Abstract
Fifty patients undergoing thoracotomy was studied to compare the effects of cryoanalgesia combined with intravenous continuous analgesia (IVCA). Patients were randomized into two groups: IVCA group and IVCA-cryo group. Subjective pain intensity was assessed on a visual analogue scale at rest (VAS-R) and during movement (VAS-M). Analgesic requirements were evaluated over the 7 days following surgery. Forced vital capacity (FVC) and forced expiratory volume in 1 sec (FEV1) were measured before operation, on the 2nd and 7th postoperative days (POD). We interviewed patients by telephone to evaluate the prevalence of post-thoracotomy pain at the 1st, 3rd, and 6th months postoperatively. No significant differences were observed between the two groups with respect to postoperative pain, analgesic requirements, side effects, respiratory complications, or prevalence of post-thoracotomy pain. However, a significant increase in FVC and FEV1 was observed on the 7th POD in IVCAc-ryo group. The incidence of the post-thoracotomy pain at the 1st, 3rd, and 6th months postoperatively was 68, 60, and 44% in IVCA group, and 88, 68, and 28% in IVCA-cryo group, respectively. Our study showed that cryoanalgesia combined with IVCA effectively restore respiratory function on 7th POD, but that it was not effective at reducing the incidence of post-thoracotomy pain.
Keywords: Analgesia, Cryotherapy, Pain, Respiratory Function Tests, Thoracotomy
INTRODUCTION
Thoracotomy causes severe postoperative pain and a marked impairment of deep breathing and coughing which leads to postoperative complications such as atelectasis, hypoxemia, pneumonia, and respiratory insufficiency (1). Effective analgesia to minimize the impairment of respiratory function and to prevent respiratory complications is mandatory. The most effective analgesic methods known for post-thoracotomy pain are epidural local anesthetic-opiate combinations and paravertebral analgesia (2, 3). However, it is necessary to use other analgesic methods such as intravenous opiate or cryoanalgesia in some patients who refuse epidural catheterization or in whom epidural catheterization is impossible because of anatomic abnormalities.
Intravenous opiate is simple and rapid in onset, but can cause nausea, vomiting, pruritus and respiratory depression because of the narrow therapeutic-respiratory depression window (4). Cryoanalgesia is relatively safe, effective and long lasting, but may be ineffective against pain mediated by the phrenic, vagus or the sympathetic nerves (4, 5), and it takes 5-11 days to achieve the analgesic effect (3, 6). Many investigators have reported upon the effect of cryoanalgesia in post-thoracotomy pain versus intravenous opiate, thoracic epidural, transcutaneous nerve stimulation (TENS), and interpleural analgesia (5-8). The reports about the efficacy of cryoanalgesia have been controversial. The analgesic efficacy of cryoanalgesia has been reported to be similar to or less than intravenous opiate and to be less than thoracic epidural (3, 5, 6, 8-10). One study found that cryoanalgesia could cause neuralgia (11), but thoracotomy itself carries a risk of producing intercostal neuralgia (12), and cryotherapy is one of the treatment modality for neuralgia in chronic pain management (13). Jones and Murrin treated patients suffered from a long-term post-thoracotomy pain by transcutaneous cryoanalgesia and reported that the pain was relieved or reduced in 70% of patients and the duration of pain relief was considerably longer (14). The efficacy of cryoanalgesia needs to be verified and no report is available upon the effect of cryoanalgesia combined with other analgesic methods.
It has been reported that preemptive analgesia or aggressive management of early postoperative pain can reduce the incidence of long-term post-thoracotomy pain (15, 16), but no study has reported upon whether long lasting cryoanalgesia can reduce its incidence.
Therefore, we undertook a study on the effects of cryoanalgesia combined with intravenous continuous analgesia (IVCA) in thoracotomy patients in whom thoracic epidural was refused or impossible. We studied whether this combination of the two methods (prompt onset and systemic analgesic effect from IVCA and the long-lasting analgesic effect of cryoanalgesia) could provide additive effects in terms of pain score and respiratory function test improvement, in an effort to reduce both the requirements for analgesics and respiratory complications during the acute postoperative period. In addition, we wanted to determine whether such a combination could reduce the incidence of long-term post-thoracotomy pain and whether it caused neuralgia.
