Abstract
Three-dimensional (3D) type I collagen gels are increasingly utilized to simulate extracellular matrix (ECM) in vivo, but little is known about the effects of age on this model. Collagen was extracted from young (4–6 months) and aged (20–24 months) mice tails and compared. The collagens appeared similar by electrophoresis. However, relative to young, aged collagen formed fibrils slower and generated 3D gels with smaller diameter, less dense fibrils (75 vs 34 nm diameter and 8 vs 3.5% area, for young and aged respectively, p < 0.02). Correspondingly, aged collagen gels were more malleable and contractible (5% vs 19% compression, p < .02, and 73% vs 15.5% area, p < .01, for young and aged, respectively). Fibroblasts cultured within young and aged collagen gels had differential expression of a limited number of genes and proteins corresponding to specific integrins and matrix components. In summary, collagen extracted from young and aged mice is an effective means to examine the influence of aging on functional properties of ECM that are relevant in vivo.
Keywords: Aged, Three-dimensional collagen, Electron microscopy, Fibroblasts, Gene expression
THERE is a great deal of interest in the effect of age on the tissue microenvironment. Most studies have focused either on the physiology and gene expression of aging cells or on tissue-associated cytokines and growth factors that regulate cell behavior (1–4). Considerably less attention has been paid to the possible effects of age on the properties of structural extracellular matrix (ECM) proteins (such as type I collagen) that surround the cells. Studies that have examined type I collagen in aging have described its expression and content in various tissues and organs; however, little is known about whether the collagen within aged tissues might exhibit, itself, an altered capacity to assemble and respond to cellular activity.
Type I collagen (hereafter referred to as “collagen”) is a rod-shaped heterotrimer composed of two alpha-1 chains and one alpha-2 chain. Collagen is the major structural ECM component in most tissues, as a consequence of its capacity to assemble (polymerize) side by side and end to end to form strong fibers (5). There is general agreement that aging is associated with a progressive decrease in collagen content in most organs (6–8), leading to diminished tissue integrity and strength, especially under stress. However, there are important exceptions to this premise, such as the increased collagen deposition that is often noted in aged hearts as a result of age-related and disease-associated (ie, hypertension) factors (9). Studies examining mechanisms of decreased collagen expression in aged tissues have noted that lower levels of fibrogenic growth factors, such as transforming growth factor-beta 1 (TGF-beta 1) (10), contribute to reductions in both collagen synthesis and subsequent scarring, particularly in the dermis, an organ that is composed largely of collagen I. In addition, impaired mechanical stimulation due to defective cell–matrix contacts might also lessen collagen production by aged cells (11). At the same time, elevated matrix metalloproteinase (MMP) activity mediates heightened degradation of collagen (12,13). It is debated whether age-related collagen breakdown results from an increase in MMP activity, decreases in MMP inhibition by tissue inhibitors of MMPs (TIMPs), or changes in associated proteins that modulate collagen stability (14,15). Moreover, the relative contribution of age and environmental insults, such as actinic damage, varies with the tissue and the host (14,15). Irrespective of the mechanisms, the end result is a looser less organized collagen network. Functional consequences associated with age-related reductions and/or physical alterations in collagen include deficits in integrin binding that contribute to slowed cell adhesion and migration (15,16).
There is increasing interest in studying cell behaviors in environments that surround the cells with an ECM similar to that found in vivo. The ECM of most supporting tissues is composed primarily of collagen. In this context, the most popular model involves cell culture within three-dimensional (3D), native fibrillar collagen hydrogels (“gels”), which are considered to be effective simulators of collagen-rich interstitial connective tissue. Indeed, in PubMed, there are currently more than 700 references that have “3D collagen” in their title or abstract. Three-dimensional collagen gel culture models have been applied to studies of aging but have focused on cells rather than the collagen, that is, comparing the behaviors of young cells with aged cells, with the collagen gel being invariant (17–19).
Studies that have examined aged ECM have utilized middle-aged rats (8–16 months) to document differences between young and aging tissues (20,21). Notably, the aged mouse (>20 months of age) provides a reproducible model of aging that can be analyzed in the context of genetic modifications (22) and is highly relevant to tissue repair and tumorigenesis (16,23–25). For the earlier reasons, in the present study, we extracted collagen from young and aged mice to specifically examine age effects on 3D collagen gels. With this model, we found significant differences with respect to fibril assembly and malleability to both external and cellular forces and to gene and protein expression by resident fibroblasts.
EXPERIMENTAL PROCEDURES
Animals
Young adult (4–6 months) and aged (20–24 months) C57Bl/6 male mice were obtained from the National Institute on Aging (NIA) Rodent Colony at Harlan Sprague Dawley (Chicago, IL) and maintained under specific pathogen-free conditions at the Harborview Medical Center Research and Training Vivarium at the University of Washington. The University of Washington Animal Welfare Committee approved all animal procedures.
Preparation of Collagen Extracts
Preliminary experiments were performed on tail tendons derived from three to five young and three to five aged euthanized mice. Final experiments (shown here) were performed on isolated tail tendons from 10 young and 10 aged euthanized mice that were processed in an identical fashion. Care was taken to have equivalent amounts of tendons from each mouse that were then pooled into young and aged groups, soaked briefly in phosphate-buffered saline, rinsed 5 minutes each in acetone and 70% isopropanol, macerated, and stirred overnight in 0.05 N acetic acid at 4°C to extract the collagen. Subsequently, the “young” and “aged” collagen extracts were centrifuged for 15 minutes at 4,000g to remove undissolved material. Total protein content was quantified by the BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). Collagen content of the extracts was quantified by the collagen-specific Sircol Assay (Accurate Chemical and Scientific Corp., Westbury, NY). All biochemical studies were performed on young and aged samples that represented equivalent collagen concentration and then repeated using equivalent total protein content.
Gel Electrophoresis and Immunoblotting
Similar amounts of total protein from young and aged collagen extracts were denatured and resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) using a 5% acrylamide separating gel and a 5% acrylamide resolving gel. After electrophoresis, the gels were stained with Imperial protein stain, a Coomassie R-250 dye–based reagent (Pierce, Rockford, IL). Another set of blots with nondenatured samples were transferred to nitrocellulose. All blots were blocked in Tris-buffered saline (TBS) with 5% nonfat dried milk/0.1% Tween-20 and then exposed overnight at 4°C to rabbit polyclonal antibodies to human collagen type III and collagen type I (Rockland, Gilbertsville, PA), each diluted at 1:5,000 in TBS. Bound antibodies were visualized with a horseradish peroxidase–conjugated antibody to rabbit IgG (Chemicon International, Temecula, CA) in conjunction with a chemiluminescence kit (Amersham Biosciences, Piscataway, NJ).
