Abstract
The aim of this study was to investigate the adjuvant effects of interferon-gamma (IFN-γ) inhalation therapy for six months in the treatment of refractory multidrug-resistant pulmonary tuberculosis (MDR-TB). Aerosolized IFN-γ was given to six MDR-TB patients with persistent positive smears and cultures despite long-term medical treatment. The patients received aerosolized two million international units of IFN-γ three times a week for 6 months while they continued on identical antituberculous chemotherapy. Before IFN-γ inhalation therapy, the patients received a median of 6.5 (range, 4 to 7) antituberculous drugs for median duration of 29 months (range,7 to 76). After IFN-γ inhalation therapy, sputum smears remained persistently positive in all patients throughout the study period. Sputum cultures were transiently negative at the 4th month in two patients, but became positive again at the end of 6 months of IFN-γ therapy. Five patients had radiological improvement including three patients who showed a decrease in the size of the cavitary lesions. Resectional surgery could be performed in one patient in whom substantial clinical and radiological improvement was noted after IFN-γ inhalation therapy. These results suggest that IFN-γ inhalation therapy may be effective for some cases of refractory MDR-TB who are otherwise not responding to conventional therapy.
Keywords: Tuberculosis, Pulmonary; Tuberculosis, Multidrug-Resistant; Interferon-gamma, Recombinant; Administration, Inhalation; Aerosols
INTRODUCTION
Multidrug-resistant tuberculosis (MDR-TB), defined as a disease caused by strains of Mycobacterium tuberculosis that are resistant to at least isoniazid and rifampin, is recognized as an important global threat (1). Current therapy for MDR-TB is the combination of second-line antituberculous drugs, and resectional surgery can be sometimes combined with antituberculous chemotherapy (2, 3). Until now, however, there has been no definite treatment modality in the patients with refractory MDR-TB, who have persistent positive sputum culture for M. tuberculosis despite prolonged antituberculous chemotherapy and who are not candidates for resectional surgery due to extensive bilateral disease.
Interferon-gamma (IFN-γ) has been shown to activate monocytes and macrophages against M. tuberculosis in vitro (4-6). Some clinical efficacy has also been shown for IFN-γ given systemically to treat disseminated M. avium complex infections in immunocompromised hosts without infection of human immunodeficiency virus (HIV) or patients with AIDS (7, 8). In addition, Condos et al. reported transient but clinically encouraging improvements in five patients with MDR-TB following one month of IFN-γ inhalation therapy. Responses included the conversion of sputum smears to negative, delayed growth of cultures, and shrinkage of cavities (9).
However, there have been no further reports about long-term effects of IFN-γ inhalation therapy in the treatment of MDR-TB. Therefore, we studied the effects of IFN-γ inhalation therapy for six months in the treatment of refractory MDR-TB.
MATERIALS AND METHODS
Six patients with sputum-smear-positive and culture-positive cavitary MDR-TB that was refractory to antituberculous chemotherapy were evaluated in an open-labeled, nonrandomized observational study. The study was approved by the institutional review boards, and informed consent was obtained from all patients.
All patients were chronic cases according to the case definition criteria of World Health Organization (10) and had a refractory MDR-TB and radiological progression of disease despite adequate chemotherapy for at least six months based on the past drug history and individual susceptibility test results. No patient was indicated for resectional surgery because of extensive bilateral disease including cavitary lesions. No patient was infected by HIV. IFN-γ inhalation therapy was added to chemotherapeutic regimens only if there was no improvement in the patient's clinical, microbiological, and radiological conditions.
Recombinant human IFN-γ (LGCI Ltd. Seoul, Republic of Korea) in a dose of two million international units was given via nebulizer three times weekly for six months. All patients continued to receive the same antituberculous medications that they had received previously. Treatment was given on an outpatient basis. Treatment and surveillance for adverse events were monitored and managed by the attending physician. Monitoring of renal and hepatic function was performed regularly during therapy. Sputum examinations and posterior-anterior chest radiographies were done bimonthly. One patient had a computed tomography scan of the chest at baseline and on completion of the IFN-γ inhalation therapy on the basis of the judgment of the treating clinician. Posterior-anterior chest radiographies and computed tomography scans examined at the baseline and after completion of treatment were reviewed by two experienced chest radiologists.
