Abstract
Passive smoking is a major cause of respiratory morbidity, and is associated with increased bronchial responsiveness in children. To evaluate the effect of smoking by a parent on asthma symptoms, atopy, and airway hyperresponsiveness (AHR), we conducted a cross-sectional survey of 503 schoolchildren that involved questionnaires, spirometry, allergy testing, and a bronchial challenge test. If the PC20 methacholine was less than 16 mg/mL, the subject was considered to have AHR. The prevalence of a parent who smoked was 68.7%. The prevalence of AHR was 45.0%. The sensitization rate to common inhalant allergens was 32.6%. Nasal symptoms such as rhinorrhea, sneezing, nasal itching, and nasal obstruction were present in 42.7%. Asthma symptoms such as cough and wheezing were present in 55.4%. The asthma symptoms were significantly more prevalent in children who had a parent who smoked than in those whose parents did not. The nasal symptoms, atopy, and AHR did not differ according to whether a parent smoked. In a multiple logistic regression model, the asthma symptoms and atopy were independently associated with AHR, when adjusted for confounding variables. Passive smoking contributed to asthma symptoms in schoolchildren and was not an independent risk factor of airway hyperresponsiveness in an epidemiological survey.
Keywords: Tobacco Smoke Pollution, Bronchial Asthma, Bronchial Hyperreactivity, Child
INTRODUCTION
The prevalence of asthma continues to rise. A number of environmental factors including air pollution, cigarette smoking, and allergen exposure have been proposed to explain the changes in the prevalence of asthma (1). The prevalence of asthma has increased in Korea from 5.7% in 1980 to 10.0% in 1995 (2).
Passive smoking is a major cause of respiratory morbidity in children. High levels of parental smoking at home are associated with a reduction in health care contacts for asthma. Children with asthma who have parents who smoke heavily may not be receiving adequate management (3). The smoking rate among parents of asthmatic children is significantly higher than among parents of normal children (4).
An increase in the total IgE in relation to active smoking has been demonstrated in the general population. An increase in IgE was also observed in first-degree relatives exposed to passive smoking (5).
Passive smoking increases the likelihood of experiencing respiratory symptoms and is associated with increased bronchial responsiveness. Decreasing involuntary exposure to tobacco smoke in the community, especially in the workplace, should improve respiratory health (6).
To investigate the effect of exposure to parental cigarette smoking on asthma symptoms, we gave schoolchildren questionnaires that asked about asthma symptoms, atopy, and airway hyperresponsiveness.
MATERIALS AND METHODS
Study population and Questionnaire
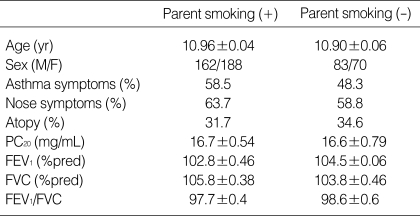
All children were 10-12 yr of the age. The question on respiratory and allergic disorders included those developed for the International Study of Asthma and Allergies in Childhood (ISAAC). The focus of this survey was on responses to the ISAAC questions on "wheeze in the last 12months", "number of wheezing attacks in the last 12 months", and on "sleepdisturbing" and "speech-limiting" wheezing in the last 12 months. Symptoms of allergic rhinitis ("sneezing or runny, or blocked nose without a cold") were assessed (7). In addition, a question on morning cough as a symptom of nonspecific airway irritation was asked ("Did you frequently cough in the morning right after waking up in the last 12 months?"). Also a question on smoking status of parents was asked. A total of 503 schoolchildren was enrolled in this study (Table 1). No subjects took any drugs within 72 hr of tests such as antihistamine, cromolyn, theophylline, sympathomimetics, which could interfere with the performance of skin tests. The all guardians signed informed consent before the study.
Table 1.
Characteristics of school children
% pred, group mean value of individual percentage predicted lung function parameter; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; M, male; F, female.