Collagen Polymerization Assay
Young and aged collagen extracts were diluted to a concentration of 0.6 mg/mL collagen in ice-cold solution of 50 mM (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 1.8 mM CaCl2, 0.81 mM MgSO4, 100 mM NaCl, and 5 mM KCl, pH 7.0. The cold extracts were added in 250 μL volumes (in triplicate) to wells of a flat-bottomed 96-well assay plate (Corning/Costar Corp., Cambridge, MA). The plate was inserted into a plate reader (OPTImax; Molecular Devices, Sunnyvale, CA) with the reading chamber electrically preheated to 37°C. Recordings were done with the absorption set to 410 nm in 30-second intervals for 30 minutes using SoftMax Pro software and repeated in three separate experiments.
Measurements of Collagen Fibril Diameter and Density in Young and Aged Collagen Gels
Young and aged collagen extracts were polymerized as described previously for malleability assays. Subsequently, the gels were dehydrated in a graded series of alcohols, then immersed 5 minutes each in 1:1 hexamethyldisilazane (HMDS):100% alcohol, 2:1 HMDS:100% alcohol, and undiluted HMDS, and the samples were embedded in Araldite. Blocks were sectioned with a diamond knife, applied to formvar-coated copper grids, and stained with uranyl acetate and lead citrate. Stained sections were viewed by transmission electron microscopy (TEM) with a 1200EXII (JEOL, Peabody, MA) at 80 kV, and images were recorded on film at a magnification of ×15,000. Film images (negatives) from two separate polymerization experiments (representing two young and two aged collagen gels) were digitally scanned, and the density and diameter of collagen fibrils were measured using Adobe Photoshop (26). Forty images from each gel and 250–400 fibrils from each image were quantified for diameter. Diameters were measured only from en face views of banded fibril segments with lengths exceeding 370 nm. The diameter of each fibril segment was measured at the center and at either ends, and the three values were averaged. To measure fibril density, the digital images were processed with a threshold filter that rendered fibrils and background as sets of white and black pixels, respectively. Density was calculated by taking the number of white pixels over the total number of pixels in randomly selected standardized fields. Fibril diameter and density measurements were expressed as mean ± standard deviation.
Assay of Malleability of Young and Aged Collagen Gels
Malleability assays were based on the centrifugation method of Nishiyama and colleagues (27). Gels were prepared from young and aged collagen extracts by combining 1 volume of extract, 1/9 volume of 10-strength NaHCO3-saturated Medium 199 (Invitrogen Corp., Grand Island, NY) and sufficient Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen), and fetal bovine serum (FBS) to yield a gel with final collagen and FBS concentrations of 0.6 mg/mL and 1%, respectively. The ice-cold mixtures were dispensed in 2 mL volumes into small, straight-walled cylindrical polypropylene centrifuge tubes, incubated for 1 hour at 37°C to polymerize the collagen, and then centrifuged at 375g for 10 minutes in a swinging bucket rotor. The volume of medium lying above the compressed gel was then measured (by weighing the fluid) and subtracted from the gel volume prior to centrifugation to yield the volume of the compressed gel in three separate experiments.
Fibroblast Cell Culture
Dermal fibroblasts from a young human donor (a 30-year-old male, AG13153) were obtained from the Aging Cell Repository, NIA, at the Coriell Institute (Camden, New Jersey). Human fibroblasts were used as they are well characterized and maintain a stable phenotype in culture. The cells were maintained in DMEM with 10% FBS, penicillin (50 U/mL; Invitrogen), streptomycin (50 μg/mL; Invitrogen), and amphotericin (2.5 μg/mL; Sigma, St. Louis, MO). Cells from confluent early-passage cultures were trypsinized and resuspended in DMEM with 1% FBS for use in collagen gel contraction assays (described later).
Assay of Cell-Mediated Collagen Gel Contraction
Young and aged collagen extracts were combined with culture medium and FBS, as described for malleability assays, and supplemented with human fibroblasts at 1.5 × 105 cells. To discount any effect due to total protein content, assays were performed with equivalent concentrations of collagen as determined by the Sircol Assay and then repeated using equivalent total protein as determined by BCA Protein Assay. Four hundred microliters of the collagen/cell suspension was then dispensed in triplicate into oil-supported gel contraction supports (assay (28)). The gels were polymerized at 37°C, incubated for 18 hours to allow cell-mediated contraction, and then fixed in 10% neutral buffered formalin. Diameters of the fixed gels from two separate experiments were measured from digital images using Adobe Photoshop.
Reverse Transcription-Polymerase Chain Reaction (RT- PCR) Arrays
Human fibroblasts (1.25 × 105 cells) were dispersed in 500 μL of 3D gels made from young and aged collagen (0.6 mg/mL collagen/1%FBS). The gels were cast in six-well plastic tissue culture plates and were not separated from the sides and bottom of the wells, which prevented gel contraction during culture. After 18 hours of culture, messenger RNA (mRNA) was isolated using an RNeasy Plus Mini-Kit (Qiagen, Valencia, CA) and complementary DNA was made using an RT2 First Strand Kit (SABiosciences, Frederick, MD). Reverse transcription-polymerase chain reaction (RT-PCR) using the Human Extracellular Matrix and Adhesion Molecules Array (84 relevant genes, 5 housekeeping genes, and 7 control genes) was performed as per protocol from SABiosciences. Two separate experiments were analyzed on different dates for each array. Fold differences were calculated using polymerase chain reaction (PCR) Array Data Analysis Web Portal (SABiosciences). Genes that were either upregulated or downregulated by more than 40% in both experiments were considered significant based on prior studies describing the effects of age on protein secretion and cellular functions (25,29).