RESULTS
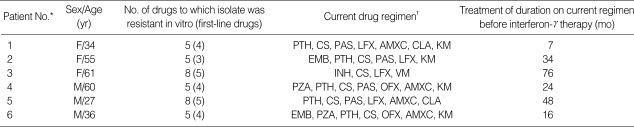
The patient's characteristics are shown in Table 1. There were three men and three women. The median duration of disease before IFN-γ inhalation therapy was 29 months (range, 7 to 76). The median number of drugs used previously was 6.5 (range, 4 to 7).
Table 1.
Characteristics of the six patients with multidrug-resistant tuberculosis treated with interferon-gamma
*The patients are numbered according to the order of entry. †PTH, prothionamide; CS, cycloserine; PAS, para-aminosalicylic acid; LFX, levofloxacin; AMXC, amoxicillin-clavulanic acid; CLA, clarithromycin; KM, kanamycin; EMB, ethambutol; INH, isoniazid; EVM, enviomycin; PZA, pyrazinamide; OFX, ofloxacin.
All patients tolerated the therapy well. Routine laboratory assessments done throughout the study showed no abnormalities.
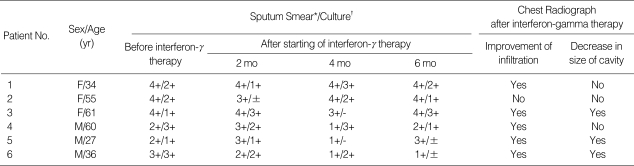
All patients were sputum-smear and sputum-culture positive despite prolonged chemotherapy. For the 6-month period of IFN-γ inhalation therapy, no patient had become sputum-smear negative. Also sputum cultures remained positive for all patients at the end of the IFN-γ inhalation therapy. In Patients 3 and 5, the sputum cultures were negative at the 4th month, but became positive again at the end of 6 months of IFN-γ inhalation therapy (Table 2).
Table 2.
Outcome of treatment of interferon-gamma Inhalation and chemotherapy in six patients with multidrug-resistant tuberculosis
*Sputum smear: ±, 1-2 organisms/300 fields; 1+, 1-9 organisms/100 fields; 2+, 1-9 organisms/10 fields; 3+, 1-9 organisms/1 field; 4+, >9 organisms/1 field (10). †Sputum culture: -, no colonies seen; ±, <50 colonies; 1+, 100-200 colonies; 3+, 200-500 colonies (almost confluence); 4+, >500 colonies (confluence) (10).
Chest radiographs done at the baseline were compared with those taken after 6 months of IFN-γ inhalation therapy. Five patients had improvement of infiltration and three patients showed decrease in the size of the cavitary lesions (Table 2).
One patient (Patient 5) who was not initially considered as a candidate for surgery successfully received pulmonary resectional surgery after 6 months of IFN-γ inhalation therapy combined with chemotherapy due to substantial clinical and radiological improvements.
This 27-yr-old immunocompetent male was presented to our hospital with a 6-yr history of refractory pulmonary tuberculosis. Pulmonary tuberculosis had been diagnosed 6 yr ago, at which time he was treated with isoniazid, rifampin, ethambutol, and pyrazinamide. Results of drug susceptibility test showed that isolated M. tuberculosis was resistant to isoniazid and rifampin and had been on various second-line antituberculous drugs since then. The sputum cultures, however, had remained persistently positive for M. tuberculosis despite a 5-yr history of medical treatment.
