Abstract
Hepatocellular carcinomas (HCCs) show genomic alterations, including DNA rearrangements associated with HBV DNA integration, loss of heterozygosity, and chromosomal amplification. The genes most frequently involved are those encoding tumor suppressors. The p16INK4A tumor suppressor gene frequently displays genetic alteration in HCC tissues. The present study was performed to examine the incidence of methylated p16INK4A in the sera of liver cirrhosis (LC) and HCC patients, and to evaluate its role as a tumor marker of HCC. The sera of 23 LC patients and 46 HCC patients were examined in this study. The methylation status of p16INK4A was evaluated by methylation-specific PCR of serum samples. Methylated p16INK4A was detected in 17.4% (4/23) of LC patients and in 47.8% (22/46) of HCC patients. No association was demonstrated between p16INK4A methylation and serum AFP level. As the status of p16INK4A methylation was not associated with serum AFP level, it may have a role as a tumor marker of HCC.
Keywords: p16INK4A Methylation; PCR, Methylation-Specific; Carcinoma, Hepatocellular; Liver Cirrhosis
INTRODUCTION
Hepatocellular carcinoma (HCC) is a common cause of mortality in cancer patients in Korea, with a death rate of approximately 21 per 100,000 (1). No treatment modality has been successful in increasing survival time in patients with advanced HCC, whereas effective treatments, such as liver transplantation, surgical resection, and local ablation therapies, are available for patients with small HCCs (2-6).
Although alpha-fetoprotein (AFP) measurement and ultrasonography are useful surveillance tests for detecting HCCs at a stage at which they may be treated, both tests have limitations: AFP shows low sensitivity and specificity, while the results of ultrasonography are dependent on the skill of those performing the examination and on the condition of the patient.
Inactivation of tumor suppressor genes is important in the development of cancers, and leads to abnormal proliferation, transformation, invasion, and metastasis (7). Inactivation of tumor suppressor genes can occur due to silencing as a result of methylation of tumor suppressor gene promoters, as well as genetic mutation, loss of heterozygosity (LOH), or deletion of homozygosity (8-10).
The tumor suppressor gene p16INK4Ais located on chromosome 9p21 and encodes the p16 protein, which binds selectively to CDK4 to inhibit activation of the CDK4/cyclin D complex in G1 phase (11). Recent studies have indicated the occurrence of structural changes in p16INK4Ain HCC (12, 13). Inactivation of this gene, which normally inhibits progression to the G1 phase of the cell cycle, is involved in the initiation of tumors. The methylation of p16INK4A is known to silence transcription of the gene (14).
The degree of p16INK4Amethylation shows a wide range of variation (from 0 to 94%) in tumor tissues of HCC patients (8, 15-22), and this change has been detected in the sera of such patients (20).
This study was performed to evaluate the incidence of methylated p16INK4Ain the sera of liver cirrhosis (LC) and HCC patients, and to examine its role as a tumor marker of HCC.
MATERIALS AND METHODS
Subjects
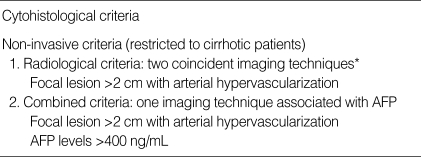
This study included 23 patients with cirrhosis and 46 with HCC. Cirrhosis patients were selected on the basis of clinical, biochemical, and radiological findings, and follow-up was performed for at least 6 months to exclude undetected cancers. HCC patients (23) were selected consecutively from those diagnosed according to the criteria of the European association for the study of the liver (EASL) (Table 1).
Table 1.
Diagnostic criteria for hepatocellular carcinoma
*Four techniques considered. US, spiral CT, MRI, and angiography.
Methods
Methylation-specific PCR (MSP) (24) was used to detect abnormal methylation of the p16INK4A gene. Serum AFP was measured by radioimmunoassay (BioSource Europe S.A., Nivelles, Belgium).