Western Blot and Zymographic Analyses
Western blotting of collagen digests was performed as noted previously. For Western blot analyses of fibroblasts and their conditioned media, human fibroblasts were cultured in duplicate for 18 hours in young or aged collagen gels. For analyses of integrins, the 18-hour gel/cell cultures were lysed in a solution of 50 mM Tris, pH 6.8/2% sodium dodecyl sulfate/5% glycerol/1% 2-mercaptoethanol/5 mM EDTA, supplemented with a protease inhibitor cocktail (Sigma). For analyses of secreted proteins (MMP-1 and secreted protein acidic and rich in cysteine [SPARC]), the gel/cell cultures were overlain with serum-free DMEM, which was collected after the 18-hour culture period. The collected cell lysates (integrins) or conditioned culture media (SPARC and MMPs) were resolved by SDS-PAGE under reducing conditions and blotted to nitrocellulose. All blots were blocked in TBS with 5% nonfat dried milk/0.1% Tween-20 and then exposed overnight at 4°C to rabbit polyclonal antibodies to human alpha-2 integrin (Chemicon), human MMP-1 (Chemicon), and human SPARC (a kind gift from Dr. E. Helene Sage, University of Washington), each diluted 1:500 in TBS. Bound antibodies were visualized with a horseradish peroxidase–conjugated antibody to rabbit IgG (Chemicon) in conjunction with a chemiluminescence kit (Amersham Biosciences).
For zymography, conditioned serum-free media from the 18-hour cultures were resolved by SDS-PAGE gels that contained 1 mg/mL of gelatin (Sigma). Subsequently, the gels were washed in 2.5% Triton X-100 for 15 minutes, incubated overnight at 37°C in 50 mM Tris, pH 7.5/5 mM CaCl2, and stained with Coomassie Brilliant Blue. MMPs with gelatinolytic activity appeared as lucent bands.
Statistics
Data represent collagen from 10 young or 10 aged mice that were pooled in equivalent amounts. Each analysis was repeated in two to three separate experiments as noted previously under specific methods. Differences between groups were determined according to a two-tailed Student’s t test with unequal variance and were considered significant when p < .05.
RESULTS
Electrophoresis and Immunoblots of Young and Aged Collagen Extracts
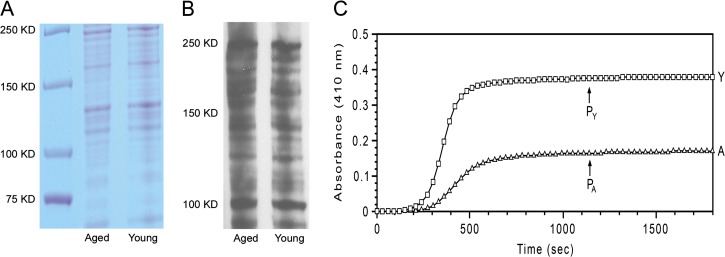
Collagen derived from young mice tails averaged an initial concentration of 1.83 mg/mL and that derived from aged mice tails averaged 1.24 mg/mL. Equivalent amounts of protein from young and aged collagen extracts resolved by polyacrylamide gel electrophoresis under denaturing conditions exhibited an essentially identical pattern of bands representing a mixture of collagen: monomeric, dimeric, and trimeric polypeptide chains (Figure 1A and B). All these bands were eliminated by exposure to collagenase (data not shown), indicating that collagen was predominant in the extracts. Moreover, bands representing collagens I and III were similar in both quality and quantity in young and aged collagen extracts. Further evaluation using Western blots demonstrated that there were no detectable laminins, fibronectins, MMP-2, MMP-9, TIMP-1, and TIMP-2 (data not shown) in the extracts from young and aged collagen.
Figure 1.
Gel profiles and polymerization of young and aged collagen extracts. Collagen extracts from young (Y) and aged (A) mice have similar band patterns, as visualized by Coomassie blue stain (panel A). Immunoblot using an antibody against collagen I (panel B) showed no significant differences in quality or quantity of the bands. Subsequent polymerization assay demonstrated that native collagen extracted from young mice (Y—squares) initiates fibril formation earlier and at a greater rate than corresponding collagen extracted from aged mice (A—triangles). The absorbance plateau (PY) of young mouse collagen is substantially higher than that of aged mouse collagen (PA), indicating a more effective assembly of collagen molecules into fibrils (panel C).
We then wished to examine features of the young and aged collagen that would induce changes in cell behavior. Isolated, native collagen molecules exposed to physiological ionic strength, pH, and temperature spontaneously polymerize into long banded fibrils that resemble collagen fibrils in vivo (26). We utilized this model to compare collagen extracts (0.6 mg/mL) from young versus aged mice (Figure 1C). Both young and aged collagen extracts exhibited typical polymerization curves—after an initial lag, there was a steep sigmoidal rise in optical density (OD) followed by a slow linear rise (plateau). The sigmoidal portion of the curve has been shown to correspond to the assembly of individual collagen molecules into fine fibrils, whereas the linear portion of the curve corresponds to the subsequent aggregation of the fibrils into larger bundles (30). Notably, the young collagen extracts initiated polymerization earlier than the aged collagen extracts. Moreover, the rate of the young collagen was three times greater than that of the aged collagen. The OD of the plateau portion of the curve reflects the fraction of gel volume occupied by fibrils (fibril density) as well as fibril thickness. The OD at the plateau of the young collagen was more than twice that of the aged collagen, indicating that the young collagen had a substantially greater extent of fibril formation than the aged collagen.
TEM Analyses of 3D Gels Made From Young and Aged Collagen
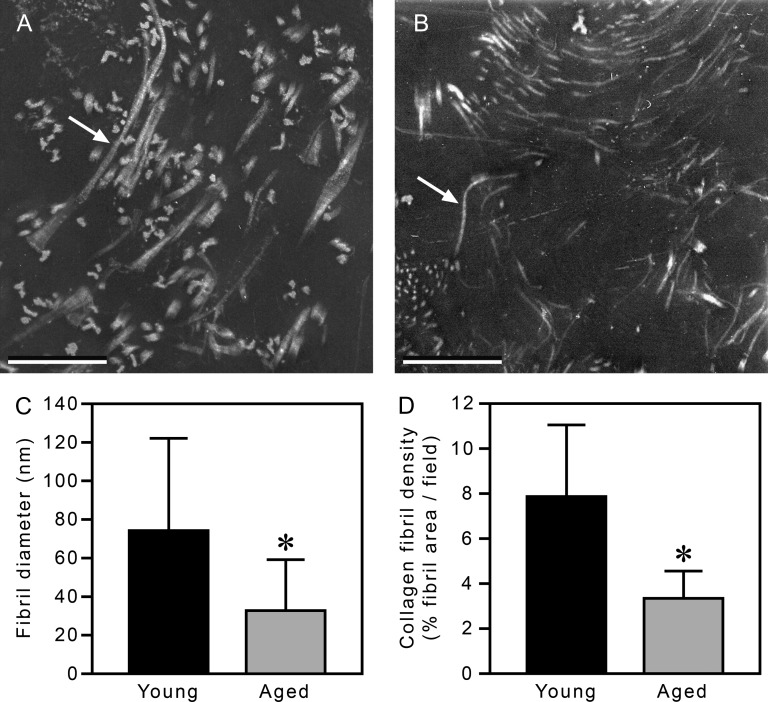
Our data suggested that collagen extracts from young mice formed fibrils more effectively than corresponding extracts from aged mice. To investigate the structural consequences of such a difference, we examined fully polymerized 3D collagen gels by TEM to directly assess fibril diameter and density (Figure 2). Gels made from young and aged collagen exhibited fibrils with 64 nm periodicity, typical of collagen gels polymerized in vitro (30) and collagen fibrils in vivo (31). The fibrils formed by young collagen extracts were substantially thicker than the fibrils formed by aged collagen extracts (Figure 2A and B). Quantification of the TEM images showed that the young collagen fibrils had an average diameter of approximately 75 nm (Figure 2C), a value within the range found in 3D gels made from rat tail tendon collagen (30,32). In contrast, the aged collagen fibrils averaged less than half the diameter of the young fibrils (∼34 nm). The gels made from young and aged collagen also had substantial differences in fibril density; the fibrils formed from aged collagen formed a network that was 45% as dense as that formed from young collagen (Figure 2D). The observations by TEM of smaller fibril diameter and lower fibril density of the aged collagen gels versus the young collagen gels were in correspondence with the observed differences in OD of the plateau regions of the polymerization assays.
