiglitazone; however, no significant change was found in the large artery elasticity. The authors explained that large arteries have a major component of fixed fibrotic tissue that probably needs more time for repair. Pistroch et al.[49] compared the glycemic control by rosiglitazone with nateglinide and demonstrated that rosiglitazone had therapeutic effects on endothelial dysfunction in diabetic patients.
Until now, no research has been published to examine the effect of rosiglitazone on arterial stiffness in IGT patients. The DREAM trial examined the effect of rosiglitazone on atherosclerosis on IGT, measured by sequential carotid ultrasound in a subset of DREAM participants, which is yet to be published.[13] A recent study by Rahman et al.[51] showed that rosiglitazone significantly reversed preclinical vasculopathy in newly diagnosed, never treated IGT individuals as evident by significant decrease in PWV and AI after 1 year of treatment.
Ramipril and Preclinical Vasculopathy
Studies that have focused on the effects of antiatherogenic effect of ACE inhibitors on diabetes are scarce and no research was done to examine the effect of ramipril on arterial stiffness in IGT patients. The antiatherogenic properties of ACE inhibitors may be mediated by the lowering of angiotensin-II and the increasing of bradykinin concentrations. These result in decreased proliferation and migration of smooth muscle cells, decreased accumulation and activation of inflammatory cells, decreased oxidative stress, and increased endothelial nitric oxide formation, leading to improved endothelial function. The observed benefits of ramipril may be largely due to a protective effect of ACE inhibitors on the arterial wall.[52] The clinical studies on vasculopathy with ramipril are summarized in Table 6.
The MICRO-HOPE trial, a substudy of the HOPE trial, is first to examine the cardioprotective effects of ramipril on diabetic patients.[39] The study reported that ramipril was beneficial for CV events and overt nephropathy in people with diabetes. The CV benefit was greater than that attributable to the decrease in blood pressure. The SECURE trial,[53] a substudy of the HOPE Study, has examined the effect of ramipril on intima-media thickening. The study found that treatment with ramipril significantly reduced the progression of carotid artery wall thickness. In a recent study, Rahman et al.[51] found that ramipril reduced large artery stiffness as shown by significant decrease of AI after 1 year of treatment in newly diagnosed, never treated IGT individuals.
Conclusion
Vascular complications are the major causes of morbidity and mortality in patients with diabetes. Limited numbers of studies with diabetic patients have shown the beneficial effects of rosiglitazone and ramipril on diabetic vasculopathy. Research finding established the fact that both drugs have the potentiality to offer novel therapeutic strategies to prediabetic vasculopathy in diabetes and IGT patients because of their antiatherogenic effects. Clinical trials are needed with IGT patients as more than 8% of adult populations worldwide have either IGT or IFG[54] and every year about 5-10% of these people would develop diabetes who would be at high risk for several chronic complications. It is noteworthy that even at the stage of IGT, before full-blown diabetes has developed, the risk of CVD is already increased by about two times compared to people with normal glucose tolerance.[55] Unless diabetic macrovasculopathy in patients with IGT are identified and treated, the enhanced risk of macrovascular complications will increase in future.[56] Further randomized controlled trials should be undertaken to show whether rosiglitazone and ramipril can prevent/reverse the preclinical vasculopathy both in diabetic and in IGT patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Roglic G, Unwin N, Bennett PH, Mathers C, Tuomilehto J, Nag S, et al. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care. 2005;28:2130. doi: 10.2337/diacare.28.9.2130. [DOI] [PubMed] [Google Scholar]
