Abstract
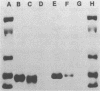
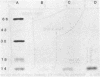
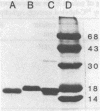
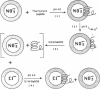
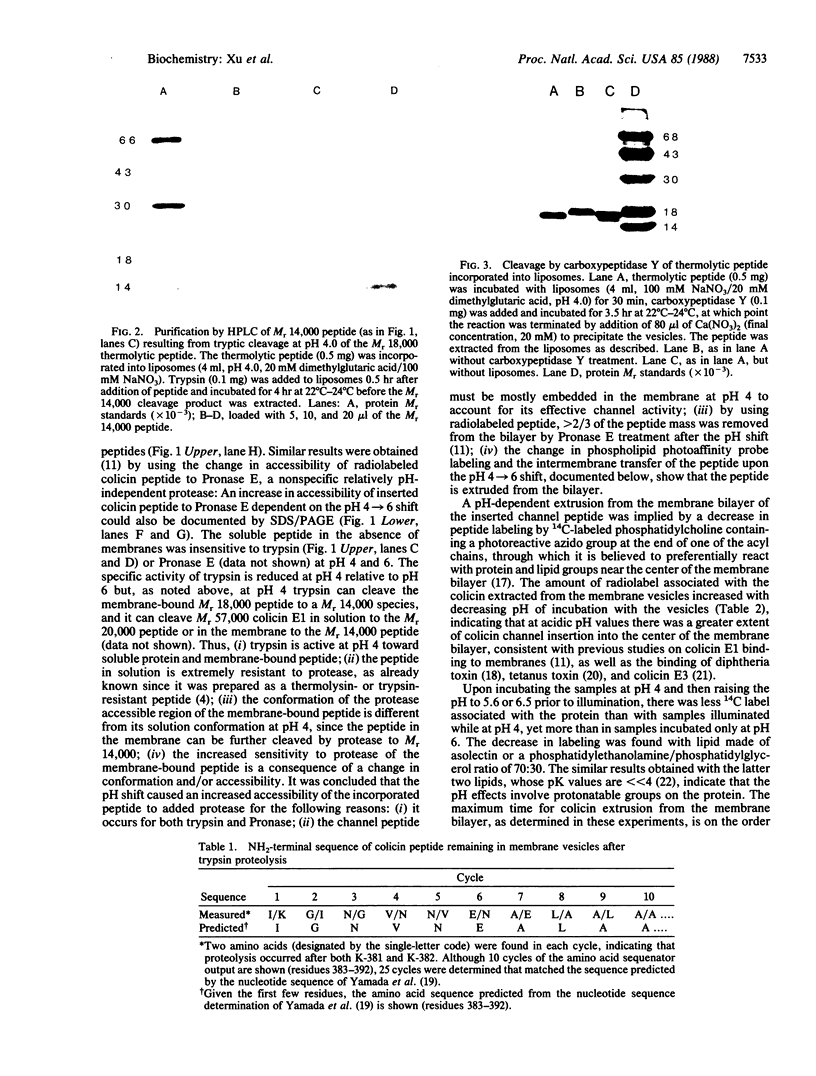
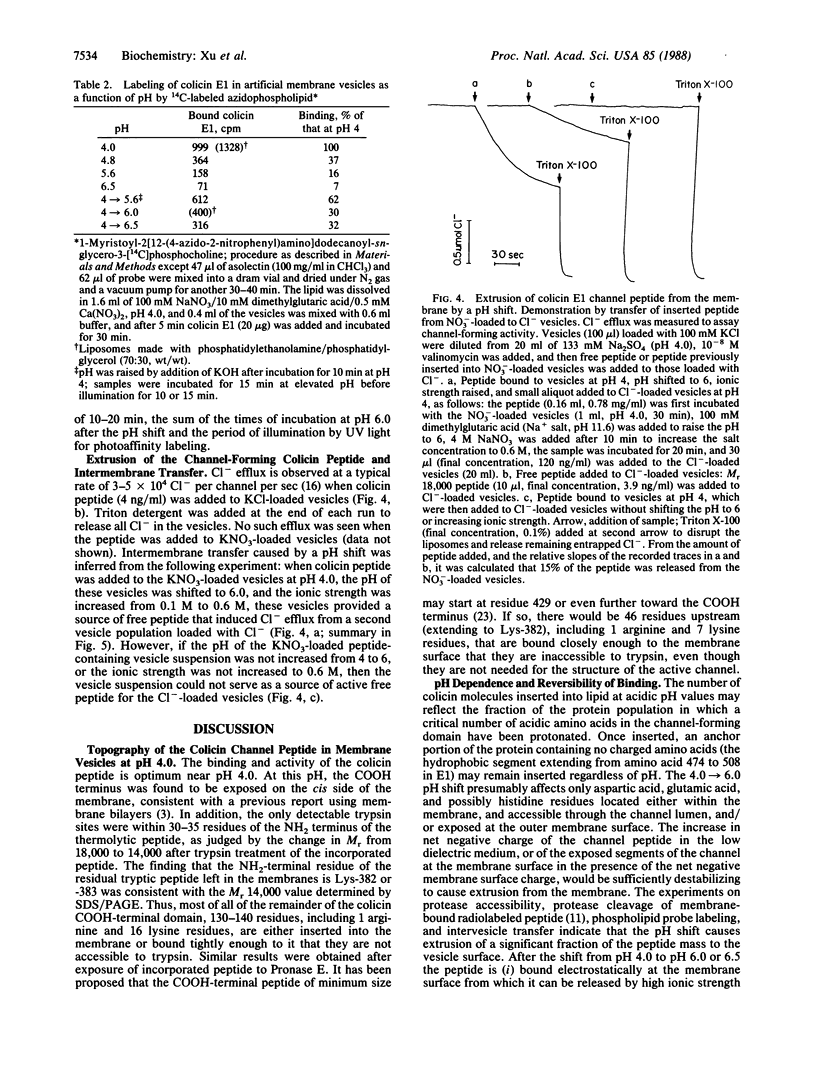
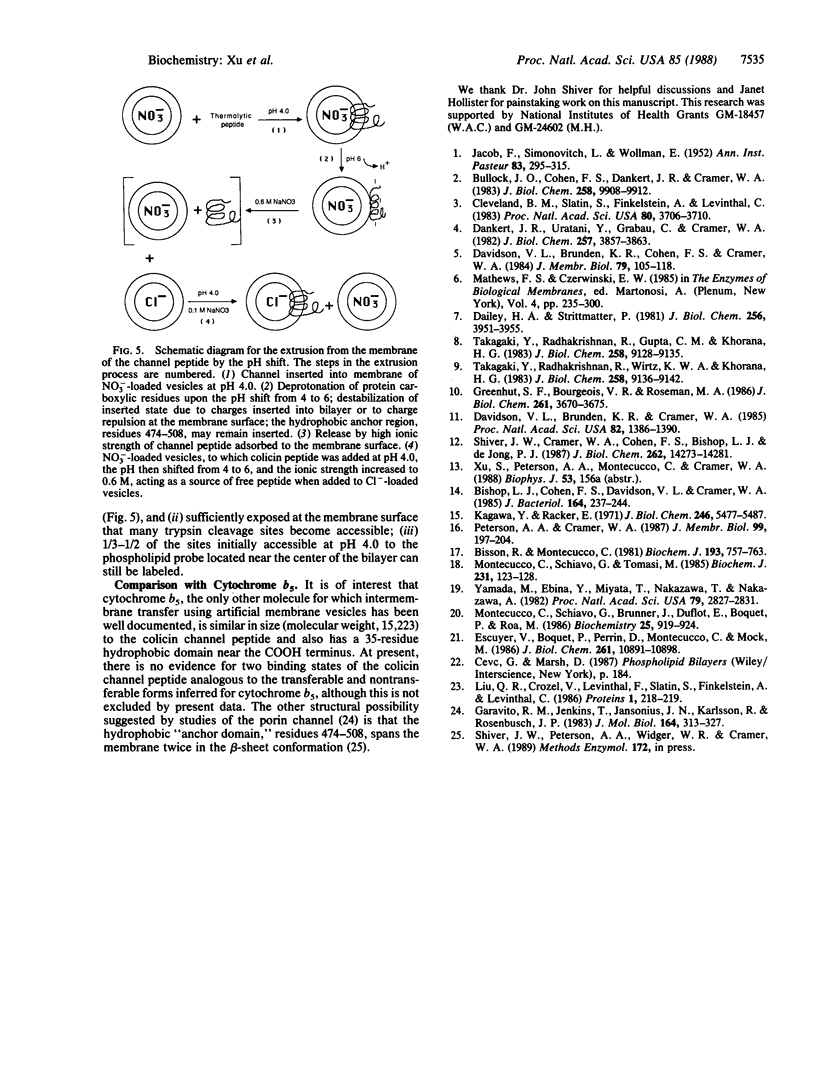
The binding of colicin E1 and its COOH-terminal channel-forming peptides to artificial membrane vesicles has an optimum at acidic pH values. The Mr 18,000 thermolytic peptide inserted into membrane vesicles at pH 4.0 has a limited accessibility to exogenous protease. It is converted by trypsin cleavage after Lys-381 and Lys-382 to a lower Mr 14,000 peptide. However, when the pH of a vesicle suspension to which peptide has been bound at pH 4.0 is shifted to 6.0, the accessibility to protease increased greatly. This was shown (i) by the large decrease in the amount of Mr 14,000 or Mr 18,000 peptide after the pH 4----6 shift and treatment with trypsin or Pronase, consistent with (ii) a previously observed decrease in membrane-bound radiolabeled peptide after protease treatment. (iii) When a photoactivable nitrene-generating phospholipid probe was used to label the colicin peptide inserted into the bilayer, the extent of labeling decreased by a factor of 3 when the pH was shifted from 4.0 to 6.5. (iv) Colicin peptide added to vesicles at pH 4.0 can "hop" to other vesicles if the pH and ionic strength of donor vesicles are successively increased. It is proposed that deprotonation of acidic residues in contact with the hydrophobic bilayer or the membrane surface destabilizes the inserted channel and causes it to be extruded from the membrane. The pH-dependent extrusion of the inserted colicin channel provides an