Figure 2.
Transmission electron microscopy analysis of three-dimensional (3D) gels made from young and aged collagen. There are more young collagen fibrils (panel A, an example is indicated by an arrow) with greater diameters than aged collagen fibrils (panel B, arrow). Quantification of the images confirmed that 3D gels made from young collagen have thicker fibrils (75 vs 34 nm, respectively, panel C) at a higher density (8% vs 3.5% area, respectively, panel D) than corresponding gels made from aged collagen. In A and B, bars = 500 nm. In C and D, data are shown as mean ± standard deviation; *p < .02.
Malleability of 3D Gels Made From Young and Aged Collagen
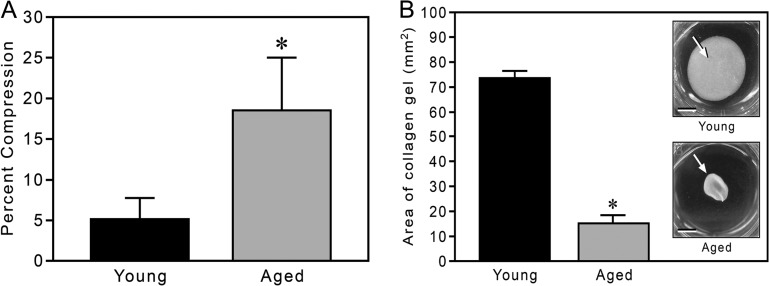
The TEM study of the 3D collagen gels was followed by functional assays of gel malleability, both to externally applied and to cell-derived forces. Initial measures were of the compressibility of similar 0.6 mg/mL gels under elevated gravity (Figure 3A). Under a load of 375g, gels made from young collagen were slightly compressed (an average of 5.2% reduction in volume), whereas those made from aged collagen were substantially more compressible, averaging an 18.6% reduction in volume.
Figure 3.
Malleability and gel contraction of three-dimensional gels made from young and aged collagen. Fully polymerized 0.6 mg/mL collagen gels were compressed by centrifugation for 10 minutes at 375g. Under elevated gravity, collagen from aged mice is more compressible than that from young mice (19% vs 5% compression, respectively). Data are shown as mean ± standard deviation; *p < .02 (panel A). Collagen gels (0.6 mg/mL) from young and aged mice were then populated with human dermal fibroblasts and cultured for 18 hours (panel B). The cells contracted the aged collagen significantly more than the young collagen (15.5% vs 73% area, respectively). Bar graph data are shown as mean ± standard deviation; *p < .01. Insets show young and aged collagen gels (arrows), respectively, after the contraction period (bars = 3 mm).
Cell-Mediated Remodeling of 3D Gels Made From Young and Aged Collagen
The physical properties of aged collagen (in the context of fibril formation, fibril diameter, fibril density, and gel malleability) pointed to an impaired capacity of aged collagen to form a 3D matrix, relative to young collagen. The consequence of such impairment to cell-mediated remodeling was examined with a 3D collagen gel contraction assay (28). Whether cells were placed in gels composed of equivalent amounts of total protein or total collagen, both young and aged (82-year-old male donor, AG04152, data not shown) human fibroblasts contracted 3D gels made from aged collagen to a significantly greater extent than corresponding gels made from young collagen; after 18 hours in vitro, fibroblasts contracted the aged collagen gels to a surface area that was as much as one fifth that of young collagen gels (Figure 3B).