Isolation and Quantification of DNA
To obtain serum, blood specimens from each patient were centrifuged at 3,000 RPM for 20 min. Sera were stored at -70℃ until DNA extraction. A total volume of 2 mL of serum was treated with an equal volume of 1% sodium dodecyl sulfate, 0.5 mg/mL proteinase K (1× SDS/PK) for over 16 hr at 58℃. The solution was extracted twice with an equal volume of PC-8 [250 mL of Aquaphenol, supplemented with pH 8.0 buffer (Qbiogene Inc., Carlsbad, CA, U.S.A.), 40 mL of distilled water, 2.5 mL of 0.5 M EDTA, and 200 mL of chloroform], and 7.5 M ammonium acetate was added to the supernatant. After addition of glycogen, the DNA was precipitated with ethanol. The DNA pellet was washed twice with 70% ethanol, dried, and dissolved in LoTE (30 mM Tris-HCl, 0.3 mM EDTA).
Modification Reaction
Bisulfite conversion of genomic DNA was performed using the reagents provided with a CpGenome™ DNA modification kit, according to the manufacturer's protocols (Intergen, Edinburgh, U.K.). The modified DNA was eluted into 20 µL of TE (10 mM Tris-HCl, 1 mM EDTA), and was either used immediately as a template for MSP or stored at -20℃.
Methylation-specific Polymerase Chain Reaction (MSP)
Bisulfite-modified DNA was amplified using the primers provided with a CpG WIZ™ p16INK4A amplification kit (Intergen, Edinburgh, U.K.). The PCR mixture contained 1× universal PCR buffer, dNTPs (each at 0.25 mM), methylated or unmethylated primer, 1 unit of AmpliTaq Gold polymerase (Perkin Elmer, Wellesley, MA), and modified DNA (100 ng) in a final volume of 12.5 µL. Water was used as a negative control, while positive control DNA was supplied with the kit. Amplification was carried out in a thermal cycler (MWG-Biotech AG, Ebersberg, Germany) as follows: 94℃ for 5 min; 35 cycles of 94℃ for 45 sec, 56℃ for 45 sec, and 72℃ for 1 min; with a final extension of 10 min at 72℃. PCR products (12.5 µL) were separated on non-denaturing 10% polyacrylamide gels, stained with ethidium bromide, and visualized under UV illumination.
Statistical Analysis
Statistical analysis was performed using the SPSS™, version 11.0 for Windows software (SPSS Inc., Chicago, IL, U.S.A.). The significance of variations between groups was examined by chi-square test, Fisher's exact test, or Student's t-test.
RESULTS
Clinical Features of Patients
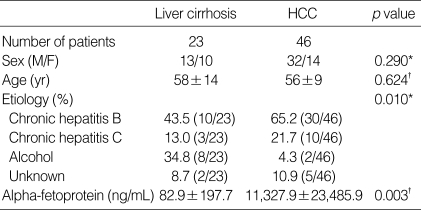
The average age of patients was 57±11 yr (mean standard deviation), and ranged from 30 to 86. The patient population consisted of 45 men and 24 women. Forty patients (58.0%) were positive for HBsAg and 13 (18.8%) were positive for anti-HCV antibody. The clinical and laboratory features of each group are shown in Table 2.
Table 2.
Clinical and laboratory features of cirrhosis and HCC patients
*Fisher's exact test. †Student's t-test.
Detection of Aberrant p16INK4A Methylation in Serum Using MSP
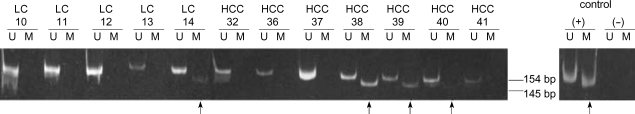
Methylated p16INK4A was detected in 47.8% (22/46) of HCC patients, a significantly higher rate than the 17.4% (4/23) seen in LC patients (p=0.014) (Fig. 1).