- 2.IDF (2007) Fact Sheet Diabetes and cardiovascular disease (CVD) Available from: http://www.idf.org/home/index.cfm?node=1158 [cited in 2007]
- 3.Cooper ME, Bonnet F, Oldfield M, Jandeleit-Dahm K. Mechanisms of diabetic vasculopathy: An overview. Am J Hypertens. 2001;14:475–86. doi: 10.1016/s0895-7061(00)01323-6. [DOI] [PubMed] [Google Scholar]
- 4.Rahman S, Rahman T, Ismail AA, Rahman AR. Diabetes-associated macrovasculopathy: Pathophysiology and pathogenesis. Diabetes, Obesity and Metabolism. 2007;9:767–80. doi: 10.1111/j.1463-1326.2006.00655.x. [DOI] [PubMed] [Google Scholar]
- 5.Nicolaides E, Jones CJH. Type 2 diabetes - implications for macrovascular mechanics and disease. Br J Diabetes Vasc Dis. 2002;2:9–12. [Google Scholar]
- 6.Cooper ME, Gilbert RE, Epstein M. Pathophysiology of diabetic nephropathy. Metabolism. 1998;47:3–6. doi: 10.1016/s0026-0495(98)90362-6. [DOI] [PubMed] [Google Scholar]
- 7.IDF Clinical Guidelines Task Force. Global guideline for Type 2 diabetes. Brussels: International Diabetes Federation; 2005. [Google Scholar]
- 8.Scheen AJ. Treatment of type 2 diabetes. Acta Clin Belg. 2003;58:318–24. doi: 10.1179/acb.2003.58.5.010. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28:S4–S36. [PubMed] [Google Scholar]
- 10.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 11.Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin converting enzyme inhibitor, Ramipril on death from cardiovascular causes, myocardial infarction, and stroke in high risk patients. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 12.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Erdmann E, Massi-Benedetti M, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 13.The DREAM Trial Investigators. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet. 2006;368:1096–105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 14.The DREAM Trial Investigators. Effect of Ramipril on the Incidence of Diabetes. N Engl J Med. 2006;355:1–12. doi: 10.1056/NEJMoa065061. [DOI] [PubMed] [Google Scholar]
- 15.Raynolds K, Goldberg RB. Thiazolidinediones: beyond glycemic control. Treatments Endocrinol. 2006;5:25–36. doi: 10.2165/00024677-200605010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Hughes K, Aw TC, Kuperan P, Choo M. Central obesity, insulin resistance, syndrome X, lipoprotein(a), and cardiovascular risk in Indians, Malays, and Chinese in Singapore. J Epidemiol Community Health. 1997;51:394–9. doi: 10.1136/jech.51.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McFarlane SI, Kumar A, Sowers JR. Mechanisms by which angiotensin-converting enzyme inhibitors prevent diabetes and cardiovascular disease. Am J Cardiol. 2003;91:H30–7. doi: 10.1016/s0002-9149(03)00432-6. [DOI] [PubMed] [Google Scholar]
- 18.The DREAM Trial Investigators. Rationale, design and recruitment characteristics of a large, simple international trial of diabetes prevention: The DREAM trial Diabetologia. 2004;47:1519–27. doi: 10.1007/s00125-004-1485-5. [DOI] [PubMed] [Google Scholar]
- 19.Carlsson PO, Barne C, Jansson L. Angiotensin II and the endocrine pancreas. Diabetologia. 1998;41:127–33. doi: 10.1007/s001250050880. [DOI] [PubMed] [Google Scholar]
- 20.Vourinen-Markkola H, Yki-Jarnen H. Antihypertensive therapy with enalapril improves glucose storage and insulin sensitivity in hypertensive patients with non-insulin-dependent diabetes mellitus. Metabolism. 1995;44:85–9. doi: 10.1016/0026-0495(95)90293-7. [DOI] [PubMed] [Google Scholar]
- 21.Henriksen EJ, Jacob S, Kinnick TR, Youngblood EB, Schmit MB, Dietze GJ. ACE inhibition and glucose transport in insulin muscle: roles of bradykinin and nitric oxide. Am J Physiol. 1999;277:R332–6. doi: 10.1152/ajpregu.1999.277.1.R332. [DOI] [PubMed] [Google Scholar]
- 22.Torlone E, Rambotti AM, Perriello G, Botta G, Santeusanio F, Brunetti P, et al. ACE inhibition increases hepatic and extrahepatic sensitivity to insulin patients with type 2 (non-insulin dependent) diabetes mellitus and arterial hypertension. Diabetologia. 1991;34:119–25. doi: 10.1007/BF00500383. [DOI] [PubMed] [Google Scholar]
