Abstract
We report on a 51-yr-old woman who developed intravascular hemolytic anemia caused by arsenic after long-term ingestion of a traditional Chinese medicine (TCM). Twelve years before the admission, she was diagnosed as neurocysticercosis. She has ingested a TCM for about 12 yr instead of undergoing medical therapy for the disease. She was presented with a severe Coombs'-negative hemolytic anemia with hemosiderinuria. The urine arsenic level was elevated suggesting the arsenic intoxication as a cause of the anemia. She was treated successfully with therapeutic red cell exchange without any sequelae.
Keywords: Anemia, Hemolytic; Arsenic; Medicine, Traditional Chinese
INTRODUCTION
Traditional Chinese medicines (TCMs) are commonly used for a wide range of conditions in east Asian countries, including Korea. TCMs contain heavy metals, such as arsenic, cadmium, mercury, and lead (1, 2). Of these, arsenic and lead can cause hematologic changes, including anemia, neutropenia, and thrombocytopenia (1, 2).
Exposure to arsenic compounds usually follows ingestion or inhalation, and can occur during the occupational exposure of metal workers or workers engaged in the manufacturing or application of arsenical pesticides and herbicides, or nonoccupational exposure resulting from the ingestion of contaminated well water, dried milk, soy sauce, or moonshine whiskey (3-5). Short-term arsenic poisoning may cause neuropathy and intravascular hemolysis, while long-term exposure is associated with the development of cancer (3-7).
Here, we report a severe hemolytic anemia induced by arsenic intoxication after the long-term ingestion of TCM.
CASE REPORT
A 51-yr-old woman was admitted to Chonnam National University Hospital with exertional dyspnea and dizziness for 4 weeks. She also complained of tingling sensations in her palms and soles that had been present for a long time. Her family had no medical problems in the past. Twelve years before the admission, she had been diagnosed as neurocysticercosis with intermittent seizure attacks. Surgery was recommended, but the patient refused and instead ingested TCMs for about 12 yr intermittently. Recently, she had ingested TCMs for 6 months because of seizures and did not ingest further TCMs after the admission. There was no history of illicit drug use, alcohol intake, or transfusion.
On the examination, the blood pressure, pulse, temperature and respirations were 130/70 mmHg, 76/min, 36℃, and 20/min, respectively. She appeared chronically ill and had anemic conjunctiva and scleral icterus. There was no palpable hepatosplenomegaly or lymphadenopathy.
She had a white cell count of 2.1×109/L (neutrophil: 54.7%, lymphocyte: 31.6%, monocyte: 9.8%, eosinophil: 0.5%), with platelets 107×109/L, a hemoglobin of 7.5 g/dL, and 11.5% reticulocytes. Blood chemistry was revealed as follows: total serum protein 6.6 g/dL, albumin 4.1 g/dL, alkaline phosphatase 62 IU/L, AST 132 IU/L, ALT 29 IU/L, total bilirubin 1.6 mg/dL (direct, 0.3 mg/dL), BUN 19.0 mg/dL, creatinine 0.7 mg/dL, and lactate dehydrogenase (LDH) 4,989 IU/L. The serum haptoglobin was 7.25 mg/dL (normal range: 30 to 200 mg/dL), and there was a positive finding of urinary hemosiderin. The coagulation profile revealed a prothrombin time of 11.4 sec (control: 12.5 sec), a partial thromboplastin time of 33.8 sec (control: 28 to 40 sec), and a fibrinogen level of 158 mg/dL. Hepatitis A, B, and C virus antigens and antibodies were all negative. Antinuclear antibodies, anti-Sm antibodies, anti-DNA antibodies, anti-cardiolipin antibodies, lupus anticoagulant, and anti-β2-glycoprotein I IgG were all negative.