Fig. 1.
Detection of aberrant p16INK4A methylation (sample numbers 14, 38, 39 and 40, as indicated with arrows) in the sera of patients with liver cirrhosis (LC) and hepatocellular carcinoma (HCC). U, unmethylated; M, methylated; bp, base pairs.
Sensitivity, Specificity, and Positive Predictive Value of the Detection of p16INK4A Methylation in the Diagnosis of Hepatocellular Carcinoma
Aberrant serum p16INK4Amethylation showed 47.8% (22/46) sensitivity, 82.6% (19/23) specificity, and 84.6% (22/26) positive predictive value in the diagnosis of HCC.
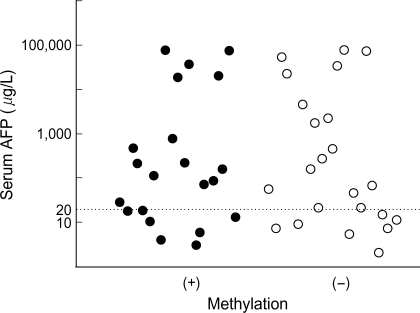
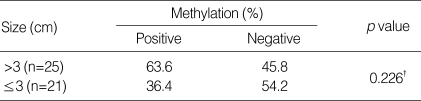
Association of p16INK4A Methylation Status and Serum AFP Level or Tumor Size in Patients with Hepatocellular Carcinoma
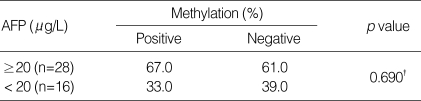
There were no associations between the status of p16INK4A methylation and serum AFP level in HCC patients (Table 3, Fig. 2) or tumor size (Table 4).
Table 3.
Association between p16INK4A methylation and serum AFP levels in patients with HCC*
*Two patients were excluded due to long interval between the AFP test date and serum sampling for methylation. †chi-square test.
Fig. 2.
Serum AFP levels of 44 HCC patients according to status of p16INK4A methylation.
Table 4.
Association between p16INK4A methylation and tumor size
*Chi-square test.
DISCUSSION
The tumor suppressor gene p16INK4A exhibits variations, including methylation, that are involved in the process of carcinogenesis. A causal relationship between genetic variation and methylation has yet to be determined. Kondo et al. (8) studied LOH, microsatellite instability, and DNA methylation in HCC and in non-cancerous surrounding tissue by microdissection. Their results suggested that methylation often occurs in both tissue types, that it precedes LOH, and that methylation is involved in the early genesis of HCC as the cause of LOH.
The reported rate of incidence of p16INK4A methylation is quite variable, ranging from 0% to 94% in HCC (8, 15, 22), and from 29.4% to 83% in cirrhosis (8, 21, 22). This may be due to the lack of a standardized method of detection and to the diversity in the clinical courses of patient groups. There have been few serum studies of p16INK4A methylation in HCC patients. Wong et al. (20) reported abnormal p16INK4A methylation in 60% of sera and in 73% of tissue in HCC patients. In the present study, abnormal p16INK4A methylation was detected in 48% of the sera of HCC patients. Further studies on plasma or sera are required; large variations in incidence may be expected due to differences in sample materials, detection methods, and/or subjects selected.
Wong et al. (20) reported that p16INK4A methylation was not detected in the plasma of patients with either liver cirrhosis or hepatitis, whereas in the present study it was detected in the sera of 17.4% of cirrhosis patients. We infer that abnormal p16INK4A methylation can also be detected in the sera of patients with cirrhosis because it is frequently found in non-tumorous tissues (8). AFP and albumin mRNA were also detected in the sera of hepatitis and cirrhosis patients, as well as in those of HCC patients (25). More meticulous methods may be required to detect p16INK4A methylation in cirrhosis patients, as the amount of circulating DNA is lower in the sera of cirrhosis patients than in that of HCC patients. The incidence of methylated p16INK4A DNA did not differ between small (≤3 cm) and large HCCs, and was not correlated with AFP levels in the sera of HCC patients. Wong et al. (26) reported a significant correlation between the methylation of circulating DNA and serum AFP levels in HCC patients. There is a danger in generalizing from this result, however, as that study included only six cases of HCC without methylation.
