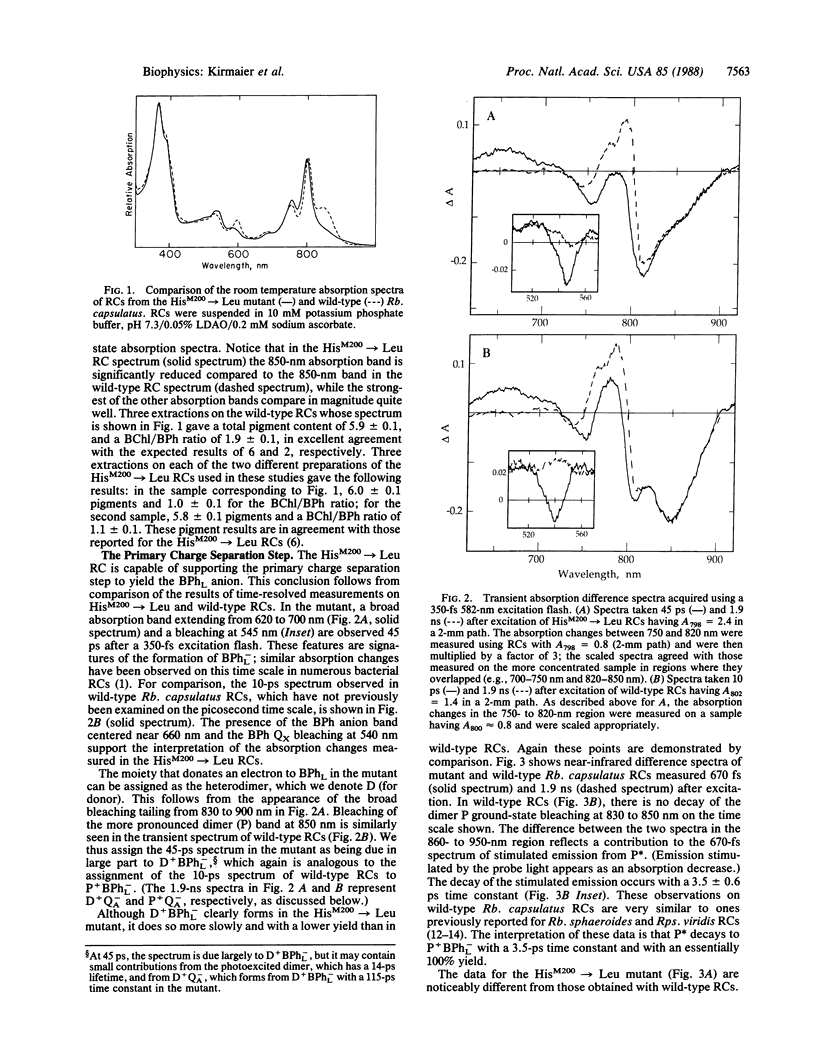
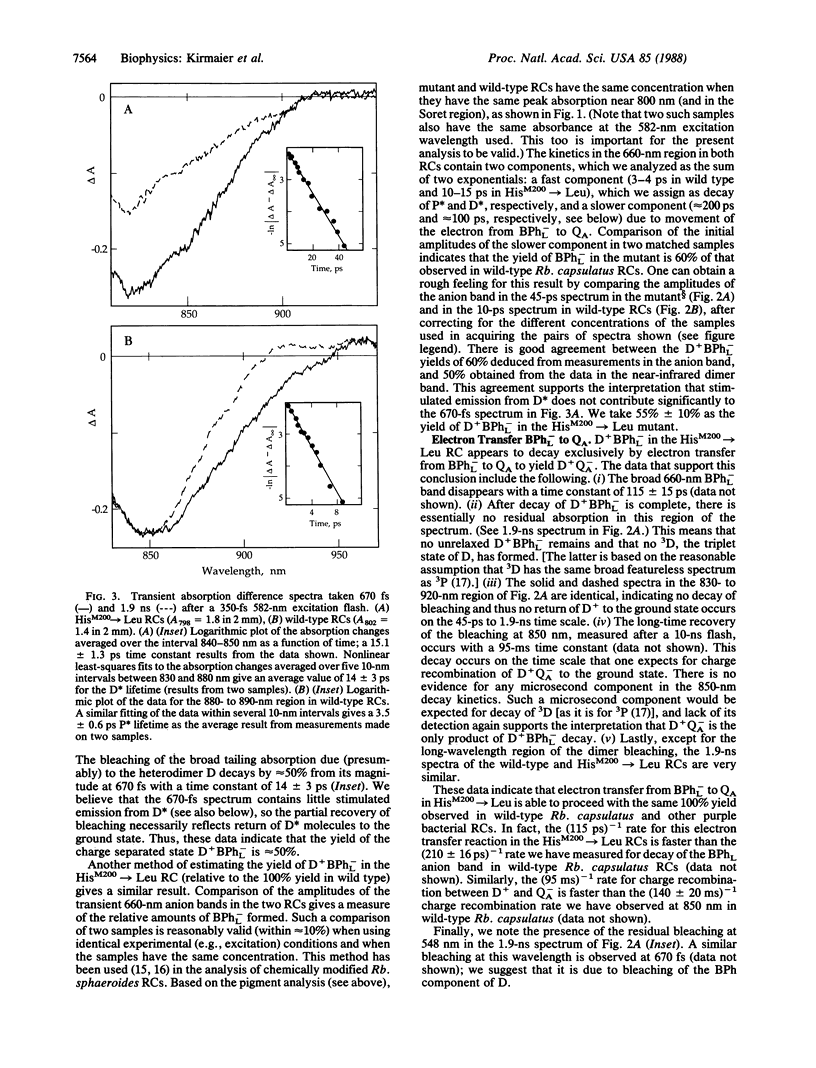
Abstract
Time-resolved optical measurements encompassing the femtoseconds to seconds time scales have been used to investigate Rhodobacter capsulatus reaction centers (RCs) in which the histidine residue at position 200 on the M polypeptide has been changed to a leucine by site-directed mutagenesis. The HisM200----Leu RC, which has a heterodimer consisting of a bacteriochlorophyll and a bacteriopheophytin, is capable of the primary photochemistry observed in wild-type Rb. capsulatus RCs, but with an overall quantum yield reduced by about half. The lower yield resides in the initial electron transfer reaction and may be associated in part with substantial charge transfer character of the excited heterodimer. These and other comparisons between Rb. capsulatus wild-type and HisM200----Leu RCs have important implications for our understanding of the mechanism of electron transfer in the RC and the efficiency of the charge separation process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G., Yeates T. O., Rees D. C., Deisenhofer J., Michel H., Huber R. Structural homology of reaction centers from Rhodopseudomonas sphaeroides and Rhodopseudomonas viridis as determined by x-ray diffraction. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8589–8593. doi: 10.1073/pnas.83.22.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylina E. J., Youvan D. C. Directed mutations affecting spectroscopic and electron transfer properties of the primary donor in the photosynthetic reaction center. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7226–7230. doi: 10.1073/pnas.85.19.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Tiede D., Tang J., Smith U., Norris J., Schiffer M. Structure of Rhodopseudomonas sphaeroides R-26 reaction center. FEBS Lett. 1986 Sep 1;205(1):82–86. doi: 10.1016/0014-5793(86)80870-5. [DOI] [PubMed] [Google Scholar]