MATERIALS AND METHODS
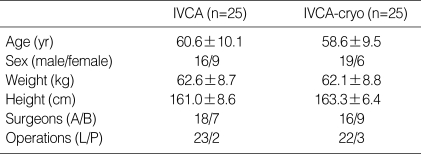
Fifty patients who underwent posterolateral thoracotomy for lung cancer were studied. Patients were excluded if they had preoperative respiratory dysfunction, were scheduled for elective postoperative mechanical ventilation, or had been receiving analgesics for chronic pain. All patients gave written informed consent to the trial that was approved by our hospital's Ethics Committee. Patients were randomized into two groups: an IVCA group and an IVCA-cryo group. There was no significant difference between the two groups in terms of age, sex, body weight, height, or distributions of surgeons or operations (Table 1).
Table 1.
Demographic data
Values are mean±SD or numbers of patients. IVCA, intravenous continuous analgesia; IVCA-cryo, intravenous continuous analgesia combined with cryoanalgesia; L/P, lobectomy/pneumonectomy. There was no significant difference between the two groups.
General anesthesia was induced with thiopental 5 mg/kg and vecuronium 0.1 mg/kg to facilitate tracheal intubation with a double-lumen endobronchial tube. Anesthesia was maintained with enflurane 0-4 vol% inspired in oxygen with one-lung ventilation. Muscular relaxation was achieved by intermittent injection of vecuronium. Before the surgical incision, a bolus of fentanyl 2 µg/kg and ketorolac 0.2 mg/kg was administered to patients in both groups intravenously, and continuous infusion was started at a rate of 0.5 mL/hr using an infuser (Portable Elastometric Infusion System, Baxter Healthcare Corporation, U.S.A.). The infuser contained fentanyl 30 µg/kg and ketorolac 3 mg/kg in 60 mL of normal saline.
Before thorax closure, three intercostal nerves were frozen in each patient in the IVCA-cryo group, using a nitrous oxide cryoprobe (SL 2000 Lloyd Neurostat, Spembly Medical Ltd., U.K.) at -20℃ for a 90 sec under direct vision. Nerves were dissected out as far back as possible to include the collateral branches, and were frozen at the operative level, and at one level above and below the incision after peeling back the pleura. A 10-sec thaw was allowed prior to probe removal in order to prevent nerve adherence.
Severity of pain was graded by the patients on a 10 cm VAS chart, one mark representing pain at rest (VAS-R) and the other pain on deep breathing and coughing (VAS-M). The VAS score was recorded daily until the 7th postoperative day (POD) and breakthrough pain when experienced by patients was treated with 0.6 µg/kg of fentanyl intravenously on demand. We investigated the required doses and numbers of fentanyl treatments, and asked about the presence and frequencies of nausea, vomiting and pruritus and also checked the incidence of respiratory depression (less than 10/min of the respiratory rate).
Forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) were recorded preoperatively, 2nd and 7th POD by a research assistant using a portable spirometer (Micro, Micro Medical Ltd, Rochester, England). Three spirometric measurements were taken while the patient was sitting upright and the means of these measurements were included in the analysis. We checked the chest radiograph on the 2nd and 7th POD and recorded the incidence of atelectasis, effusion, and pneumonia. Patients who required the assistance of a mechanical ventilator because of respiratory insufficiency, and who received talc pleurodesis because of a consistent air leak during the postoperative period were excluded.
Patients were contacted by telephone on the 1st, 3rd, and 6th months postoperatively by a research assistant. A standardized questionnaire was administered to each patient, which assessed the absence or presence and intensity of pain and numbness in the region of the thoracotomy scar at rest and during deep breathing and coughing. All patients reporting pain rated it on a 0-10 numerical rating scale (NRS).
Using a double blind method, research assistant and nurses were unaware of the patient group assignments.