example of dynamic properties of an intrinsic membrane protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop L. J., Bjes E. S., Davidson V. L., Cramer W. A. Localization of the immunity protein-reactive domain in unmodified and chemically modified COOH-terminal peptides of colicin E1. J Bacteriol. 1985 Oct;164(1):237–244. doi: 10.1128/jb.164.1.237-244.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson R., Montecucco C. Photolabelling of membrane proteins with photoactive phospholipids. Biochem J. 1981 Mar 1;193(3):757–763. doi: 10.1042/bj1930757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock J. O., Cohen F. S., Dankert J. R., Cramer W. A. Comparison of the macroscopic and single channel conductance properties of colicin E1 and its COOH-terminal tryptic peptide. J Biol Chem. 1983 Aug 25;258(16):9908–9912. [PubMed] [Google Scholar]
- Cleveland M. V., Slatin S., Finkelstein A., Levinthal C. Structure-function relationships for a voltage-dependent ion channel: properties of COOH-terminal fragments of colicin E1. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3706–3710. doi: 10.1073/pnas.80.12.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey H. A., Strittmatter P. Orientation of the carboxyl and NH2 termini of the membrane-binding segment of cytochrome b5 on the same side of phospholipid bilayers. J Biol Chem. 1981 Apr 25;256(8):3951–3955. [PubMed] [Google Scholar]
- Dankert J. R., Uratani Y., Grabau C., Cramer W. A., Hermodson M. On a domain structure of colicin E1. A COOH-terminal peptide fragment active in membrane depolarization. J Biol Chem. 1982 Apr 10;257(7):3857–3863. [PubMed] [Google Scholar]