Our study suggested that methylated p16INK4A DNA may play an important role as a tumor marker in detection of HCC. The state of serum p16INK4A methylation discriminated HCC from cirrhosis with a sensitivity of 47.8% and a specificity of 82.6%. As there is no correlation between serum methylation and AFP, it may be a useful complementary tool to the AFP test.
References
- 1.Korea National Statistical Office. Korea Statistical Yearbook. Daejeon: 2002. [Google Scholar]
- 2.Shiina S, Tagawa K, Unuma T, Terano A. Percutaneous ethanol injection therapy for treatment of hepatocellular carcinoma. Am J Reontgenol. 1990;154:947–951. doi: 10.2214/ajr.154.5.2157329. [DOI] [PubMed] [Google Scholar]
- 3.Okuda S. Local ablation therapy for hepatocellular carcinoma. Semin Liver Dis. 1999;19:323–328. doi: 10.1055/s-2007-1007121. [DOI] [PubMed] [Google Scholar]
- 4.Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi CG, Lai CL. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology. 2000;31:330–335. doi: 10.1002/hep.510310211. [DOI] [PubMed] [Google Scholar]
- 5.Nagasue N, Uchida M, Makino Y, Takemoto Y, Yamanoi A, Hayashi T, Chang YC, Kohno H, Nakamura T, Yukaya H. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology. 1993;105:488–494. doi: 10.1016/0016-5085(93)90724-q. [DOI] [PubMed] [Google Scholar]
- 6.Shimada M, Takenaka K, Gion T, Fujiwara Y, Kajiyama K, Maeda T, Shirabe K, Nishizaki T, Yanaga K, Sugimachi K. Prognosis of recurrent hepatocellular carcinoma: a 10-year surgical experience in Japan. Gastroenterology. 1996;111:720–726. doi: 10.1053/gast.1996.v111.pm8780578. [DOI] [PubMed] [Google Scholar]
- 7.Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutation in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855–4878. [PubMed] [Google Scholar]
- 8.Kondo Y, Kanai Y, Sakamoto M, Mizokami M, Ueda R, Hirohashi S. Genetic instability and aberrant DNA methylation in chronic hepatitis and cirrhosis: A comprehensive study of loss of heterozygosity and microsatellite instability at 39 loci and DNA hypermethylation on 8 CpG islands in microdissected specimens from patients with hepatocellular carcinoma. Hepatology. 2000;32:970–979. doi: 10.1053/jhep.2000.19797. [DOI] [PubMed] [Google Scholar]
- 9.Chaubert P, Gayer R, Zimmermann A, Fontolliet C, Stamm B, Bosman F, Shaw P. Germ-line mutations of the p16INK4 (MTS1) gene occur in a subset of patients with hepatocellular carcinoma. Hepatology. 1997;25:1376–1381. doi: 10.1002/hep.510250613. [DOI] [PubMed] [Google Scholar]
- 10.Merlo A, Herman JG, Mao L, Lee DJ, Gabrielson E, Burger PC, Baylin SB, Sidransky D. 5' CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med. 1995;1:686–692. doi: 10.1038/nm0795-686. [DOI] [PubMed] [Google Scholar]
- 11.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 12.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 13.Tannapfel A, Wittekind C. Genes involved in hepatocellular carcinoma: deregulation in cell cycling and apoptosis. Virchows Arch. 2002;440:345–352. doi: 10.1007/s00428-002-0617-x. [DOI] [PubMed] [Google Scholar]