Inter-group demographics and difference in the incidence of side effects, respiratory complications and long-term postoperative pain and numbness were compared using the t-test and the chi-square test, respectively. For VAS scores, analgesic requirements and spirometry data, repeated measures analysis of variance and Dunnet's test were used for intra-group comparisons, and the Mann-Whitney rank sum test with Bonferroni's correction was used for inter-group comparisons. A p value<0.05 was taken to indicate a significant difference. Data are presented as means±SD or as numbers of patients.
RESULTS
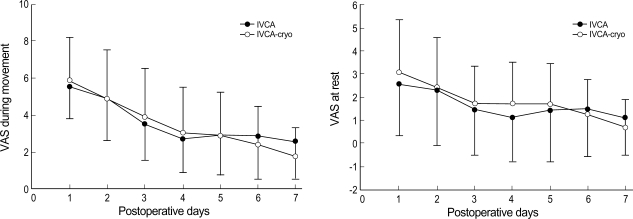
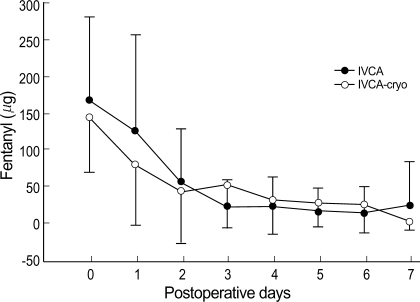
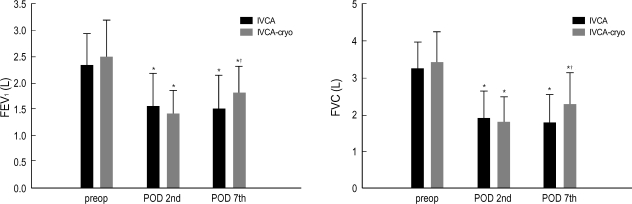
No statistically significant difference was found between the two groups with respect to VAS-R or VAS-M (Fig. 1), and the groups were similar in terms of analgesics requirements (Fig. 2). FVC and FEV1 increased significantly in IVCA-cryo group on the 7th POD (Fig. 3). On the contrary, FVC and FEV1 on the 2nd POD was slightly lower in IVCA-cryo group than in IVCA group, but this difference was not statistically significant. The incidence of pruritus, nausea, vomiting, atelectasis, pneumonia and effusion was similar in the two groups (Table 2).
Fig. 1.
Visual analogue scale at rest and during movement show no difference between the two groups. Data expressed as mean±SD.
Fig. 2.
Analgesics requirement shows no difference between the two groups. Data expressed as mean±SD.
Fig. 3.
Forced vital capacity (FVC) and forced expiratory volume in 1 sec (FEV1) on the 7th POD are significantly increased in the IVCA-cryo group. Data expressed as mean±SD. *; p<0.05 versus the preoperative value. †; p<0.05 versus the IVCA group.
Table 2.
Incidence of side effects and complications
Values are number of patients. IVCA, intravenous continuous analgesia; IVCA-cryo, intravenous continuous analgesia combined with cryoanalgesia. There was no significant difference between the two groups.
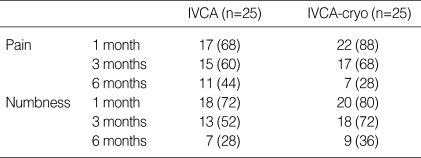
The incidence of long-term post-thoracotomy pain and numbness in the 1st, 3rd, and 6th months was shown in Table 3. NRS-R and NRS-M in the 1st, 3rd, and 6th months were 2.1/3.9, 1.5/2.6, and 1.3/2.2 in the IVCA group and 1.6/3.4, 1.1/2.7, and 0.8/1.8 in the IVCA-cryo group, respectively. There was no statistical difference during the entire observation period between the two groups.
Table 3.
Long-term follow-up
Values are numbers (%) of patients. IVCA, intravenous continuous analgesia; IVCA-cryo, intravenous continuous analgesia combined with cryoanalgesia. There was no significant difference between the two groups.
