Abstract
Gastrointestinal stromal tumors (GISTs) typically occur late in life; however, there are also reports of these tumors in pediatric patients and young adults. This rare, but fascinating subset of GISTs, have distinct clinical-pathological and molecular deviations from their adult counterparts. The majority of pediatric GIST patients are female and often present with multi-focal tumors that are completely or partly epithelioid in nature. These young patients often have metastatic disease, but still follow a slow course of disease progression. Molecularly, most pediatric GISTs lack the gain-of-function mutations in the KIT and PDGFRA genes, which are commonly found in adult cases. Recent expression profiling and genomic studies of pediatric GISTs show distinct molecular signatures, suggesting a unique origin as compared to adult GISTs. We and others have shown that the insulin-like growth factor 1 receptor (IGF1R) may have a prominent role in driving those adult and pediatric GISTs that lack KIT and PDGFRA activating mutations and clinical trials are currently being designed to exploit these types of discoveries.
Keywords: GISTs, pediatric, young adult, imatinib mesylate
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the digestive tract, with an estimated annual occurrence of 3,300-6,000 in the United States [1]. GISTs were originally described by Mazur and Clark in 1983 based on the presence of smooth muscle and neural elements in these tumors [2], however unlike bona fide smooth muscle tumors, GISTs typically show only partial smooth muscle differentiation or lack it completely [3]. GISTs are believed to arise from the Interstitial Cells of Cajal (ICC) [4], the pacemaker cells of the gut, or from interstitial mesenchymal precursor stem cells [5] that also have the potential of giving rise to cells in the omentum and peritoneal surfaces. GISTs express and are clinically diagnosed by immunohistochemical staining of CD117, the 145 kDa transmembrane glycoprotein KIT. The combination of these histopathologic and immunohistochemical characteristics allows for a distinction to be made between GISTs and other soft tissue sarcomas, such as leiomyosarcomas and leiomyomas. The most common primary sites for these neoplasms are the stomach (60-70%) [1, 6], followed by the small intestine (25-35%) [1, 6, 7], and to a much lesser degree the colon and rectum (10%) [8]. GISTs have also been observed in the mesentery, omentum, esophagus, and the peritoneum [6, 9, 10].
The majority (~80%) of GISTs possess gain-of-function mutations in KIT in either exons 9, 11, 13 or 17, causing constitutive activation of the kinase receptor, whereas smaller subsets of GISTs possess either gain-of-function mutations in PDGFRA (exons 12, 14, or 18) (~5-8%) or no mutations in either KIT or PDGFRA and are commonly referred to as wild-type (WT) GISTs (~12-15%) [1, 11]. The primary treatment for GIST is surgical resection, which is often not curative in high risk GIST due to a high incidence of reoccurrence [12, 13]. Since 2002, imatinib mesylate (IM), an oral 2-phenylaminopyrimidine derivative that works as a selective inhibitor against mutant forms of type III tyrosine kinases such as KIT, PDGFRA, and BCR/ABL, has become a standard treatment for patients with metastatic and/or unresectable GIST, with objective responses or stable disease obtained in >80% of patients [13]. Response to IM has been correlated to the genotype of a given tumor [14]. GIST patients with exon 11 KIT mutations have the best overall response and longest disease-free survival interval, while other KIT mutation types and WT GIST have worse prognoses.
The majority of GISTs present later in life with a median age at presentation of ~sixty-one years; however, GISTs have also been observed in the pediatric population (~1-2%). Regardless of the rarity of pediatric GISTs, they are an extremely interesting entity to study because they have distinct clinical-pathological and molecular deviations from adult GISTs. Molecular genetic studies of pediatric GISTs are underway and should help to further elucidate the pathogenesis of these tumors and provide additional insights into the origins of adult onset GISTs.
Clinical-Pathological Characteristics of Pediatric GISTs
To date, approximately 150 reported pediatric (<18 years) and young adult (<30 years) GIST cases have been reported in the last forty years. Until about three years ago, the literature mostly consisted of case reports describing single or small series of pediatric GIST cases; however, recent publications by Miettinen et al. (2005) [15], Prakash et al. (2005) [16], Janeway et al. (2007) [17], and Agaram et al. (2008) [18] have expanded the number of diagnosed cases. It is now clear that GISTs that occur in the pediatric population are distinct in both clinical and molecular aspects from their adult counterparts.