- Davidson V. L., Brunden K. R., Cramer W. A. Acidic pH requirement for insertion of colicin E1 into artificial membrane vesicles: relevance to the mechanism of action of colicins and certain toxins. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1386–1390. doi: 10.1073/pnas.82.5.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson V. L., Brunden K. R., Cramer W. A., Cohen F. S. Studies on the mechanism of action of channel-forming colicins using artificial membranes. J Membr Biol. 1984;79(2):105–118. doi: 10.1007/BF01872115. [DOI] [PubMed] [Google Scholar]
- Escuyer V., Boquet P., Perrin D., Montecucco C., Mock M. A pH-induced increase in hydrophobicity as a possible step in the penetration of colicin E3 through bacterial membranes. J Biol Chem. 1986 Aug 15;261(23):10891–10898. [PubMed] [Google Scholar]
- Garavito R. M., Jenkins J., Jansonius J. N., Karlsson R., Rosenbusch J. P. X-ray diffraction analysis of matrix porin, an integral membrane protein from Escherichia coli outer membranes. J Mol Biol. 1983 Feb 25;164(2):313–327. doi: 10.1016/0022-2836(83)90079-7. [DOI] [PubMed] [Google Scholar]
- Greenhut S. F., Bourgeois V. R., Roseman M. A. Distribution of cytochrome b5 between small and large unilamellar phospholipid vesicles. J Biol Chem. 1986 Mar 15;261(8):3670–3675. [PubMed] [Google Scholar]
- JACOB F., SIMINOVITCH L., WOLLMAN E. Sur la biosynthèse d'une colicine et sur son mode d'action. Ann Inst Pasteur (Paris) 1952 Sep;83(3):295–315. [PubMed] [Google Scholar]
- Liu Q. R., Crozel V., Levinthal F., Slatin S., Finkelstein A., Levinthal C. A very short peptide makes a voltage-dependent ion channel: the critical length of the channel domain of colicin E1. Proteins. 1986 Nov;1(3):218–229. doi: 10.1002/prot.340010304. [DOI] [PubMed] [Google Scholar]
