Abstract
Non-myeloablative allogeneic peripheral stem cell transplantation (NST) is a novel therapeutic strategy for patients with hematologic malignancies. Whether non-myeloablative transplants are associated with increased risk of cytomegalovirus (CMV) infections is unknown. To clarify this issue, we compared the outcome of CMV infection following 24 allogeneic non-myeloablative peripheral blood stem cell transplants and 40 conventional bone marrow transplants (CBT). The NST regimen consisted of busulfan (4mg/kg/day), fludarabine (30mg/m2) and anti-thymocyte globulin (10 mg/kg). Twelve patients (50%) in the NST group and 17 (43%) in the CBT group developed positive antigenemia before day 100 (p=0.60). The time to the first appearance of positive antigenemia was not different between these two groups (p=0.40), and two groups showed similar initial and maximal antigenemia values (p=0.56 and p=0.68, respectively). Only one case of CMV colitis developed in the CBT group whereas CMV disease did not develop in the NST group. Although statistically insignificant, the treatment response against CMV antigenemia using ganciclovir was in favor of NST group. In conclusion, there was no difference in the risk of CMV infection between NST group and CBT group. Further prospective and controlled study is needed to clarify the impact of non-myeloablative procedure on the outcome of CMV infection.
Keywords: Cytomegalovirus, Hematopoietic Stem Cell Transplantation, Bone Marrow Transplantation, Ganciclovir
INTRODUCTION
Non-myeloablative allogeneic peripheral stem cell transplantation (NST) is an emerging treatment modality for malignant and nonmalignant hematologic disorders. Because of their low toxicity, nonmyeloablative preparative regimens are being explored in patients who are not eligible for conventional hematopoietic stem cell transplantation owing to age or medical contraindications (1-4). However, several problems need to be resolved in this procedure.
Although antiviral prophylaxis has led to a significant reduction in early cytomegalovirus (CMV) disease after hematopoietic stem cell transplantation (HSCT), CMV infection continues to be a major cause of morbidity and mortality in patients undergoing this procedure (5). Several studies have found a high rate of CMV infection after non-myeloablative stem cell transplantation (NST) (6-8). However, it is still controversial whether non-myeloablative procedure increases the risk of CMV infection after HSCT, since there have been few studies comparing NST and conventional HSCT. Therefore, we examined whether NST affected the outcomes of CMV infection in patients undergoing allogeneic HSCT. The primary end point of this study was to compare the incidence of CMV infection and disease between NST group and CBT group. Secondly, we compared the time to first detection of CMV antigenemia, initial and maximal CMV antigen values and serial change of CMV antigen values after pre-emptive therapy using ganciclovir between these two groups.
MATERIALS AND METHODS
Patients
We retrospectively analyzed the medical records of 64 consecutively registered CMV-seropositive patients who had undergone allogeneic HSCT from HLA-identical or one-locus mismatched sibling at the Asan Medical Center between July 1999 and July 2001. Forty patients underwent conventional bone marrow transplantation (CBT group), whereas 24 patients underwent non-myeloablative peripheral stem cell transplantation (NST group). Patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
GVHD, graft-versus-host disease; CsA, cyclosporin; mTX, methotrexate.
*Others included Non-Hodgkin's lymphoma (n=6), Hodgkin's disease (n=3), multiple myeloma (n=3), paroxysmal nocturnal hemoglobinuria (n=2) in NST group, and one case of non-Hodgkin's lymphoma in CBT group.
Transplantation procedures
The NST regimen consisted of busulfan (4 mg/kg/day p.o. on days -7 to -6), fludarabine (30 mg/m2/day i.v. on days -7 to -2), and anti-thymocyte globulin (ATG: 10 mg/kg/day for on days -5 to -2). Granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral stem cells from donors were infused on day 0 and 1. In the CBT group, all patients received Bu-Cy regimen (busulfan 4 mg/kg/day p.o. on days -7 to -4 and cyclophosphamide i.v. 60 mg/kg/day on days -3 to -2) and received bone marrow stem cell from donors.
GVHD prophylaxis and treatment
All patients received prophylactic therapy with cyclosporine alone or cyclosporine and methotrexate for graft-versus-host disease (GVHD) prophylaxis. Cyclosporine 1.5 mg/kg was given intravenously every 12 hr starting on day -1, then switched to an oral dose of two times the intravenous dose when oral intake became feasible. If there was no evidence of GVHD, the cyclosporine dose was tapered by 10% each month starting on day 60. Intravenous methotrexate was given at a dose of 15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6 and 11. Patients who developed grade II-IV acute GVHD were treated with methylpredinisolone at 1-2 mg/kg/day.