Pulmonary function study
Spirometry was performed with SensorMedics 2200 spirometer (Cardiopulmonary care company™, Yorba Linda, California, U.S.A.). Baseline measurements of VC and FEV1 were selected according to American Thoracic Society criteria (8) and reference values were taken from Choi et al. (9).
Allergy skin prick test
Allergy skin prick tests were performed with eleven common allergen extracts [Dermatophagoides farinae, Dermatophagoides pteronyssinus, Aspergillus spp, alder, birch, hazel, rye, timothy, mugwort, ragweed, Blatella germinica; Allergopharma Co, Reinbek, Germany], and histamine (1 mg/mL) and saline as a positive control and a negative control. The reactions were read 15 min later. When the wheal size was equal or greater than that of histamine (positive control), it was read as 3+. Atopy was defined as a reactor who showed ≥3+ response to one or more allergens on skin prick tests (10).
Airway hyperresponsiveness
Methacholine challenge tests were carried out by a modified method described by Chai et al. (11). Concentrations of 0.075, 1.25, 2.5, 5, 10 and 25 mg/mL methacholine were prepared by dilution with buffered saline. A Micro-dosimeter (S&M Instrument Co, Doylestown, PA) was used to deliver the aerosol generated by a DeVilbiss 646 nebulizer. Subjects inhaled 5 breaths of increasing concentrations of methacholine until FEV1 fell by more than 20% of its basal value or the highest concentration was reached. The largest value of triplicate FEV1 at 30 or 90 or 180 sec after each inhalation was adopted for analysis. If PC20 was less than 16 mg/mL, a subject was considered to have AHR.
Statistical analyses
All data were analyzed using the SPSS version 7.5 for Windows. Each biochemical assays were repeated at least two times. Data are expressed as mean±standard error of mean (SEM). Statistical analysis was performed by Student's t test, Mann-Whitney U test. In multivariate analysis, logistic regression was performed with a stepwise selection method with p<0.05 for entry into the model. Atopy and asthma symptoms were treated as binary outcome variables in logistic regression analysis. A p-value of <0.05 considered to be significant.
RESULTS
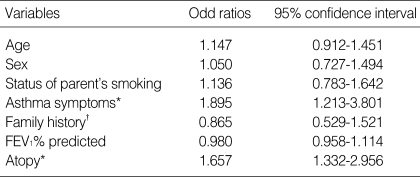
The characteristics of children of school age were shown in Table 1. The prevalence of parent's smoking was 68.7% (346/503). The prevalence of AHR was 45.0% (227/503). The sensitization rate (skin prick test A/H ≥3+) to common inhalant allergens were 32.6% (164/503). Nasal symptoms such as rhinorrhea, sneezing, nasal itching, and nasal obstruction were present in 42.7%. Asthma symptoms such as coughing, wheezing, and chest tightness were present in 55.4%. The asthma symptoms were significantly more prevalent in children who had a parent who smoked than in those whose parents did not [(205/350 (58.5%) vs. 74/157 (47.1%), p=0.016)]. The nasal symptoms, atopy, and AHR did not differ according to whether a parent smoked. In a multiple logistic regression model, the asthma symptoms and atopy were independently associated with AHR, when adjusted for confounding variables (OR=1.895; 95% CI=1.213-3.801, p=0.046; OR=1.657; 95% CI=1.332-2.956, p=0.029, Table 2).
Table 2.
Multivariate logistic regression on airway hyperresponsiveness
Stepwise logistic regression was used to select factors significantly associated AHR risk (*p<0.05). †defined by having or not parent's allergic diseases.
DISCUSSION
This epidemiological survey demonstrated that passive smoking contributes to asthma symptoms and is not an independent risk factor of AHR in schoolchildren.