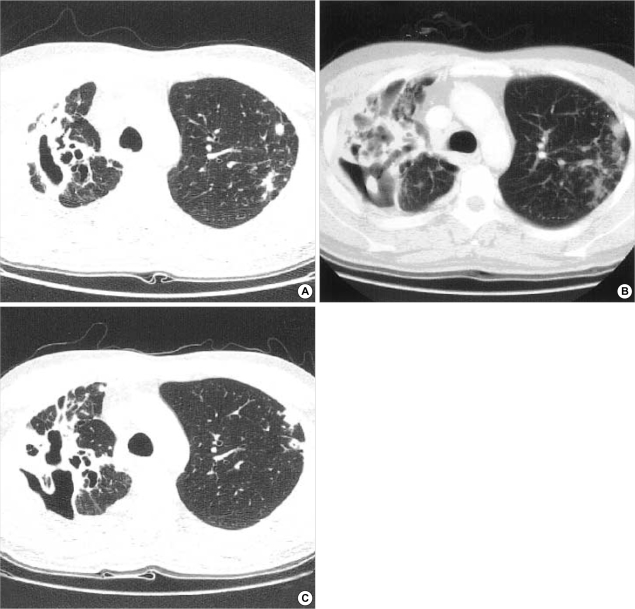
Upon presentation to our hospital, sputum examination showed smear and culture positivity and his chest radiography revealed a large cavity in the right upper lobe and progressive infiltrations in the left upper lobe and both lower lobes (Fig. 1A). Recent drug susceptibility test revealed extensive resistance to isoniazid, rifampin, ethambutol, pyrazinamide, streptomycin, and ofloxacin. Prothionamide, cycloserine, para-aminosalicylic acid, levofloxacin, amoxicillin-clavulonic acid, and clarithromycin were prescribed. Six months later, he became clinically worse, with cough, sputum, and anorexia. Sputum smears for acid-fast bacilli were positive, and cultures yielded growth of M. tuberculosis. The extent of radiographic infiltrations, especially on the left side, had increased (Fig. 1B). At that time, resectional surgery could not be considered due to progressive bilateral lesions.
Fig. 1.
27-yr-old male patient with multidrug-resistant tuberculosis. (A) Computed tomographic scan of the chest showed multiple cavities in the right upper lobe and small nodular infiltrations in the left upper lobe. (B) The extent of radiographic infiltrations of the left upper lobe increased despite 6 months of antituberculous chemotherapy. (C) After 6 months of IFN-γ inhalation therapy, computed tomographic scan showed reduction in the size of cavitary lesions in the right upper lobe and improvement of nodular infiltrates in the left upper lobe.
The patient received IFN-γ inhalation therapy combined with previously prescribed chemotherapy. Six months later there was a decrease of cavity size in the right upper lobe and, more importantly, improvement of nodular infiltrates in the left upper lobe and both lower lobes (Fig. 1C). Resectional surgery was considered because of previous failure of medical treatment alone. Right upper lobectomy and wedge resection of the right lower lobe were successfully performed without perioperative complications. Negative conversion of sputum smear and culture was established postoperatively.
DISCUSSION
MDR-TB is a serious life-threatening condition that is associated with a high degree of morbidity and mortality. In 1993, the National Jewish Center for Immunology and Respiratory Medicine reported 22% mortality attributable to MDR-TB and only 56% of MDR-TB patients were disease free after a mean follow-up period of 51 months (12).
Recently, the development of modern immunology has led to new investigational therapeutic approach, that is cytokine therapy, in the treatment of MDR-TB. One of the targeted cytokines is IFN-γ (9, 13, 14). There is a scientific rationale for the use of IFN-γ in MDR-TB. By activating macrophages and promoting a range of host immune responses, IFN-γ may provide an effective adjunct to antituberculous agents in patients who are not responding to conventional courses of therapy. IFN-γ enhances antigen processing and presentation to lymphocytes through the induction of major histocompatibility complex class II antigens. It also acts on T lymphocytes, stimulating a differentiation toward a Th1-type of immune response (6, 9, 13, 14).