Supportive care
All patients were treated as inpatients in private rooms. Patients received antibacterial and antifungal prophylaxis with oral ciprofloxacin at 1,000 mg/day and fluconazole 100 mg/day until the absolute neutrophil count (ANC) was over 3,000/µL. Prophylaxis against herpes simplex was performed with acyclovir 750 mg/m2 i.v. from days -7 to 14 and then switched to an oral dose at 600 mg/day until the end of immunosuppressive therapy. Intravenous globulin 500 mg/kg was given once every 2 weeks from day -7 until day 120, then monthly until day 180.
CMV antigenemia assay
All patients were monitored for CMV infection using a CMV pp 65 antigenemia assay once a week from day -7 until day 100 after BMT. Patients with a positive antigenemia test were monitored twice weekly. The CMV antigenemia assay was performed according to the method described previously (9). In brief, 2×105 peripheral blood leukocytes were attached to slides using a cytocentrifuge and fixed with formaldehyde. The cells were sequentially incubated with Clonab CMV monoclonal antibodies C10/11 (Biotest AG, Dreieich, Germany). After incubation, the slides were washed with phosphate buffered saline (PBS) and then stained using alkaline phosphatase anti-alkaline phosphatase (APAAP) technique. If the target antigen (CMV matrix protein pp 65) is present, a red reaction product forms at leukocyte. Reading was done under a microscope to search for red reaction-positive cells. Records were reported as the number of pp 65-positive cells per 2×105 cells examined.
Preemptive therapy
Ganciclovir treatment was started on the day of the first positive antigenemia findings for all patients with positive CMV antigenemia at an induction dose of 5 mg/kg intravenously twice daily for 1 week. Maintenance gancicovir therapy was continued until day 100 after transplantation with doses of 5 mg/kg/day 5 to 6 days per week.
A neutropenic episode was defined as a decrease of the ANC to 1,000/µL for 2 consecutive days. If the ANC dropped below 1,000/µL for 3 consecutive days, G-CSF was administered. If the ANC dropped below 500/µL for >2 consecutive days in spite of therapy, treatment with ganciclovir was temporarily discontinued until the ANC returned to a level 1,000/µL on 2 successive days.
Definition of CMV disease
CMV pneumonia was diagnosed on the basis of signs and symptoms compatible with a diagnosis of pneumonia (dyspnea, interstitial infiltrates on chest radiography) and a bronchoalveolar lavage fluid or lung biopsy specimen positive for CMV by culture or immunohistology. Diagnosis of CMV colitis was based on gastrointestinal signs or symptoms and histologic demonstration of CMV.
Statistical Analysis
Time to the documentation of first positive CMV antigenemia was estimated by the Kaplan-Meier method and the differences between 2 groups were compared using the logrank test. We accepted a level of p<0.05 as statistically significant. To compare the mean levels of CMV antigenemia, we used a Mann-Whitney U test. The differences of treatment response for positive CMV antigenemia were compared using repeated ANOVA. We performed the analysis using the SPSS statistical program for Windows version 10.0.
RESULTS
Transplant outcome
All patients achieved engraftment except 1 patient in the NST group. The NST group (median 11days) showed faster neutrophil engraftment than CBT group (median 14 days). Seven (29%) in the NST group and 5 (12%) in the CBT group developed acute GVHD III-IV. After a median follow-up of 575 days (range 44-1146) for surviving patients, 30 CBT patients are alive with an actuarial survival of 75%. In NST, 8 patients are alive with a median follow up of 234 days (range 49-1106) with an actuarial survival of 33%. Of 10 deaths in CBT and 16 deaths in NST, 6 were due to relapse or disease progression. Of the remaining 20 patients, 5 died of acute GVHD, 8 patients died of infection, one patient died of graft failure.