- Fajer J., Brune D. C., Davis M. S., Forman A., Spaulding L. D. Primary charge separation in bacterial photosynthesis: oxidized chlorophylls and reduced pheophytin. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4956–4960. doi: 10.1073/pnas.72.12.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmaier C., Holten D., Debus R. J., Feher G., Okamura M. Y. Primary photochemistry of iron-depleted and zinc-reconstituted reaction centers from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6407–6411. doi: 10.1073/pnas.83.17.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lösche M., Feher G., Okamura M. Y. The Stark effect in reaction centers from Rhodobacter sphaeroides R-26 and Rhodopseudomonas viridis. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7537–7541. doi: 10.1073/pnas.84.21.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. L., Breton J., Hoff A. J., Migus A., Antonetti A. Femtosecond spectroscopy of electron transfer in the reaction center of the photosynthetic bacterium Rhodopseudomonas sphaeroides R-26: Direct electron transfer from the dimeric bacteriochlorophyll primary donor to the bacteriopheophytin acceptor with a time constant of 2.8 +/- 0.2 psec. Proc Natl Acad Sci U S A. 1986 Feb;83(4):957–961. doi: 10.1073/pnas.83.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson W. W., Clayton R. K., Cogdell R. J. Excited states of photosynthetic reaction centers at low recox potentials. Biochim Biophys Acta. 1975 May 15;387(2):265–278. doi: 10.1016/0005-2728(75)90109-7. [DOI] [PubMed] [Google Scholar]
- Straley S. C., Parson W. W., Mauzerall D. C., Clayton R. K. Pigment content and molar extinction coefficients of photochemical reaction centers from Rhodopseudomonas spheroides. Biochim Biophys Acta. 1973 Jun 28;305(3):597–609. doi: 10.1016/0005-2728(73)90079-0. [DOI] [PubMed] [Google Scholar]
- Van der Rest M., Gingras G. The pigment complement of the photosynthetic reaction center isolated from Rhodospirillum rubrum. J Biol Chem. 1974 Oct 25;249(20):6446–6453. [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Feher G. Primary structure of the reaction center from Rhodopseudomonas sphaeroides. Proteins. 1986 Dec;1(4):312–325. doi: 10.1002/prot.340010405. [DOI] [PubMed] [Google Scholar]
- Woodbury N. W., Becker M., Middendorf D., Parson W. W. Picosecond kinetics of the initial photochemical electron-transfer reaction in bacterial photosynthetic reaction centers. Biochemistry. 1985 Dec 17;24(26):7516–7521. doi: 10.1021/bi00347a002. [DOI] [PubMed] [Google Scholar]