DISCUSSION
Cryoanalgesia was introduced as a mean of achieving pain relief after the development of the first cryoprobe in 1961 by Cooper, and the demonstration by Lloyd that prolonged analgesia could be obtained following a single freeze of a peripheral nerve. Lloyd et al. (17) stressed the safety aspect of the procedure and reported that nerve function always returned and neuroma formation did not occur.
When nerve fibers are progressively cooled, the dissociation of sensory modalities and a loss of motor function often occurs above 10℃, complete temporary conduction block occurs at temperatures between 0℃ and -20℃, and at temperatures below -20℃, persistent conduction block occurs and recovers with axonal regeneration (7). Zhou et al. (18) reported that cryotherapy at -60℃ to -100℃ injured myelinated fibers selectively, but complete regeneration occurred within 60 days. These workers felt that the technique might be suitable for short period analgesia for postoperative pain. Moreover, cryolesioning at -140℃ to -180℃ caused complete nerve fiber necrosis with subsequent incomplete regeneration, which might be suitable for lasting pain relief in those with intractable pain.
According to Evans et al. (19), once the critical temperature of -20℃ has been reached in tissues, no additional benefit is derived by prolonging the duration of exposure or by repeating the freeze thaw cycle. Moreover, to inhibit saltatory conduction for myelinated fibers, a lesion of 3 mm diameter will suffice, and this can be achieved by a 1 min freeze (7, 19). Lloyd et al. (17) advocated two freeze cycles of 2 min' each, whereas Wood et al. (20) recommended two 1-min freezes, though Katz et al. (21) achieved good results after a 1 min freeze. In patients who receive transcutaneous cryoanalgesia, close approximation between the cryoprobe and the nerves may be difficult, and repeated cycles might be necessary. In the present study, we performed cryoanalgesia under direct vision and considered one 90 sec at -20℃ freeze sufficient.
The efficacy of cryoanalgesia remains controversial. Several studies have demonstrated that it is less effective than epidural analgesia (2, 3, 8-10), though its effect was found to be similar to intravenous morphine (5), whereas Muller et al. (11) found that it has no analgesic effect and causes moderate to severe neuralgiform pain. However, none of the patients in the present study complained of symptoms suggestive of severe neuralgic pain. Katz et al. (21) reported that patients underwent thoracotomy and who received cryoanalgesia had less postoperative pain and that their requirements for analgesics were reduced by 50% versus those who did not receive the cryoanalgesia, and that a return of sensation occurred within 30 days. Moreover, patients receiving cryoanalgesia were found not to perform well on the 1st POD, but by the 5th POD, cryoanalgesia was found to be significantly effective at managing post-thoracotomy pain (6). According to Brichon et al. (3) cryoanalgesia provided a slight improvement in postoperative pain and marginally reduced analgesic requirements in the early PODs, but achieved a better pain score than epidural analgesia on the 11th and 12th PODs.
Our results showed that cryoanalgesia combined on IVCA had no effect in terms of reducing analgesic requirements or upon improving pain score, but it did improve the respiratory function test results on the 7th POD. Moreover, these results were comparable with the results of Rooney et al. (6) in terms of the delayed onset of the effect. But it is questionable that pain score and analgesic requirements did not show the same improvement. We thought that was because much of the pain in thoracotomy came from straining of the ligaments during rib retraction and such pain was mediated by posterior primary rami, which were not affected by intercostal nerve freezing.
We interviewed patients on the 1st, 3rd, and 6th months postoperatively by telephone to determine whether cryoanalgesia could prevent or reduce the incidence of post-thoracotomy pain. In our study, the incidences of long-term post-thoracotomy pain were 68%, 60%, and 44% in the IVCA group and 88%, 68%, and 28% in the IVCA-cryo group on the 1st, 3rd, and 6th months, which suggests that cryoanalgesia is not helpful at preventing or reducing post-thoracotomy pain. Although it was not statistically significant, the incidence of long-term post-thoracotomy pain was even higher in IVCA-cryo group than in IVCA group on the 1st and 3rd months. Long-term pain scores in the IVCA-cryo group were slightly lower than those in the IVCA group, but this difference was statistically insignificant.