Differential Expression of Genes by Fibroblasts Placed in Young and Aged 3D Collagen Gels
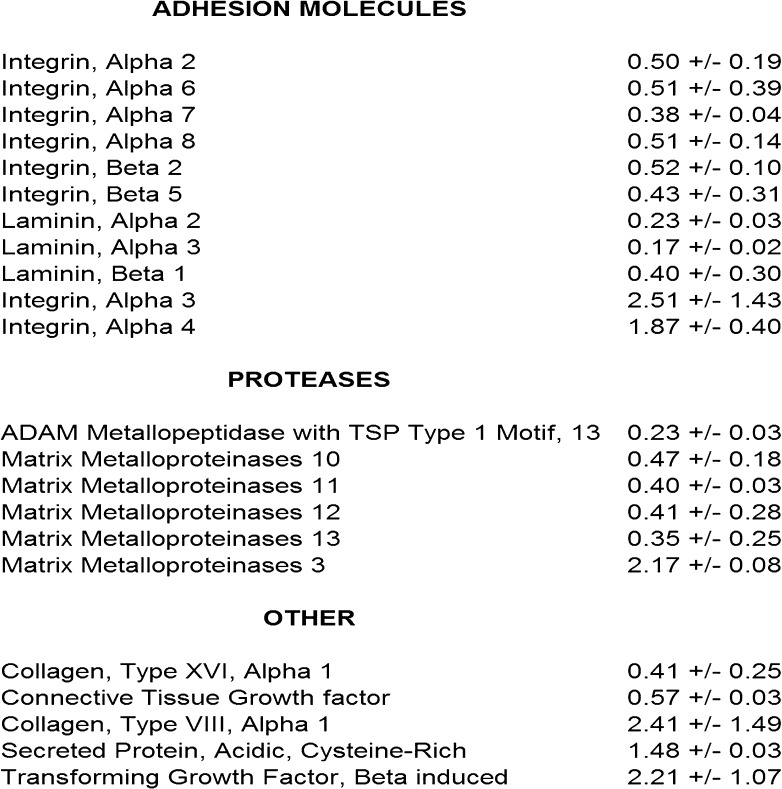
It is generally accepted that gene expression in cells can be significantly influenced by the composition of the ECM with which they associate (33,34). In the context of the significant age-related changes in ECM noted previously, the subsequent influence of young and aged collagen on relevant gene expression in fibroblasts from the young donor was evaluated. Fibroblasts were placed overnight in 3D gels made from young and aged collagen, and transcripts were measured using pathway RT-PCR arrays for 84 genes corresponding to relevant ECM and adhesion molecules (Figure 4). Genes that were upregulated or downregulated by more than 40% in fibroblasts cultured in aged relative to young collagen in two separate experiments were considered significant—a threshold selected based on prior data that aging confers biologically significant changes at that level (35,36). As expected, fibroblasts expressed many molecules (MMP-1 and MMP-2) to a similar extent in both young and aged collagen gels. However, relative to young collagen gels, integrin subunits alpha-3 and alpha-4 were upregulated in aged collagen gels, whereas integrin subunits alpha-2, alpha-6, alpha-7, alpha-8, beta-2, and beta-5 were downregulated in aged collagen gels. When cultured in aged collagen gels relative to young collagen gels, fibroblasts expressed increased levels of mRNA for SPARC, a secreted nonstructural (matricellular) ECM component highly expressed in inflammation and repair of aged tissues (37), and TGF-beta 1 (38), a multifunctional growth factor associated with ECM turnover and remodeling. Relative to fibroblasts cultured in young collagen gels, fibroblasts cultured in aged collagen gels upregulated mRNA for MMP-3 (a broad-spectrum MMP) but downregulated mRNAs for MMP-10, MMP-11, MMP-12, MMP-13, and a disintegrin and metalloproteinase with thrombospondin type I motif–13 (ADAMTS-13).
Figure 4.
Differential expression of genes representing extracellular matrix proteins and adhesion molecules in three-dimensional gels made from young and aged collagen. Shown are genes that differ by at least 40% in two separate experiments when fibroblasts are placed overnight in young versus aged collagen under identical conditions. Note that genes for ITGA2, ITGA6, ITGA7, ITGA8, ITGB2, ITGB5, MMP-10, MMP-11, MMP-12, MMP-13, and ADAMTS-13 are increased in young collagen and ITGA3, ITGA4, SPARC, TGF-β1, and MMP-3 are increased in aged collagen. ITGA = integrin alpha; ITGB = integrin beta; MMP = matrix metalloproteinase; SPARC = secreted protein acidic and rich in cysteine; TGF-β1 = transforming growth factor-beta 1.
RT-PCR Confirmation by Western Blotting and Zymography
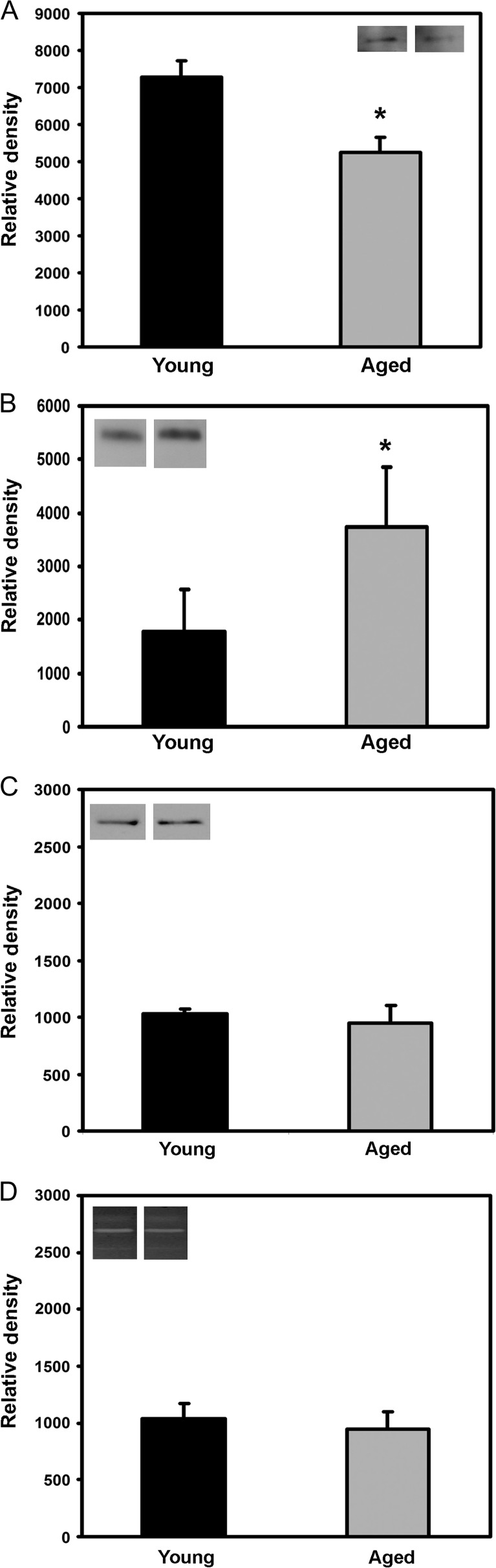
A subset of the ECM components, MMPs, and integrins assayed by the PCR arrays were validated by Western blot and zymography (Figure 5). The subset was selected based on relevance to collagen I and aging (3,16,22,39). In correspondence with the array data, Western blots of cell lysates and conditioned media demonstrated a decrease in levels of integrin alpha-2 (Figure 5A), an increase in SPARC (Figure 5B), and unaltered MMP-1 expression (Figure 5C) in fibroblasts cultured in aged versus young collagen gels. Zymography confirmed that fibroblasts expressed both pro- and active MMP-2 at similar levels (Figure 5D) and no detectable MMP-9 activity after culture in both young and aged collagen gels.
Figure 5.
Expression of proteins by fibroblasts placed in three-dimensional gels made from young and aged collagen. Histograms represent Western blots and zymographic activity of selected proteins that show increased (integrin alpha-2, panel A), decreased (secreted protein acidic and rich in cysteine, panel B), and similar (MMP-1 [panel C] and MMP-2 [panel D]) expression by fibroblasts placed in young versus aged collagen, respectively. Data are shown as mean ± standard deviation; *p < .05. MMP = matrix metalloproteinase.