- 23.Nolan JJ, Jones NP, Patwardhan R, Deacon LF. Rosiglitazone taken once daily provides effective glycaemic control in patients with type 2 diabetes. Diabet Med. 2000;17:287–94. doi: 10.1046/j.1464-5491.2000.00269.x. [DOI] [PubMed] [Google Scholar]
- 24.Patel J, Miller E, Patwardhan R. Rosiglitazone (BRL49653) monotherapy has significant glucose lowering effect in type 2 diabetic patients. Diabetes. 1998;47:A17. [Google Scholar]
- 25.Raskin P, Rappaport EB, Cole ST, Yan Y, Patwardhan R, Freed M. Rosiglitazone short-term monotherapy lowers fasting and post-prandial glucose in patients with Type II diabetes. Diabetologia. 2000;43:278–84. doi: 10.1007/s001250050045. [DOI] [PubMed] [Google Scholar]
- 26.Phillips LS, Grunberger G, Miller E, Patwardham R, Rappaport EB, Salzman A. Once- and twice daily doing of rosiglitazone improves glycemic control in patients with type 2 diabetes. Diabetes Care. 2001;124:308–15. doi: 10.2337/diacare.24.2.308. [DOI] [PubMed] [Google Scholar]
- 27.Lebovitz HE, Dole JF, Patwardhan R, Rappaport EB, Freed MI for the rosiglitazone clinical trials study group Rosiglitazone monotherapy is effective in patients with type 2 diabetes. J Clin Endocrinol Metab. 2001;86:280–8. doi: 10.1210/jcem.86.1.7157. [DOI] [PubMed] [Google Scholar]
- 28.Charbonnel B, Lonnqvist F, Jones NP, Patwardhan R. Rosiglitazone is superior to glyburide in reducing fasting plasma glucose after 1 year of treatment in type 2 diabetic patients. Diabetes. 1999;48:All4. [Google Scholar]
- 29.Hanefeld M, Patwardhan R, Jones NP for the Rosiglitazone Clinical Trial Group A one-year study comparing the efficacy and safely of rosiglitazone and glibenclamide in the treatment of type 2 diabetes. Nutr Metab Cardiovasc Dis. 2007;17:13–23. doi: 10.1016/j.numecd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 31.Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Jones NP, et al. Rosiglitazone evaluated for cardiovascular outcomes: An interim analysis. N Engl J Med. 2007;357:28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- 32.Hansson L, Lindholm LH, Niskanen L, Lanke J, Hedner T, Niklason A, et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: The Captopril Prevention Project (CAPPP) randomised trial. Lancet. 1999;353:611–6. doi: 10.1016/s0140-6736(98)05012-0. [DOI] [PubMed] [Google Scholar]
- 33.ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 34.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 35.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 36.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, et al. CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: The CHARM-Overall programme. Lancet. 2003;362:759–66. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 37.Trevisan R, Tiengo A. Effect of low-dose ramipril on microalbuminuria in normotensive or mild hypertensive non-insulin-dependent diabetic patients: North-East Italy Microalbuminuria Study Group. Am J Hypertens. 1995;8:876–83. doi: 10.1016/0895-7061(95)00162-i. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen FS, Sato A, Ali S, Tarnow L, Smidt UM, Kastrup J, et al. Beneficial impact of ramipril on left ventricular hypertrophy in normotensive nonalbuminuric NIDDM patients. Diabetes Care. 1998;21:804–9. doi: 10.2337/diacare.21.5.804. [DOI] [PubMed] [Google Scholar]
- 39.The Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICROHOPE substudy. Lancet. 2000;355:253–9. [PubMed] [Google Scholar]
- 40.Takagi T, Akasaka T, Yamamuro A, Honda Y, Hozumi T, Morioka S, et al. Troglitazone reduces neointimal tissue proliferation after coronary stent implantation in patients with non-insulin dependent diabetes mellitus: A Serial Intravascular Ultrasound Study. J Am Coll Cardiol. 2000;36:1529–35. doi: 10.1016/s0735-1097(00)00895-0. [DOI] [PubMed] [Google Scholar]
- 41.Takagi T, Yamamuro A, Tamita K, Yamabe K, Katayama M, Morioka S, et al. Impact of troglitazone on coronary stent implantation using small stents in patients with type 2 diabetes mellitus. Am J Cardiol. 2002;89:318–22. doi: 10.1016/s0002-9149(01)02232-9. [DOI] [PubMed] [Google Scholar]