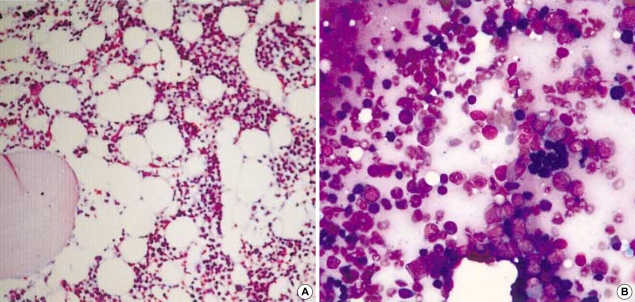
The peripheral blood smear showed polychromatophilia, anisocytosis, and macrocytosis. Both direct and indirect Coombs' test results were negative. Osmotic fragility and glucose-6-phosphate dehydrogenase activity were appropriate to the reticulocyte count. Hemoglobin electrophoresis was normal. Sucrose lysis and Ham's test were negative. The serum ceruloplasmin level and urine copper excretion were not elevated. A bone marrow biopsy showed a mild hypocellular marrow with 40% cellularity and erythroid hyperplasia (Fig. 1). However, numerous investigations failed to reveal a cause for the hemolysis.
Fig. 1.
Bone marrow biopsy shows mild hypocellular marrow with 40% cellularity (A) (H&E stain, ×100) and erythroid hyperplasia (B) (H&E stain, ×400).
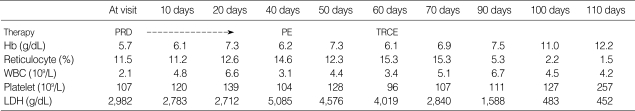
She was treated empirically with prednisolone 1 mg/kg per oral daily in three divided doses for 6 weeks, but there was no improvements in the hemolytic anemia. Subsequently, four sessions of plasma exchange were performed as a salvage therapeutic intervention, resulting in mild improvement in the hemolysis. We measured the levels of some heavy metals found in TCMs. There was increased urinary excretion of arsenic of 67.2 µg/day (normal range; 0-25 µg/day). Other heavy metals included: serum cadmium 3.1 µg/dL (0-10 µg/dL), serum lead 5 µg/dL (0-60 µg/dL), and urine lead 6 µg/L (0-150 µg/L). Her hemolytic anemia improved gradually after therapeutic red cell exchange of 450 mL. After 1 month, her hemoglobin rose to 11.0 g/dL, and the reticulocyte count and urinary excretion of arsenic decreased to 2.2% and 13.0 µg/day, respectively. Her clinical course is described in Table 1. She is currently being followed monthly and there has been no deterioration in her condition.
Table 1.
Hospital course of the patient: The hemolytic episode improved gradually after therapeutic red cell-exchange
PRD, prednisolone; PE, plasma exchange; TRCE, therapeutic red cell-exchange.
DISCUSSION
Our patient was initially presented with severe intravascular hemolysis of unknown etiology. It is necessary to obtain a thorough history from patients, but physicians in east Asian countries often encounter difficulties in taking past histories from patients who frequently conceal traditional management method of diseases, including the ingestion of TCMs.
Arsenic intoxication mainly occurs through occupational exposure, including work with smelters, flue maintenance operations, pesticides, herbicides, and preservatives. In addition, non-occupational exposure, such as ingestion of contaminated well water, dried milk, soy sauce, and moonshine whiskey, has the potential to cause arsenic intoxication (3, 4). The arsenic compounds absorbed after ingestion or inhalation are readily taken up by red blood cells and then deposited in the liver, kidney, muscle, bone, skin, and hair (4).
Acute arsenic intoxication presents with gastrointestinal symptoms (nausea, vomiting, or diarrhea), neurologic symptoms (peripheral neuropathy, seizure, or coma), and other manifestations (facial edema, intravascular hemolysis, hepatomegaly, renal failure, and circulatory collapse preceding death) (4, 7). In chronic arsenic intoxication, skin, heart, lung, kidney, hematopoietic system, and neurologic system can be affected. Epidemiological studies have shown an association between malignancies, such as lung cancer, leukemia, lymphoma, and angiosarcoma of the liver, and arsenic exposure (3, 4, 8, 9). The hematologic manifestations of acute and chronic arsenic intoxication include intravascular hemolysis, leukopenia, and thrombocytopenia (3, 4).
Our patient ingested TCM for about 12 yr intermittently, and continuously for the most recent 6 months. Although hemolytic manifestations occur mainly in acute arsenic intoxication, it is unclear whether the hemolytic anemia in our patient was induced by the recent or long-term ingestion of TCMs. Her bone marrow hypocellularity, peripheral pancytopenia, and long-term peripheral neuropathy were thought to reflect chronic intoxication for 12 yr. In contrast, the intravascular hemolytic anemia was thought to reflect acute intoxication for the last 6 months. Our patient is therefore suggesting to have coexisting acute and chronic intoxication.