Incidence and levels of CMV Antigenemia
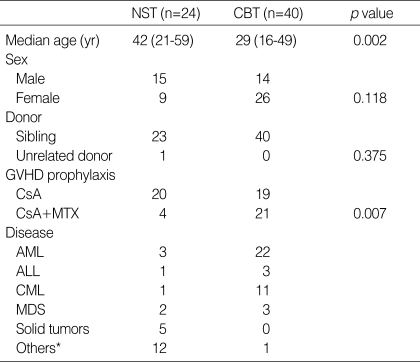
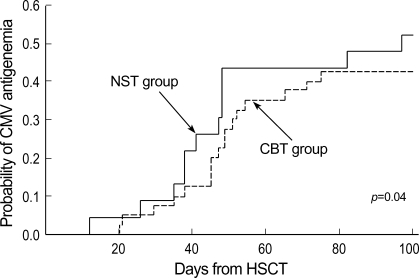
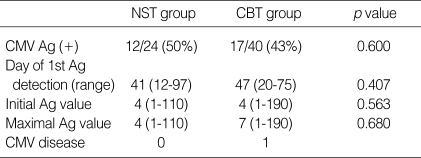
In the NST group, 12 patients (50%) developed antigenemia at a median onset of 44 days (range 12-97). Seventeen patients (43%) developed CMV antigenemia at a median onset of 47 days (range 20-75) in the CBT group. The time to the first appearance of positive antigenemia was not different between these two groups (p=0.40, Fig. 1). The median number of initial CMV antigen value of NST group was 4 (range 1-110) and that of CBT group was 4 (range 1-190). The median number of maximal CMV antigen of NST group was 8 (range 1-110) and that of CBT group was 7 (range 1-190). There was no significant difference in the degree of initial and maximal CMV antigen value between NST group and CBT group (Fig. 2).
Fig. 1.
Probability of developing positive CMV antigenemia after non-myeloablative and conventional transplantation shown by Kaplan-Meier curves.
Fig. 2.
The initial (1st Ag) and maximum (Max Ag) antigenemia values, compared between non-myeloablative and conventional transplantation procedures.
Serial CMV antigenemia values after pre-emptive therapy using ganciclovir
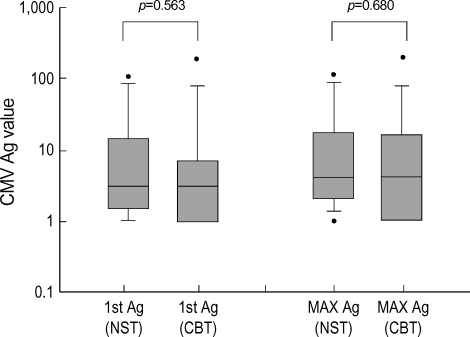
Of the 12 NST group patients who had CMV antigenemia, 9 patients received pre-emptive therapy using ganciclovir and one patient developed neutropenia. In the CBT group, 14 patients received pre-emptive therapy and 3 patients developed neutropenia. The serial antigen value declined to baseline within 7 weeks in all of the patients undergoing pre-emptive therapy. The serial change of antigenemia values during pre-emptive therapy using ganciclovir is shown in Fig. 3. Although statistically insignificant, there was a trend for the treatment response using ganciclovir in favor of NST group (p=0.168).
Fig. 3.
Serial changes in antigenemia values in patients receiving ganciclovir were compared between non-myeloablative and conventional transplantation.
CMV disease
Among the patients who received pre-emptive therapy, no patient developed CMV disease in the NST group whereas one patient developed CMV colitis in the CBT group. The patient who had CMV colitis suffered from acute GVHD before the onset of disease and she was treated with combination of ganciclovir and intravenous immunoglobulin. She recovered from CMV colitis after 1 month of therapy.
DISCUSSION
Following conventional allogeneic hematopoietic stem cell transplantation, all patients experience a period of profound neutropenia and immunodeficiency that are responsible for serious infection. Over the past years several groups have developed non-myeloablative regimens, allowing engraftment of donor hematopoietic stem cells with lesser toxicity compared with conventional HSCT. This reduced extrahematologic toxicity may lead to a reduction in infectious complication post transplant. However, in earlier studies, it was reported that the risk of CMV infection had been increased in patients undergoing NST. One potential assumption is the possible delay of immune reconstitution after NST. Since the NST regimen is primarily based on the concept of intensifying immunosuppression to enhance engraftment of donor cells rather than cytoreduction, it is likely that CMV infection will increase during this procedure.