IFN-γ has been used for the prophylactic treatment of chronic granulomatous disease and long-term follow-up of these patients who have received up to 10 yr of IFN-γ has shown no unexpected toxicity (15). These data indicated that long-term IFN-γ therapy appears safe and well tolerated in humans.
IFN-γ has been used successfully as an adjuvant in the treatment of human mycobacterial infections; leprosy, nontuberculous mycobacterial infection, and tuberculosis (7-9, 13, 16). However, clinical experience with IFN-γ for the treatment of pulmonary tuberculosis is still limited. Raad et al. described a patient with acute lymphocytic leukemia in remission who had MDR-TB involving the brain and spinal cord that was steroid-dependent. Subcutaneous IFN-γ therapy allowed rapid reduction in steroid dose and led to cure without any exacerbation of central nervous system inflammation (13). Condos et al. used IFN-γ aerosol for four weeks in five patients with long-standing MDR-TB refractory to medical treatment (9). Sputum acid-fast-bacillus smears became negative and the size of cavitary lesions was reduced in all patients suggesting that IFN-γ might be useful as an adjunctive therapy in patients with MDR-TB. With this short course of therapy, culture conversion did not occur and all patients again had positive smears after cessation of IFN-γ. However, no patient worsened during therapy, and there were no significant systemic or local complaints. These data suggested that IFN-γ inhalation therapy could be well tolerated and useful in some patients with MDR-TB.
The six patients described here had persistent MDR-TB that was refractory to appropriate therapy with conventional antituberculous agents. After the six-month therapy, sputum smears of our patients were persistently positive for the entire six months and sputum cultures were positive at the end of the period in all patients in spite of transient negative conversion in two patients. These results were not consistent with the results of Condos et al. which showed transient clearing of smears in all patients. The difference between our experience and that of Condos et al. may be related to the aerosol dose of IFN-γ that our patients received; our patients were taking two million international units of IFN-γ which were equivalent to 100 µg and Condos et al. used 500 µg of IFN-γ.
In particular, all patients except one patient showed radiological responses. The most obvious responses were improvements of infiltration such as nodules, reticulonodular opacities, which were noticed in five (83%) patients. However, reduction in the size of cavitary lesions was observed in only three (50%) patients.
Interestingly, one patient showed substantial clinical and radiological improvement and then resectional surgery could be performed. To our knowledge, this is the first reported case of refractory MDR-TB in the lung, which was successfully treated with adjunctive IFN-γ inhalation therapy combined with surgical management and antituberculous drugs. This result suggested that IFN-γ inhalation therapy may play a role of the bridge to pulmonary resection in selected patients who have initially equivocal indication of resectional surgery due to bilateral active disease. These findings suggested that IFN-γ inhalation therapy may be a powerful adjunct to conventional therapy, especially in the patients with less destructive parenchymal lesion such as nodular infiltration without cavitary lesions which have a large burden of mycobacterium.
The current study is limited by the small sample size, but the results suggest that IFN-γ inhalation therapy might be an acceptable adjunctive therapy. The distinctive role of IFN-γ in the adjunctive treatment of MDR-TB should be further investigated to determine the criteria of potential candidates and optimal duration of therapy.
Footnotes
This study was supported by the grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea. (00-PJ1-PG1-CH03-0001).