DISCUSSION
There is increasing appreciation of the time and cost efficiencies provided by initial use of 3D models to simulate the ECM environment present in native tissues in vivo. Although subsequent validation in vivo is critical (28,32,40,41), these systems have been used to effectively study cell attachment, spreading, and migration as well as more complex morphogenetic behaviors elicited from isolated cell populations and tissue explants (42). Despite the limitations of 3D models, it is generally accepted that they are particularly useful in studies of aging due to the numerous constraints (availability, cost, and variability) in the use of aged hosts (43). We and others have shown that there are significant age-associated differences in the behavior of cells and tissue explants in 3D collagen gels, which include diminished capacities for migration, collagen contraction, expression of MMPs, and morphogenesis by aged cells and tissues relative to their young counterparts, in a manner and quantity similar to complementary in vivo studies (17–19,29).
In order to better understand age effects on 3D collagen itself, we examined collagen from young and aged mice and its subsequent effects on function and gene/protein expression in resident cells. We observed that collagen extracted from aged mice had similar appearance to that of young mice with respect to expression of collagens I and III. Moreover, associated matrix proteins, for example, fibronectin and laminin, were not detectable by traditional immunostaining methods. We then shifted analyses to features that would directly affect cellular functions, such as polymerization and malleability. Collagen from aged mice formed fibrils less effectively than collagen extracts from young mice (as indicated in vitro by a longer time to initiate fibril formation, a slower rate of polymerization, and a lower OD after fibril formation). Such deficiencies corresponded to the formation of aged 3D collagen gels composed of a looser network of thinner collagen fibrils (as revealed by TEM) than was found in young gels formed under similar conditions. The appearance of the aged collagen gels by electron microscopy correlates with analyses of middle-aged tissues and organs in vivo (20,21) that have shown decreased fibril size and density with aging.
Collagen fibril diameter has been shown to correlate directly with tensile strength of collagen polymerized in vitro (40). As expected, the aged collagen gels, with their thinner fibrils (and less dense network), were more malleable than the young gels, exhibiting increased compressibility under elevated gravity. In keeping with these data, aged gels were significantly more contractible by resident fibroblasts than the young gels. Collagen gel contraction assays measure the ability of cells to adhere to and pull against surrounding collagen fibrils (44). In such assays, gels composed of relatively loose collagen networks are typically contracted by cells to a greater extent than denser gels (45).
The polymerization, fibril organization, and malleability characteristics of the aged collagen extracts in vitro are in accordance with long-standing observations that collagen in aged tissues and organs is less dense and more disorganized (7,8). It is also thought that the reduced strength of cross-linked collagen from aged tissues, relative to young tissues, reflects both decreased fibril diameter and density. These changes with age contribute to age-associated changes in the mechanical properties of skin and deficiencies in wound repair (11,12,15).
It is hoped that 3D models can elucidate mechanisms that are relevant in vivo (45). Accordingly, the underlying reasons that aged collagen forms fibrils less effectively than corresponding extracts from young tissue is the subject of ongoing analyses in our laboratory. Collagen polymerization is inhibited by denaturation or proteolysis of the native collagen molecule. Therefore, deficits in aged collagen in vitro may point to the accumulation of structural defects at the level of individual collagen molecules in vivo. Such defects could be caused by aberrant synthesis or assembly of the collagen heterotrimer by aging fibroblasts and/or by damage to intact collagen molecules by proteolytic enzymes. The latter accumulate as collagen fibrils age within tissues, but their activity is not readily detectable in subsequent extracts. Examination by Western blotting did not demonstrate significant differences in collagens I or III or detectable levels of other common ECM molecules. However, other minor components of the ECM, as well as posttranslational modifications of the collagen chains, are still under evaluation.
Diminished collagen content and density in most aged tissues (the cardiovascular system is a notable exception) (13,47) have significant clinical implications beyond tissue repair. Indeed, even during wound healing, it is notable that the decrease in collagen synthesis that contributes to delays in healing also results in less scarring and fibrosis (10). Decreased or disordered collagen may influence tumor growth in aged tissues; however, the effects of such alterations are largely a matter of conjecture and may depend on the tumor cell type. For example, we found robust tumor angiogenesis and growth of prostate tumors that had a highly collagenous matrix in aged mice (23). One could surmise that a looser collagen network in the vicinity of tumors would offer decreased support for cell migration and subsequent tumor progression (47). Conversely, a looser ECM might facilitate tumor growth and angiogenesis (24).
Certain genes and their protein products are known to be upregulated by cells cultured in contact with polymerized collagen gels (33,48–51) relative to rigid (eg, plastic) substrata. In the present study, we show that age-associated differences in collagen itself can influence gene expression; fibroblasts cultured in aged versus young collagen gels exhibited key differences in the expression of many genes representing ECM components, MMPs, and integrin subunits. As an example, the expression of genes for TGF-beta 1 and SPARC was significantly increased by fibroblasts cultured in aged collagen gels relative to young collagen gels. TGF-beta 1 modulates integrin (52) and MMP activity, thereby acting as a profibrotic growth factor (53). Moreover, TGF-beta 1 stimulates collagen synthesis, as well as gel contraction by aged fibroblasts in vitro, and enhances wound repair in aged rats (35,54). SPARC is highly expressed by aged and activated tissues and is associated with increased production and maturation of collagen during tissue repair (40,55,56). In contrast, the expression of most integrin subunits was increased in young collagen relative to aged collagen. The positive feedback interactions among the expression of integrins, MMPs, and collagen are well described (57). It is probable that the increase in alpha-2 integrin in cells placed in young collagen reflects increased ECM engagement when cells are exposed to the denser ECM provided by young collagen gels.
Collectively, our array and protein data indicate that the unique 3D microenvironment provided by young relative to aged collagen in vitro influences the synthesis of some (but not all) molecules that regulate cell/ECM attachment (eg, integrins) and ECM repair and turnover (eg, SPARC) in a manner similar to that expected from studies in vivo (11,12,14,15,29). Of note, young cells were in contact with the young and aged collagen for only a brief period of time (∼18 hours). Accordingly, the effect of prolonged exposure to aged collagen on the phenotype of the young fibroblasts (and, conversely, the influence of young collagen on aged fibroblasts) was not examined. This type of investigation is an area of high gerontological relevance (58,59) and is presently being examined in our laboratory.
In summary, collagen extracted from aged mice differs markedly from that of young mice with respect to polymerization, structural and physical properties, and influence on gene and protein expression by resident fibroblasts. ECM components extracted from young and aged mice, used in combination with 3D gel models in vitro, may prove to be an effective means to examine the influence of aging (as well as the influence of experimentally induced genetic modifications) on functional properties of ECM that are relevant in vivo.