Katz et al. (16) followed up 30 thoracotomy patients by telephone over 1.5 yr in an effort to identify predictors of long-term post-thoracotomy pain and reported that early postoperative pain was the only significant predictor, and that preemptive analgesia or analgesic requirements did not produce significant difference. They concluded that aggressive management of early postoperative pain might reduce the likelihood of long-term post-thoracotomy pain.
Post-thoracotomy pain syndrome (PTPS), which recurs or persists along the thoracotomy incision over at least the first postoperative 2 months is influenced by extent of the incision, muscle sparing versus dividing, rib resection, and video-assisted thoracic surgery versus thoracotomy. Prevention is the best strategy, and aggressive management of early postoperative pain and preemptive analgesia are important in prevention, and ketamine, an NMDA antagonist is known as useful in its treatment (22). Overall incidences of PTPS ranged widely from 2% to 67% because of the varying definitions uses for PTPS and the diversity of operating procedures and analgesic methods. Kalso et al. (12) reported that the incidence of persistent post-thoracotomy pain lasting for more than 6 months was 44%. In the study of Dajczman et al. (23), 30% of patients suffered pain for more than four years post-thoracotomy, and the pain was generally not severe, but a small percentage of patients experienced persistent, moderately disabling pain. In our study, on the 3rd month, 60%, and 68% of patients suffered pain in the IVCA and in the IVCA-cryo group, and on the 6th month, 44%, and 28% of patients experienced pain. Though this pain was mild, less than three by the NRS, the combination of cryoanalgesia did not reduce its incidence.
More than 2/3 of patients in the IVCA and IVCA-cryo groups complained of numbness around the incision area on the 1st month, and this proportion decreased to 28% and 36% on the 6th month, respectively. No significant difference between the two groups were evident, thus it is presumed that the numbness was caused not only by the cryoanalgesia but also by nerve injury during rib resection.
Conclusively, cryoanalgesia combined with IVCA improved respiratory function partially, but had no ameliorative effect on pain scores and did not reduce analgesics requirement during the acute postoperative period. Moreover, it failed to reduce the incidence of long-term post-thoracotomy pain, but did not cause neuralgic pain.
References
- 1.Sabanathan S, Eng J, Mearns AJ. Alterations in respiratory mechanics following thoracotomy. J R Coll Surg Edinb. 1990;35:144–150. [PubMed] [Google Scholar]
- 2.Richardson J, Sabanathan S, Shah R. Post-thoracotomy spirometric lung function: the effect of analgesia. A review. J Cardiovasc Surg. 1999;40:445–456. [PubMed] [Google Scholar]
- 3.Brichon PY, Pison C, Chaffanjon P, Fayot P, Buchberger M, Neron L, Bocca A, Verdier J, Sarrazin R. Comparison of epidural analgesia and cryoanalgesia in thoracic surgery. Eur J Cardiothorac Surg. 1994;8:482–486. doi: 10.1016/1010-7940(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 4.Benumof JL. Anesthesia for thoracic surgery. 2nd ed. Philadelphia: W.B. Saunders Company; 1995. p. 757. [Google Scholar]
- 5.Orr IA, Keenan DJM, Dundee JW. Improved pain relief after thoracotomy: use of cryoprobe and morphine infusion. Br Med J. 1981;283:945–948. doi: 10.1136/bmj.283.6297.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rooney SM, Jain S, McCormack P, Bains MS, Martini N, Goldiner PL. A comparison of pulmonary function tests for postthoracotomy pain using cryoanalgesia and transcutaneous nerve stimulation. Ann Thorac Surg. 1986;41:204–207. doi: 10.1016/s0003-4975(10)62670-7. [DOI] [PubMed] [Google Scholar]