The prevalence of asthma has increased dramatically in recent years, especially among children. In our study, the prevalence of a PC20 less than 16 mg/mL was 45.0%, which represents the AHR prevalence among schoolchildren according to the American Thoracic Society criteria (12). Long-term follow up to clarify the association between AHR and asthma in schoolchildren is needed. The factors leading to the increase in asthma and allergies have not been identified, although exposure related to general changes in the domestic environment is likely involved. Environmental factors play an important role in the development and manifestation of allergic conditions in genetically predisposed subjects (13). Passive smoking increases the risk of allergic sensitization. It also increases the frequency and severity of symptoms in children with asthma (14). In this study, the observed prevalence of atopy in schoolchildren was 32.6%, and atopy was an independent risk factor in AHR, indicating that atopy plays an important role in the development of asthma in schoolchildren. We did not observe an effect of parental smoking on atopy in schoolchildren. Exposure to tobacco smoke has been associated with a decline in peak flow and with increases in respiratory symptoms and bronchodilator use in asthmatic children (15). In most of the families that we studied in which a parent smoked, the father smoked (97%). Although parental smoking did not affect AHR, it was associated with asthma symptoms. Whether a parent smoked had no effect on AHR in children. Therefore, our data suggest that parental smoking does not cause asthma, but aggravates it.
The risk of asthma in Nepalese children was lower in subjects exposed to cattle kept inside the house and higher in subjects exposed to passive smoking and indoor use of smoky fuels (16). Another study found that the risk factors for childhood asthma increased with increasing numbers of smokers and the total minutes spent smoking by relatives in front of both the child and the mother while she was pregnant with the child (17). We did not measure the total minutes or amount of smoking by parents in front of children. Further study is needed to clarify the effects of exposure time and the amount of passive smoking on AHR. A greater baseline exposure to environmental tobacco smoke has also been related with poorer physical health and asthma-specific quality-of-life at longitudinal follow up (18). In our study, having a parent who smoked was not a risk factor for AHR, but contributed to asthma symptoms. This suggests that it is necessary to prohibit parents from smoking to prevent asthma-related symptoms in children. These findings emphasize the need for continuing effort to decrease exposure to passive smoke among schoolchildren and to increase understanding of the harmful health effects of smoking exposure. Parents of children with asthma should be advised not to smoke, especially in rooms that their children use.
Kurukulaaratchy et al. (19) reported factors that influenced symptom expression in children with bronchial hyperresponsiveness at 10 yr of age. When only factors present in the first 4 yr of life were considered, parental smoking at 4 yr of age, maternal asthma, and atopic sensitization at 4 yr of age were independently associated with symptomatic AHR at 10 yr of age. In our study, although asthma symptoms and atopy were independent risk factors for AHR, parents' smoking status was not. We evaluated exposure to passive smoking with a questionnaire that did not distinguish between current and former smokers. Further studies are needed to clarify the role of passive smoking in the development of AHR.
In conclusion, we found that passive smoking was associated with asthma symptoms and that parents' smoking was not a risk factor in AHR, suggesting that further effort should be made to convince the parents of asthmatic children not to smoke in the house.
Footnotes
This study was supported by a grant of the Korean Health 21 R 4D Project, Ministry of Health and Welfare, Republic of Korea (01-PJ3-PG6-01GN04-0003).