FUNDING
The study was funded by National Institutes of Health grants R01AG015837 (M.J.R.), R21AG024458 (M.J.R.), and U54CA126540 (P.S.N.).
Acknowledgments
The authors wish to acknowledge the laboratory of Dr. Stephen Plymate for technical assistance.
References
- 1.Begley LA, Kasina S, MacDonald J, Macoska JA. The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine. 2008;43(2):194–199. doi: 10.1016/j.cyto.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67(7):3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 4.Rivard A, Fabre J, Silver M, et al. Aged-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 5.Chung LW, Baseman A, Assikis V, Zhau HE. Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol. 2005;173:10–20. doi: 10.1097/01.ju.0000141582.15218.10. [DOI] [PubMed] [Google Scholar]
- 6.Johnson BD, Page RC, Narayanan AS, Pieters HP. Effects of donor age on protein and collagen synthesis in vitro by human diploid fibroblasts. Lab Invest. 1986;55:490–496. [PubMed] [Google Scholar]
- 7.Mays PK, McAnulty RJ, Campa JS, Laurent GJ. Age-related alterations in collagen and total protein metabolism determined in cultured rat dermal fibroblasts: age-related trends parallel those observed in rat skin in vivo. Int J Biochem Cell Biol. 1995;27:937–945. doi: 10.1016/1357-2725(95)00056-u. [DOI] [PubMed] [Google Scholar]
- 8.Shuster S, Black MM, McVitie E. The influence of age and sex on skin thickness, skin collagen and density. Br J Dermatol. 1975;93:639–643. doi: 10.1111/j.1365-2133.1975.tb05113.x. [DOI] [PubMed] [Google Scholar]
- 9.Gazoti Debessa CR, Mesiano Maifrino LB, Rodrigues de Souza R. Age related changes of the collagen network of the human heart. Mech Ageing Dev. 2001;122:1049–1058. doi: 10.1016/s0047-6374(01)00238-x. [DOI] [PubMed] [Google Scholar]
- 10.Ashcroft GS, Dodsworth J, van Boxtel E, et al. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat Med. 1997;3:1209–1215. doi: 10.1038/nm1197-1209. [DOI] [PubMed] [Google Scholar]
- 11.Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861–1868. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballas CB, Davidson JM. Delayed wound healing in aged rats is associated with increased collagen gel remodeling and contraction by skin fibroblasts, not with differences in apoptotic or myofibroblast cell populations. Wound Repair Regen. 2001;9:223–237. doi: 10.1046/j.1524-475x.2001.00223.x. [DOI] [PubMed] [Google Scholar]
- 13.West MD, Pereira-Smith OM, Smith JR. Replicative senescence of human skin fibroblasts correlates with a loss of regulation and overexpression of collagenase activity. Exp Cell Res. 1989;184:138–147. doi: 10.1016/0014-4827(89)90372-8. [DOI] [PubMed] [Google Scholar]
- 14.Hornebeck W. Down-regulation of tissue inhibitor of matrix metalloprotease-1 TIMP-1 in aged human skin contributes to matrix degradation and impaired cell growth and survival. Pathol Biol (Paris) 2003;51:569–573. doi: 10.1016/j.patbio.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144:666–672. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed MJ, Ferara NS, Vernon RB. Impaired migration, integrin function, and actin cytoskeletal organization in dermal fibroblasts from a subset of aged human donors. Mech Ageing Dev. 2001;122:1203–1220. doi: 10.1016/s0047-6374(01)00260-3. [DOI] [PubMed] [Google Scholar]
- 17.Arthur WT, Vernon RB, Sage EH, Reed MJ. Growth factors reverse the impaired sprouting of microvessels from aged mice. Microvasc Res. 1998;55:260–270. doi: 10.1006/mvre.1998.2078. [DOI] [PubMed] [Google Scholar]
- 18.Koike T, Vernon RB, Hamner MA, Sadoun E, Reed MJ. MT1-MMP, but not secreted MMPs, influences the migration of human microvascular endothelial cells in 3-dimensional collagen gels. J Cell Biochem. 2002;86:748–758. doi: 10.1002/jcb.10257. [DOI] [PubMed] [Google Scholar]
- 19.Zhu WH, Iurlaro M, MacIntyre A, Fogel E, Nicosia RF. The mouse aorta model: influence of genetic background and aging on bFGF- and VEGF-induced angiogenic sprouting. Angiogenesis. 2003;6:193–199. doi: 10.1023/B:AGEN.0000021397.18713.9c. [DOI] [PubMed] [Google Scholar]
- 20.Jiang ST, Liao KK, Liao MC, Tang MJ. Age effect of type I collagen on morphogenesis of Mardin-Darby canine kidney cells. Kidney Int. 2000;57:1539–1548. doi: 10.1046/j.1523-1755.2000.00998.x. [DOI] [PubMed] [Google Scholar]
- 21.Wu CC, Ding SJ, Wang YH, Tang MJ, Chang HC. Mechanical properties of collagen gels derived from rats of different ages. J Biomater Sci Polym Ed. 2005;16:1261–1275. doi: 10.1163/156856205774269494. [DOI] [PubMed] [Google Scholar]
- 22.Reed MJ, Bradshaw AD, Shaw M, et al. Enhanced angiogenesis characteristic of SPARC-null mice disappears with age. J Cell Physiol. 2005;204:800–807. doi: 10.1002/jcp.20348. [DOI] [PubMed] [Google Scholar]
- 23.Reed MJ, Karres N, Eyman D, Cruz A, Brekken RA, Plymate S. The effects of aging on tumor growth and angiogenesis are tumor-cell dependent. Int J Cancer. 2007;120:753–760. doi: 10.1002/ijc.22351. [DOI] [PubMed] [Google Scholar]
- 24.Sprenger CC, Plymate SR, Reed MJ. Extracellular influences on tumour angiogenesis in the aged host. Br J Cancer. 2008;98:250–255. doi: 10.1038/sj.bjc.6604144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest. 1999;79:1479–1487. [PubMed] [Google Scholar]
- 26.Sanders JE, Goldstein BS. Collagen fibril diameters increase and fibril densities decrease in skin subjected to repetitive compressive and shear stresses. J Biomech. 2001;34:1581–1587. doi: 10.1016/s0021-9290(01)00145-2. [DOI] [PubMed] [Google Scholar]
- 27.Nishiyama T, McDonough AM, Bruns RR, Burgeson RE. Type XII and XIV collagens mediate interactions between banded collagen fibers in vitro and may modulate extracellular matrix deformability. J Biol Chem. 1994;269:28193–28199. [PubMed] [Google Scholar]
- 28.Vernon RB, Gooden MD. New technologies in vitro for analysis of cell movement on or within collagen gels. Matrix Biol. 2002;21:661–669. doi: 10.1016/s0945-053x(02)00091-4. [DOI] [PubMed] [Google Scholar]