- 7.Evans PJD. Cryoanalgesia. The application of low temperatures to nerves to produce anaesthesia or analgesia. Anaesthesia. 1981;36:1003–1013. doi: 10.1111/j.1365-2044.1981.tb08673.x. [DOI] [PubMed] [Google Scholar]
- 8.Miguel R, Hubbell D. Pain management and spirometry following thoracotomy: a prospective, randomized study of four techniques. J Cardiothorac Vasc Anesth. 1993;7:529–534. doi: 10.1016/1053-0770(93)90308-8. [DOI] [PubMed] [Google Scholar]
- 9.Gough JD, Williams AB, Vaughan RS, Khalil JF, Butchart EG. The control of post-thoracotomy pain. A comparative evaluation of thoracic epidural fentanyl infusions and cryo-analgesia. Anaesthesia. 1988;43:780–783. doi: 10.1111/j.1365-2044.1988.tb05757.x. [DOI] [PubMed] [Google Scholar]
- 10.Choi IC, Min HK, Shim JY, Kim JU, Lee C, Park SI. The comparison of cryoanalgesia and epidural analgesia in thoracotomy. Korean J Anesthesiol. 2000;39:83–90. [Google Scholar]
- 11.Muller LC, Salzer GM, Ransmayr G, Neiss A. Intraoperative cryoanalgesia for postthoracotomy pain relief. Ann Thorac Surg. 1989;48:15–18. doi: 10.1016/0003-4975(89)90169-0. [DOI] [PubMed] [Google Scholar]
- 12.Kalso E, Perttunen K, Kaasinen S. Pain after thoracic surgery. Acta Anaesthesiol Scand. 1992;36:96–100. doi: 10.1111/j.1399-6576.1992.tb03430.x. [DOI] [PubMed] [Google Scholar]
- 13.Watson CPN, Loeser JD. Herpes zoster and postherpetic neuralgia. In: Loeser JD, Butler SH, Chapman CR, Turk DC, editors. Bonica's management of pain. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2001. p. 429. [Google Scholar]
- 14.Jones MJ, Murrin KR. Intercostal block with cryotherapy. Ann R Coll Surg Engl. 1987;69:261–262. [PMC free article] [PubMed] [Google Scholar]
- 15.Obata H, Saito S, Fujita N, Fuse Y, Ishizaki K, Goto F. Epidural block with mepivacaine before surgery reduces long-term post-thoracotomy pain. Can J Anaesth. 1999;46:1127–1132. doi: 10.1007/BF03015520. [DOI] [PubMed] [Google Scholar]
- 16.Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996;12:50–55. doi: 10.1097/00002508-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd JW, Barnard JDW, Glynn CJ. Cryoanalgesia. A new approach to pain relief. Lancet. 1976;2:932–934. doi: 10.1016/s0140-6736(76)90893-x. [DOI] [PubMed] [Google Scholar]
- 18.Zhou L, Kambin P, Casey KF, Bonner FJ, O'Brien E, Shao Z, Ou S. Mechanism research of cryoanalgesia. Neurol Res. 1995;17:307–311. doi: 10.1080/01616412.1995.11740333. [DOI] [PubMed] [Google Scholar]
- 19.Evans PJD, Lloyd JW, Green CJ. Cryoanalgesia: the response to alterations in freeze cycle and temperature. Br J Anaesth. 1981;53:1121–1127. doi: 10.1093/bja/53.11.1121. [DOI] [PubMed] [Google Scholar]
- 20.Wood CJ, Lloyd JW, Evans PJD, Bullingham RES, Britton BJ, Finch DRA. Cryoanalgesia and day-case herniorrhaphy. Lancet. 1979;2:479. doi: 10.1016/s0140-6736(79)91539-3. [DOI] [PubMed] [Google Scholar]
- 21.Katz J, Nelson W, Forest R, Bruce DL. Cryoanalgesia for post-thoracotomy pain. Lancet. 1980;1:512–513. doi: 10.1016/s0140-6736(80)92766-x. [DOI] [PubMed] [Google Scholar]
- 22.d'Amours RH, Riegler FX, Little AG. Pathogenesis and management of persistent postthoracotomy pain. Chest Surg Clin N Am. 1998;8:703–722. [PubMed] [Google Scholar]
- 23.Dajczman E, Gordon A, Kreisman H, Wolkove N. Long-term post-thoracotomy pain. Chest. 1991;99:270–274. doi: 10.1378/chest.99.2.270. [DOI] [PubMed] [Google Scholar]