Mohty et al. reported a high frequency of CMV infections after NST using a regimen consisting of fludarabine, busulfan and ATG (6). A higher incidence of early and late CMV infection was also observed after a conditioning regimen with fludarabine, melphalan, and Campath 1H, a monoclonal anti-CD52 antibody (7). In all of these NST protocol, anti-thymocyte globulin or CAMPATH 1H was used in the conditioning regimens for promoting engraftment, which may lead to delayed reconstitution of CMV-specific T cell responses post transplant. On the other hand, Junghanss et al. observed a trend toward less CMV infection and disease after NST using low-dose TBI with fludarabine (10). Nakamura et al. also showed a reduced incidence of CMV reactivation after conditioning with fludarabine and cyclophosphamide (11). Therefore, the risk of CMV infection appears to be influenced by the agents used in the NST regimen.
In a recent study of Fred Hutchison Cancer Center, comparing 56 recipients of an NST with 112 matched recipients of a myeloablative transplant found a lower probability of CMV antigenemia, viremia in the former group (12). They reported a similar incidence of CMV disease between the two groups and delayed onset of the disease in the NST group. They assumed that host hematopoietic cells, especially T lymphocytes, are not immediately eradicated by NST regimen if anti-lymphocyte antibody was not used; host T cells were present in the peripheral blood for up to 6 months. In contrast, conventional myeloablative conditioning regimens lead to early and complete disappearance of host hematopoiesis. On the basis of these findings, it is conceivable that the prolonged presence of host immunity after NST may provide some protection against early CMV infection as compared with conventional myeloablative transplantation.
We used anti-thymocyte globulin in NST group as a conditioning regimen. However, there was no significant difference in the outcome of CMV infection between NST and CBT group in our study. The frequency of CMV antigenemia, time to the first appearance of positive antigenemia after HSCT, initial and maximal CMV antigen values did not differ between these two groups. Although statistically insignificant, the treatment response against CMV infection was in favor of NST group rather than CBT group. These results may be explained in the following.
Our study had some limitations. The patient number was small and this study was a retrospective analysis. The main shortcoming of our study is the differences in baseline patient characteristics between the two transplant groups. Since NST allografts are currently offered to patients who are poor candidates for conventional myeloablative therapies due to older age or medical co-morbidity, we were unable to perform a well-matched cohort study. Some variables that may influence the outcomes of CMV infection favor the NST group; others favor the CBT group. Age has been described as a risk factor for CMV disease (13). Median age of NST group was much higher than CBT group which may have influenced the rate of CMV infection. In addition, in our study, acute GVHD was much more frequent in the NST group. Acute GVHD is a well known risk factor of CMV infection and CMV disease. So this result may negatively impact the outcome of CMV infection in NST group. In a recent study from Germany, the incidence of CMV antigenemia and CMV-related interstitial pneumonia was lower in alloPBSCT compared to BMT recipients; suggesting more rapid immune reconstitution following PBSCT compared to BMT(14). All of the CBT group patients in our study received bone marrow stem cells from donors whereas NST group patients received peripheral blood stem cells, a fact favoring the NST group. Some variables favor NST group and others favor CBT group. Overall they may have resulted in no difference for the risk of CMV infection between the two group.
In conclusion, our study showed that a new approach NST did not increase the risk of CMV infection and disease compared with CBT. Besides, the treatment response against CMV infection using ganciclovir seemed to be in favor of NST. However, our study had limitation mentioned above. Large, prospective and well controlled studies are needed to clarify the impact of NST procedure on the outcome of CMV infection.
Table 2.
The comparison of CMV infection between NST and CBT group
Ag, Antigen.