- 29.Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J Histochem Cytochem. 2003;51:1119–1130. doi: 10.1177/002215540305100902. [DOI] [PubMed] [Google Scholar]
- 30.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vernon RB, Gooden MD, Lara SL, Wight TN. Native fibrillar collagen membranes of micron-scale and submicron thicknesses for cell support and perfusion. Biomaterials. 2005;26:1109–1117. doi: 10.1016/j.biomaterials.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Haas TL, Davis SJ, Madri JA. Three-dimensional type I collagen lattices induce coordinate expression of matrix metalloproteinases MT1-MMP and MMP-2 in microvascular endothelial cells. J Biol Chem. 1998;273:3604–3610. doi: 10.1074/jbc.273.6.3604. [DOI] [PubMed] [Google Scholar]
- 34.Schonherr E, Schaefer L, O’Connell BC, Kresse H. Matrix metalloproteinase expression by endothelial cells in collagen lattices changes during co-culture with fibroblasts and upon induction of decorin expression. J Cell Physiol. 2001;187:37–47. doi: 10.1002/1097-4652(2001)9999:9999<::AID-JCP1048>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 35.Puolakkainen PA, Reed MJ, Gombotz WR, Twardzik DR, Abrass IB, Sage HE. Acceleration of wound healing in aged rats by topical application of transforming growth factor-beta1. Wound Repair Regen. 1995;3:330–339. doi: 10.1046/j.1524-475X.1995.t01-1-30314.x. [DOI] [PubMed] [Google Scholar]
- 36.Reed MJ, Corsa A, Pendergrass W, Penn P, Sage EH, Abrass IB. Neovascularization in aged mice: delayed angiogenesis is coincident with decreased levels of transforming growth factor beta1 and type I collagen. Am J Pathol. 1998;152:113–123. [PMC free article] [PubMed] [Google Scholar]
- 37.Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001;107:1049–1054. doi: 10.1172/JCI12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wells RG, Discher DE. Matrix elasticity, cytoskeletal tension, and TGF-beta: the insoluble and soluble meet. Sci Signal. 2008;1:13. doi: 10.1126/stke.110pe13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parrinello S, Coppe JP, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118:485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradshaw AD, Puolakkainen P, Dasgupta J, Davidson JM, Wight TN, Helene Sage E. SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J Invest Dermatol. 2003;120:949–955. doi: 10.1046/j.1523-1747.2003.12241.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koike T, Vernon RB, Gooden MD, Sadoun E, Reed MJ. Inhibited angiogenesis in aging: a role for TIMP-2. J Gerontol A Biol Sci Med Sci. 2003;589:B798–B805. doi: 10.1093/gerona/58.9.b798. [DOI] [PubMed] [Google Scholar]
- 44.Harris AK, Stopak D, Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1981;290:249–251. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- 45.Vernon RB, Sage EH. Contraction of fibrillar type I collagen by endothelial cells: a study in vitro. J Cell Biochem. 1996;60:185–197. doi: 10.1002/(sici)1097-4644(19960201)60:2<185::aid-jcb3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 46.Lakatta EG. Arterial aging is risky. J Appl Physiol. 2008;105:1321–1322. doi: 10.1152/japplphysiol.91145.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Provenzano PP, Inman DR, Eliceiri KW, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Broberg A, Heino J. Integrin alpha2beta1-dependent contraction of floating collagen gels and induction of collagenase are inhibited by tyrosine kinase inhibitors. Exp Cell Res. 1996;228:29–35. doi: 10.1006/excr.1996.0295. [DOI] [PubMed] [Google Scholar]
- 49.Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol. 2003;149:1309–1323. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langholz O, Rockel D, Mauch C, et al. Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by alpha 1 beta 1 and alpha 2 beta 1 integrins. J Cell Biol. 1995;131:1903–1915. doi: 10.1083/jcb.131.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomasek JJ, Halliday NL, Updike DL, et al. Gelatinase A activation is regulated by the organization of the polymerized actin cytoskeleton. J Biol Chem. 1997;272:7482–7487. doi: 10.1074/jbc.272.11.7482. [DOI] [PubMed] [Google Scholar]
- 52.Riikonen T, Koivisto L, Vihinen P, Heino J. Transforming growth factor-beta regulates collagen gel contraction by increasing alpha 2 beta 1 integrin expression in osteogenic cells. J Biol Chem. 1995;270:376–382. doi: 10.1074/jbc.270.1.376. [DOI] [PubMed] [Google Scholar]
- 53.Verrecchia F, Mauviel A. Transforming growth factor-beta and fibrosis. World J Gastroenterol. 2007;13:3056–3062. doi: 10.3748/wjg.v13.i22.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reed MJ, Vernon RB, Abrass IB, Sage EH. TGF-beta 1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblasts from young and aged donors. J Cell Physiol. 1994;158:169–179. doi: 10.1002/jcp.1041580121. [DOI] [PubMed] [Google Scholar]
- 55.Rentz TJ, Poobalarahi F, Bornstein P, Sage EH, Bradshaw AD. SPARC regulates processing of procollagen I and collagen fibrillogenesis in dermal fibroblasts. J Biol Chem. 2007;282:22062–22071. doi: 10.1074/jbc.M700167200. [DOI] [PubMed] [Google Scholar]
- 56.Schellings MW, Vanhoutte D, Swinnen M, et al. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med. 2009;16:113–123. doi: 10.1084/jem.20081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riikonen T, Westermarck J, Koivisto L, Broberg A, Kahari VM, Heino J. Integrin alpha 2 beta 1 is a positive regulator of collagenase (MMP-1) and collagen alpha 1(I) gene expression. J Biol Chem. 1995;270:13548–13552. doi: 10.1074/jbc.270.22.13548. [DOI] [PubMed] [Google Scholar]
- 58.Balducci L, Ershler WB. Cancer and ageing: a nexus at several levels. Nat Rev Cancer. 2005;5:655–662. doi: 10.1038/nrc1675. [DOI] [PubMed] [Google Scholar]
- 59.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]