- Montecucco C., Schiavo G., Brunner J., Duflot E., Boquet P., Roa M. Tetanus toxin is labeled with photoactivatable phospholipids at low pH. Biochemistry. 1986 Feb 25;25(4):919–924. doi: 10.1021/bi00352a027. [DOI] [PubMed] [Google Scholar]
- Montecucco C., Schiavo G., Tomasi M. pH-dependence of the phospholipid interaction of diphtheria-toxin fragments. Biochem J. 1985 Oct 1;231(1):123–128. doi: 10.1042/bj2310123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A. A., Cramer W. A. Voltage-dependent, monomeric channel activity of colicin E1 in artificial membrane vesicles. J Membr Biol. 1987;99(3):197–204. doi: 10.1007/BF01995700. [DOI] [PubMed] [Google Scholar]
- Shiver J. W., Cramer W. A., Cohen F. S., Bishop L. J., de Jong P. J. On the explanation of the acidic pH requirement for in vitro activity of colicin E1. Site-directed mutagenesis at Glu-468. J Biol Chem. 1987 Oct 15;262(29):14273–14281. [PubMed] [Google Scholar]
- Takagaki Y., Radhakrishnan R., Gupta C. M., Khorana H. G. The membrane-embedded segment of cytochrome b5 as studied by cross-linking with photoactivatable phospholipids. J Biol Chem. 1983 Aug 10;258(15):9128–9135. [PubMed] [Google Scholar]
- Takagaki Y., Radhakrishnan R., Wirtz K. W., Khorana H. G. The membrane-embedded segment of cytochrome b5 as studied by cross-linking with photoactivatable phospholipids. II. The nontransferable form. J Biol Chem. 1983 Aug 10;258(15):9136–9142. [PubMed] [Google Scholar]
- Yamada M., Ebina Y., Miyata T., Nakazawa T., Nakazawa A. Nucleotide sequence of the structural gene for colicin E1 and predicted structure of the protein. Proc Natl Acad Sci U S A. 1982 May;79(9):2827–2831. doi: 10.1073/pnas.79.9.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]