References
- 1.Espinal MA, Laszlo A, Simonsen L, Boulahbal F, Kim SJ, Reniero A, Hoffner S, Rieder HL, Binkin N, Dye C, Williams R, Raviglione MC. Global trends in resistance to antituberculosis drugs. World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. N Engl J Med. 2001;344:1294–1303. doi: 10.1056/NEJM200104263441706. [DOI] [PubMed] [Google Scholar]
- 2.Iseman MD. Treatment of multidrug-resistant tuberculosis. N Engl J Med. 1993;329:784–791. doi: 10.1056/NEJM199309093291108. [DOI] [PubMed] [Google Scholar]
- 3.An CH, Ahn JW, Kang KW, Kang SJ, Lim YH, Suh GY, Chung MP, Kim HJ, Kwon OJ, Rhee CH. The role of resectional surgery for the treatment of localized multidrug resistant pulmonary tuberculosis. Tuberc Respir Dis. 2000;49:676–683. [Google Scholar]
- 4.Barnes PF, Lu S, Abrams JS, Wang E, Yamamura M, Modlin RL. Cytokine production at the site of disease in human tuberculosis. Infect Immun. 1993;61:3482–3489. doi: 10.1128/iai.61.8.3482-3489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan CF, Prendergast TJ, Wiebe ME, Stanley ER, Platzer E, Remold HG, Welte K, Rubin BY, Murray HW. Activation of human macrophages. Comparison of other cytokines with interferon-gamma. J Exp Med. 1984;160:600–605. doi: 10.1084/jem.160.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonecini-Almeida MG, Chitale S, Boutsikakis I, Geng J, Doo H, He S, Ho JL. Induction of in vitro human macrophage anti-Mycobacterium tuberculosis activity: requirement for IFN-gamma and primed lymphocytes. J Immunol. 1998;160:4490–4499. [PubMed] [Google Scholar]
- 7.Holland SM, Eisenstein EM, Kuhns DB, Turner ML, Fleisher TA, Strober W, Gallin JI. Treatment of refractory disseminated nontuberculous mycobacterial infection with interferon gamma. A preliminary report. N Engl J Med. 1994;330:1348–1355. doi: 10.1056/NEJM199405123301904. [DOI] [PubMed] [Google Scholar]
- 8.Squires KE, Brown ST, Armstrong D, Murphy WF, Murray HW. Interferon-gamma treatment for Mycobacterium avium-intracellulare complex bacillemia in patients with AIDS. J Infect Dis. 1992;166:686–687. doi: 10.1093/infdis/166.3.686. [DOI] [PubMed] [Google Scholar]
- 9.Condos R, Rom WN, Schluger NW. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-gamma via aerosol. Lancet. 1997;349:1513–1515. doi: 10.1016/S0140-6736(96)12273-X. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization; International Union Against Tuberculosis and Lung Disease; Royal Netherlands Tuberculosis Association. Revised international definitions in tuberculosis control. Int J Tuberc Lung Dis. 2001;5:213–215. [PubMed] [Google Scholar]
- 11.American Thoracic Society. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 12.Goble M, Iseman MD, Madsen LA, Waite D, Ackerson L, Horsburgh CR., Jr Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N Engl J Med. 1993;328:527–532. doi: 10.1056/NEJM199302253280802. [DOI] [PubMed] [Google Scholar]
- 13.Raad I, Hachem R, Leeds N, Sawaya R, Salem Z, Atweh S. Use of adjunctive treatment with interferon-γ in an immunocompromised patient who had refractory multidrug-resistant tuberculosis of the brain. Clin Infect Dis. 1996;22:572–574. doi: 10.1093/clinids/22.3.572. [DOI] [PubMed] [Google Scholar]
- 14.Holland SM. Cytokine therapy of mycobacterial infections. Adv Intern Med. 2000;45:431–452. [PubMed] [Google Scholar]
- 15.Bemiller LS, Roberts DH, Starko KM, Curnutte JT. Safety and effectiveness of long-term interferon gamma therapy in patients with chronic granulomatous disease. Blood Cells Mol Dis. 1995;21:239–247. doi: 10.1006/bcmd.1995.0028. [DOI] [PubMed] [Google Scholar]
- 16.Sampaio EP, Moreira AL, Sarno EN, Malta AM, Kaplan G. Prolonged treatment with recombinant interferon gamma induces erythema nodosum leprosum in lepromatous leprosy patients. J Exp Med. 1992;175:1729–1737. doi: 10.1084/jem.175.6.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]