- 14.Razin A. CpG methylation, chromatin structure and gene silencing: a three-way connection. EMBO J. 1998;17:4905–4907. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JR, Kim SY, Kim MJ, Kim JH. Alterations of CDKN2 (MTS1/p16INK4A) gene in paraffin-embedded tumor tissues of human stomach, lung, cervix and liver cancers. Exp Mol Med. 1998;30:109–114. doi: 10.1038/emm.1998.16. [DOI] [PubMed] [Google Scholar]
- 16.Kita R, Nishida N, Fukuda Y, Azechi H, Matsuoka Y, Komeda T, Sando T, Nakao K, Ishizaki K. Infrequent alterations of the p16INK4A gene in liver cancers. Int J Cancer. 1996;67:176–180. doi: 10.1002/(SICI)1097-0215(19960717)67:2<176::AID-IJC4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 17.Lin YW, Chen CH, Huang GT, Lee PH, Wang JT, Chen DS, Lu FJ, Sheu JC. Infrequent mutations and no methylation of CDKN2A (p16/MTS1) and CDKN2B(p15/MTS2) in hepatocellular carcinoma in Taiwan. Eur J Cancer. 1998;34:1789–1795. doi: 10.1016/s0959-8049(98)00189-0. [DOI] [PubMed] [Google Scholar]
- 18.Chaubert P, Gayer R, Zimmermann A, Fontolliet C, Stamm B, Bosman F, Shaw P. Germ-line mutations of the p16INK4 (MTS1) gene occur in a subset of patients with hepatocellular carcinoma. Hepatology. 1997;25:1376–1381. doi: 10.1002/hep.510250613. [DOI] [PubMed] [Google Scholar]
- 19.Liew CT, Li HM, Lo KW, Leow CK, Chan JY, Hin LY, Lau WY, Lai PB, Lim BK, Huang J, Leung WT, Wu S, Lee JC. High frequency of p16INKA4 gene alterations in hepatocellular carcinoma. Oncogene. 1999;18:789–795. doi: 10.1038/sj.onc.1202359. [DOI] [PubMed] [Google Scholar]
- 20.Wong IH, Lo YM, Zhang J, Liew CT, Ng MH, Wong N, Lai PB, Lau WY, Hjelm NM, Johnson PJ. Detection of aberrant p16 methylation in the plasma and serum of liver cancers patients. Cancer Res. 1999;59:71–73. [PubMed] [Google Scholar]
- 21.Roncalli M, Bianchi P, Bruni B, Laghi L, Destro A, Di Gioia S, Gennari L, Tommasini M, Malesci A, Coggi G. Methylation framework of cell cycle gene inhibitors in cirrhosis and associated hepatocellular carcinoma. Hepatology. 2002;36:427–432. doi: 10.1053/jhep.2002.34852. [DOI] [PubMed] [Google Scholar]
- 22.Kaneto H, Sasaki S, Yamamoto H, Itoh F, Toyota M, Suzuki H, Ozeki I, Iwata N, Ohmura T, Satoh T, Karino Y, Satoh T, Toyota J, Satoh M, Endo T, Omata M, Imai K. Detection of hypermethylation of the p16(INK4A) gene promoter in chronic hepatitis and cirrhosis associated with hepatitis B or C virus. Gut. 2001;48:372–377. doi: 10.1136/gut.48.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodes J EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 24.Herman JG, Gaff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong IH, Lau WY, Leung T, Johnson PJ. Quantitative comparison of alpha-fetoprotein and albumin mRNA levels in hepatocellular carcinoma/adenoma, non-tumor liver and blood: implications in cancer detection and monitoring. Cancer Lett. 2000;156:141–149. doi: 10.1016/s0304-3835(00)00473-0. [DOI] [PubMed] [Google Scholar]
- 26.Wong IH, Lo YM, Lai PB, Johnson PJ. Relationship of p16 methylation status and serum alpha-fetoprotein concentration in hepatocellular carcinoma patients. Clin Chem. 2000;46:1420–1422. [PubMed] [Google Scholar]