- 42.Minamikawa J, Tanaka S, Yamauchi M, Inoue D, Koshiyama H. Potent inhibitory effect of troglitazone on carotid arterial wall thickness in type 2 diabetes. J Clin Endocrinol Metab. 1998;83:1818–20. doi: 10.1210/jcem.83.5.4932. [DOI] [PubMed] [Google Scholar]
- 43.Koshiyama H, Shimono D, Kuwamura N, Minamikawa J, Nakamura Y. Inhibitory effect of pioglitazone on carotid arterial wall thickness in type 2 diabetes. J Clin Endocrinol Metab. 2001;86:3452–56. doi: 10.1210/jcem.86.7.7810. [DOI] [PubMed] [Google Scholar]
- 44.Satoh N, Ogawa Y, Usui T, Tagami T, Kono S, Uesugi H, et al. Antiatherogenic Effect of Pioglitazone in Type 2 Diabetic Patients Irrespective of the Responsiveness to Its Antidiabetic Effect. Diabetes Care. 2003;26:2493–9. doi: 10.2337/diacare.26.9.2493. [DOI] [PubMed] [Google Scholar]
- 45.Langenfeld MR, Forst T, Hohberg C, Kann P, Lübben G, Konrad T, et al. Pioglitazone decreases carotid intima-media thickness independently of glycemic control in patients with type 2 diabetes: Results from a controlled randomized study. Circulation. 2005;111:2525–31. doi: 10.1161/01.CIR.0000165072.01672.21. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe I, Tani S, Anazawa T, Kushiro T, Kanmatsuse K. Effect of pioglitazone on arteriosclerosis in comparison with that of glibenclamide. Diabetes Res Clin Pract. 2005;68:104–10. doi: 10.1016/j.diabres.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 47.Kim SG, Ryu OH, Kim HY, Lee KW, Seo JA, Kim NH, et al. Effect of rosiglitazone on plasma adiponectin level and arterial stiffness in subjects with prediabetes or non-diabetic metabolic syndrome. Eur J Endocrin. 2006;154:433–40. doi: 10.1530/eje.1.02100. [DOI] [PubMed] [Google Scholar]
- 48.Shargorodsky M, Wainstein J, Gavish D, Leibovitz E, Matas Z, Zimlichman R. Treatment With Rosiglitazone Reduces Hyperinsulinemia and Improves Arterial Elasticity in Patients With Type 2 Diabetes Mellitus. Am J Hyperten. 2003;16:617–22. doi: 10.1016/s0895-7061(03)00911-7. [DOI] [PubMed] [Google Scholar]
- 49.Pistrosch F, Passauer J, Fischer S, Fuecker K, Hanefeld M, Gross P. In Type 2 diabetes rosiglitazone therapy for insulin resistance ameliorates endothelial dysfunction independent of glucose control. Diabetes Care. 2004;27:484–90. doi: 10.2337/diacare.27.2.484. [DOI] [PubMed] [Google Scholar]
- 50.Hetzel J, Balletshofer B, Rittig K, Walcher D, Kratzer W, Hombach V, et al. Rapid Effects of Rosiglitazone Treatment on Endothelial Function and Inflammatory Biomarkers. Arterioscler Thromb Vasc Biol. 2005;25:1804–9. doi: 10.1161/01.ATV.0000176192.16951.9a. [DOI] [PubMed] [Google Scholar]
- 51.Rahman S, Ismail AAS, Ismail SB, Naing NN, Rahman ARA. Effect of Rosiglitazone and Ramipril on Preclinical Vasculopathy in Newly Diagnosed, Untreated T2DM and IGT Patients: One-year Randomised, Double-blind and Placebo-controlled Study. Eur J Clin Pharmacol. 2007;63:733–41. doi: 10.1007/s00228-007-0315-3. [DOI] [PubMed] [Google Scholar]
- 52.Lonn EM, Yusuf S, Jha P, Montague TJ, Teo KK, Benedict CR, et al. Emerging role of angiotensin-converting enzyme inhibitors in cardiac and vascular protection. Circulation. 1994;90:2056–69. doi: 10.1161/01.cir.90.4.2056. [DOI] [PubMed] [Google Scholar]
- 53.Lonn E, Yusuf S, Dzavik V, Doris C, Yi Q, Smith S, et al. Effects of ramipril and vitamin E on atherosclerosis: The study to evaluate carotid untrasound changes in patients with ramipril and vitamin E (SECURE) Circulation. 2001;103:919–25. doi: 10.1161/01.cir.103.7.919. [DOI] [PubMed] [Google Scholar]
- 54.Secree R, Shaw J, Zimmet P. Diabetes and impaired glucose tolerance: Prevalence and projections. In: Allgot B, Gan D, King H, editors. Diabetes atlas. 2nd ed. Brussels: International Diabetes Federation; 2003. pp. 17–71. [Google Scholar]
- 55.IDF (2001) Brussels. International Diabetes Federation; 2001. Diabetes and cardiovascular disease: A time to act. [Google Scholar]
- 56.Simpson RW, Shaw JE, Zimmet PZ. The prevention of type 2 diabetes lifestyle change or pharmacotherapy? A challenge for the 21st century. Diabetes Res Clin Pract. 2003;59:165–80. doi: 10.1016/s0168-8227(02)00275-9. [DOI] [PubMed] [Google Scholar]