References
- 1.Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, Varadi G, Kirschbaum M, Ackerstein A, Samuel S, Amar A, Brautbar C, Ben-Tal O, Eldor A, Or R. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic disease. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 2.McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, Chauncey TR, Gooley TA, Hegenbart U, Nash RA, Radich J, Wagner JL, Minor S, Appelbaum FR, Bensinger WI, Bryant E, Flowers ME, Georges GE, Grumet FC, Kiem HP, Torok-Storb B, Yu C, Blume KG, Storb RF. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 3.Khouri IF, Keating M, Korbling M, Przepiorka D, Anderlini P, O'Brien S, Giralt S, Ippoliti C, von Wolff B, Gajewski J, Donato M, Claxton D, Ueno N, Andersson B, Gee A, Champlin R. Transplant lite: Induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor cell transplantation as treatment for lymphoid malignancy. J Clin Oncol. 1998;16:2817–2824. doi: 10.1200/JCO.1998.16.8.2817. [DOI] [PubMed] [Google Scholar]
- 4.Giralt S, Estey E, Albitar M, van Besien K, Rondon G, Anderlini P, O'Brien S, Khouri I, Gajewski J, Mehra R, Claxton D, Andersson B, Beran M, Przepiorka D, Koller C, Kornblau S, Korbling M, Keating M, Kantarjian H, Champlin R. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997;89:4531–4536. [PubMed] [Google Scholar]
- 5.Park SH, Lee HG, Choi SC, Lee HR, Kim KH, Nam EM, Kim WS, Yoon SS, Kang WK, Kim SW, Park KC. Cytomegalovirus infection and disease after allogeneic hematopoietic stem cell transplantation; analysis of risk factors. Korean J Hematop Stem cell Transplant. 2001;5:23–31. [Google Scholar]
- 6.Mohty M, Faucher C, Vey N, Stoppa AM, Viret F, Chabbert I, Chabannon C, Bouabdallah R, Ladaique P, Collet L, Zandotti C, Maraninchi D, Blaise D. High rate of secondary viral and bacterial infections in patients undergoing allogeneic bone marrow mini-transplantation. Bone Marrow Transplant. 2000;26:251–255. doi: 10.1038/sj.bmt.1702509. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti S, Mackinnon S, Chopra R, Kottaridis PD, Peggs K, O'Gorman P, Chakraverty R, Marshall T, Osman H, Mahendra P, Craddock C, Waldmann H, Hale G, Fegan CD, Yong K, Goldstone AH, Linch DC, Milligan DW. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution. Blood. 2002;99:4357–4363. doi: 10.1182/blood.v99.12.4357. [DOI] [PubMed] [Google Scholar]
- 8.Nakai K, Kanda Y, Mineishi S, Saito T, Ohnishi M, Niiya H, Chizuka A, Takeuchi T, Matsubara H, Kami M, Makimoto A, Tanosaki R, Kunitoh H, Tobinai K, Takaue Y. Suspected delayed immune recovery against cytomegalovirus after reduced-intensity stem cell transplantation using anti-thymocyte globulin. Bone Marrow Transplant. 2002;29:237–241. doi: 10.1038/sj.bmt.1703351. [DOI] [PubMed] [Google Scholar]
- 9.Boeckh M, Bowden RA, Goodrich JM, Pettinger M, Meyers JD. Cytomegalovirus antigen detection in peripheral blood leukocytes after allogeneic marrow transplantation. Blood. 1992;80:1358–1364. [PubMed] [Google Scholar]
- 10.Junghanss C, Boeckh M, Carter R. Incidence of herpesvirus infections following nonmyeloablative allogeneic stem cell transplantation. Blood. 2000;96(Suppl 1):188a. doi: 10.1182/blood.v99.6.1978. (Abstr. 805) [DOI] [PubMed] [Google Scholar]
- 11.Nakamura R, Cortez K, Solomon S, Battiwalla M, Gill VJ, Hensel N, Childs R, Barrett AJ. High-does acyclovir and pre-emptive ganciclovir to prevent cytomegalovirus disease in myeloablative and non-myeloablative allogeneic stem cell transplantation. Bone Marrow Transplant. 2002;30:235–242. doi: 10.1038/sj.bmt.1703648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Junghanss C, Boeckh M, Carter RA, Sandmaier BM, Maris MB, Maloney DG, Chauncey T, McSweeney PA, Little MT, Corey L, Storb R. Incidence and outcome of cytomegalovirus infections following nonmyeloablative compared with myeloablative allogeneic stem cell transplantation, a matched control study. Blood. 2002;99:1978–1985. doi: 10.1182/blood.v99.6.1978. [DOI] [PubMed] [Google Scholar]
- 13.Einsele H, Hebart H, Kaufmann-Schneider C, Sinzger C, Jahn G, Bader P, Klingebiel T, Dietz K, Loffler J, Bokemeyer C, Muller CA, Kanz L. Risk factors for treatment failures in patients receiving PCR-based preemptive therapy for CMV infection. Bone Marrow Transplant. 2000;25:757–763. doi: 10.1038/sj.bmt.1702226. [DOI] [PubMed] [Google Scholar]
- 14.Trenschel R, Ross S, Husing J, Ottinger H, Elmaagacli A, Roggendorf M, Schaefer UW, Runde V. Reduced risk of persisting cytomegalovirus pp65 antigenemia and cytomegalovirus interstitial pneumonia following allogeneic PBSCT. Bone Marrow Transplant. 2000;25:665–672. doi: 10.1038/sj.bmt.1702216. [DOI] [PubMed] [Google Scholar]