The most useful laboratory test for confirming recent arsenic exposure is the total urine arsenic level (1, 4, 10). Nonexposed persons have levels below 10 µg/g of creatinine (g Cr), while persons exposed to 0.01 mg/µL have levels of 50 µg/g Cr, and acute poisoning is caused at 1,000 µg/g Cr or higher levels (4). Another diagnostic method for arsenic intoxication, especially the systemic absorption of arsenic, is measuring arsenic levels in the hair and nails (3, 4). Therapeutic red cell-exchange is used to treat patients with hemolytic anemia due to arsenic intoxication, despite insufficient evidence of its effectiveness (11). In addition, chelating agents, including D-penicillamine, dimercaprol, dimercaptosuccinic, or dimercaptopanesulfonic acid, used clinically in chronic arsenic poisoning, but the chelating agents is not effective for treating established arsenical peripheral neuropathies or arsine poisoning (3, 4).
Plasmapheresis given to our patient as an empirical treatment was not an effective intervention for the arsenic intoxication. However, the therapeutic red cell-exchange resulted in gradual improvement of her hemolytic anemia within a few weeks without any complications. In this patient, we performed the therapeutic red cell-exchange with half volume of total red cell, as described a previous report in a patients with malaria (12).
In summary, we report a case of arsenic intoxication after long-term ingestion of TCM presenting with intravascular hemolysis that was treated successfully with therapeutic red cell-exchange.
References
- 1.Ernst E. Toxic heavy metals and undeclared drugs in Asian herbal medicines. Trends Pharmacol Sci. 2002;23:136–139. doi: 10.1016/S0165-6147(00)01972-6. [DOI] [PubMed] [Google Scholar]
- 2.Ernst E, Thompson Coon J. Heavy metals in traditional Chinese medicines: a systematic review. Clin Pharmacol Ther. 2001;70:497–504. doi: 10.1067/mcp.2001.120249. [DOI] [PubMed] [Google Scholar]
- 3.Hall AH. Chronic arsenic poisoning. Toxicol Lett. 2002;128:69–72. doi: 10.1016/s0378-4274(01)00534-3. [DOI] [PubMed] [Google Scholar]
- 4.Gochfeld M. Chemical agents. In: Brooks S, Gochfeld M, Herzstein J, et al., editors. Environmental medicine. St. Louis: Mosby; 1995. pp. 592–614. [Google Scholar]
- 5.Tay CH, Seah CS. Arsenic poisoning from anti-asthmatic herbal preparations. Med J Aust. 1975;2:424–428. [PubMed] [Google Scholar]
- 6.Wong ST, Chan HL, Teo SK. The spectrum of cutaneous and internal malignancies in chronic arsenic toxicity. Singapore Med J. 1998;39:171–173. [PubMed] [Google Scholar]
- 7.Cuncha J, Pereira L, Pun MI, Lopes V, Vong SK. Arsenic and acute lethal intoxication. Hong Kong Pharm J. 1998;7:50–53. [Google Scholar]
- 8.Bates MN, Smith AH, Hopenhayn-Rich C. Arsenic ingestion and internal cancers: a review. Am J Epidemiol. 1992;135:462–476. doi: 10.1093/oxfordjournals.aje.a116313. [DOI] [PubMed] [Google Scholar]
- 9.Chiou HY, Hsueh YM, Liaw KF, Horng SF, Chiang MH, Pu YS, Lin JS, Huang CH, Chen CJ. Incidence of internal cancers and ingested inorganic arsenic: a seven-year follow-up study in Taiwan. Cancer Res. 1995;55:1296–1300. [PubMed] [Google Scholar]
- 10.Johnson LR, Farmer JG. Use of human metabolic studies and urinary arsenic speciation in assessing arsenic exposure. Bull Environ Contam Toxicol. 1991;46:53–61. doi: 10.1007/BF01688254. [DOI] [PubMed] [Google Scholar]
- 11.Valbonesi M, Bruni R. Clinical application of therapeutic erythrocytapheresis. Transfus Sci. 2000;22:183–194. doi: 10.1016/s0955-3886(00)00042-4. [DOI] [PubMed] [Google Scholar]
- 12.Whang DH, Kim SH, Song JH, Kim DW. Therapeutic RBC exchange in a patient with severe Plasmodium falciparum infection. Korean J Blood Transfusion. 1997;8:147–151. [Google Scholar]