References
- 1.Black PN, Sharpe S. Dietary fat and asthma: is there connection? Eur Respir J. 1997;10:6–12. doi: 10.1183/09031936.97.10010006. [DOI] [PubMed] [Google Scholar]
- 2.Lee SI, Shin MH, Lee HB, Lee JS, Son BK, Koh YY, Kim KE, Ahn YO. Prevalences of symptoms of asthma and other allergic diseases in Korean children: A nationwide questionnaire survey. J Korean Med Sci. 2001;16:155–164. doi: 10.3346/jkms.2001.16.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crombie IK, Wright A, Irvine L, Clark RA, Slane PW. Does passive smoking increase the frequency of health service contacts in children with asthma? Thorax. 2001;56:9–12. doi: 10.1136/thorax.56.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Dawood K. Parental smoking and the risk of respiratory symptoms among schoolboys in Al-Khobar City, Saudi Arabia. J Asthma. 2001;38:149–154. doi: 10.1081/jas-100000033. [DOI] [PubMed] [Google Scholar]
- 5.Oryszczyn MP, Annesi-Maesano I, Charpin D, Paty E, Maccario J, Kauffmann F. Relationships of active and passive smoking to total IgE in adults of the Epidemiological Study of the Genetics and Environment of Asthma, Bronchial Hyperresponsiveness, and Atopy (EGEA) Am J Respir Crit Care Med. 2000;161:1241–1246. doi: 10.1164/ajrccm.161.4.9905027. [DOI] [PubMed] [Google Scholar]
- 6.Janson C, Chinn S, Jarvis D, Zock JP, Toren K, Burney P. Effect of passive smoking on respiratory symptoms, bronchial responsiveness, lung function, and total serum IgE in the European Community Respiratory Health Survey: a cross-sectional study. Lancet. 2001;358:2103–2109. doi: 10.1016/S0140-6736(01)07214-2. [DOI] [PubMed] [Google Scholar]
- 7.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, Mitchell EA, Pearce N, Sibbald B, Stewart AW, Strachan D, Weiland SK, Williams HC. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 8.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 9.Choi IS, Greville HW, Park KO. Addition of peak expiratory flow rate to the selection criteria of the representative spirometric result. Chonnam J Med Sci. 1990;3:23–28. [Google Scholar]
- 10.Crockcroft DW, Murdock KY, Berscheid BA. Relationship between atopy and bronchial responsiveness to histamine in a random population. Ann Allergy. 1984;53:26–29. [PubMed] [Google Scholar]
- 11.Chai H, Farr RS, Froehlich LA, Mathison DA, McLean JA, Rosenthal RR, Sheffer AL, Spector SL, Townley RG. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol. 1975;56:323–327. doi: 10.1016/0091-6749(75)90107-4. [DOI] [PubMed] [Google Scholar]
- 12.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 13.Yemaneberhan H, Bekele Z, Venn A, Lewis S, Parry E, Britton J. Prevalence of wheeze and asthma and relation to atopy in urban and rural Ethiopia. Lancet. 1997;350:85–90. doi: 10.1016/S0140-6736(97)01151-3. [DOI] [PubMed] [Google Scholar]
- 14.Strachen DP, Cook DG. Health effects of passive smoking. 6. Parental smoking and childhood asthma: longitudinal and case-control studies. Thorax. 1998;53:204–212. doi: 10.1136/thx.53.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz J, Timonen KL, Pekkanen J. Respiratory effects of environmental tobacco smoke in a panel study of asthmatic and symptomatic children. Am J Respir Crit Care Med. 2000;161:802–806. doi: 10.1164/ajrccm.161.3.9901002. [DOI] [PubMed] [Google Scholar]
- 16.Melsom T, Brinch L, Hessen JO, Schei MA, Kolstrup N, Jacobsen BK, Svanes C, Pandey MR. Asthma and indoor environment in Nepal. Thorax. 2001;56:477–481. doi: 10.1136/thorax.56.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng T, Niu S, Lu B, Fan X, Sun F, Wang J, Zhang Y, Zhang B, Owens P, Hao L, Li Y, Leaderer B. Childhood asthma in Beijing, China: a population-based case-control study. Am J Epidemiol. 2002;156:977–983. doi: 10.1093/aje/kwf127. [DOI] [PubMed] [Google Scholar]
- 18.Eisner MD, Yelin EH, Katz PP, Earnest G, Blanc PD. Exposure to indoor combustion and adult asthma outcomes: environmental tobacco smoke, gas stoves, and woodsmoke. Thorax. 2002;57:973–978. doi: 10.1136/thorax.57.11.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurukulaaratchy RJ, Matthews S, Waterhouse L, Arshad SH. Factors influencing symptom expression in children with bronchial hyperresponsiveness at 10 years of age. J Allergy Clin Immunol. 2003;112:311–316. doi: 10.1067/mai.2003.1623. [DOI] [PubMed] [Google Scholar]