The most frequently cited presenting symptom of pediatric GIST patients is severe chronic anemia (hemoglobin: <8 g/dL) and associated symptoms such as paleness, fatigue and vertigo. Additional symptoms include abdominal pain, vomiting and abdominal distention. GISTs in pediatric and young adults have a definite predilection towards females. Of all reported cases approximately seventy-five percent were females, in contrast to the literature on adult GISTs where there are reports of male predominance [19, 20] and other studies showing an equal male to female ratio [21, 22]. In addition, the majority of female pediatric GISTs exhibit either purely epithelioid or epithelioid/spindle mixed cytology, in comparison to adult counterparts that typically have spindle cell morphology. Interestingly, pediatric GISTs from males do not seem to exhibit this propensity towards epithelioid cell morphology [18, 23]. Immunohistochemical (IHC) analyses have shown that all of the pediatric GISTs examined are positive for CD117 (KIT), while other markers such as CD34, DOG1, vimentin and smooth muscle actin (SMA) are also frequently found in these tumors (>80%, >80%, >65%, and >40%, respectively) [15, 16, 24-26]. The majority of pediatric tumors tested by IHC were negative for S-100, desmin, neuron-specific enolase (NSE) and cytokeratins.
The majority of pediatric GISTs tend to arise in the stomach. The exact frequency is difficult to determine because some of the studies describing large samples of pediatric GISTs included only GISTs of gastric origin. However, it appears that the ratio of gastric pediatric GISTs to those located in other sites (e.g., bowel, omentum, colon) ranges between 2:1 and 6:1. Furthermore, many of these gastric tumors present with multifocal nodular growth [16, 18, 27-31], which is rarely seen in adult sporadic GIST. These GISTs typically present as a single primary solid tumor. There also seems to be a higher frequency of metastases in pediatric GISTs as compared to adult GISTs [16, 18, 29]. However, the risk of developing metastatic disease in children is difficult to predict based on the standard criteria of mitotic index, tumor site, and tumor size [15]. Interestingly, this increased rate of metastasis does not translate to poorer prognoses as seen in adult cases. Many pediatric GISTs, even those with metastases, follow an indolent course clinically and have favorable long-term prognoses [15, 18].
GISTs in young adults (defined as >17 - <30 years) appear to be a more heterogeneous subset of GISTs, some resembling pediatric GISTs and some having characteristics more in common with late onset GISTs. For example, Miettinen's study of 44 pediatric and young adult GIST cases reported only 6/31 (19%) were males in the pediatric group whereas 6/13 (46%) of the young adult GISTs were males [15]. In addition, there were a higher percentage of tumors with purely spindle cytology in the young adult subset in comparison to the pediatric group. In the subset of young adult cases they described, both Prakash and Agaram found a greater proportion of male patients presenting with non-gastric tumors having spindle cell morphology, as well as a lower incidence of multi-focal tumors, as compared to the group of pediatric patients [16, 18].
Carney Triad
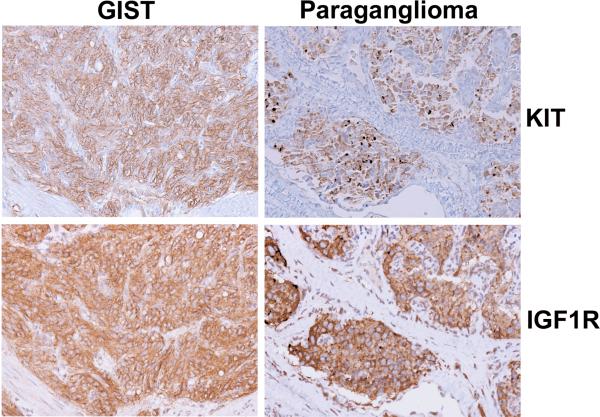
The aforementioned hallmarks of pediatric GISTs, such as early onset, female predilection and presence of multifocal disease, prompted the search for association to other syndromes such as familial GIST syndrome, neurofibromatosis type I (NF1), and Carney's triad. Familial GIST syndrome can be ruled out because it is associated with inherited germline mutations in KIT or PDGFRA [11] and the majority of pediatric cases are WT. In addition, pediatric GISTs probably are not associated with NF1. GISTs that arise as part of this syndrome, though typically multifocal with WT KIT/PDGFRA genotypes, also tend to arise in the bowel and do not typically metastasize [32]. However, an association between pediatric GISTs and GISTs of the Carney triad has been reported [15, 18, 24, 25, 33]. This syndrome, originally described by Carney in 1977, comprises the triad of GIST, paraganglioma, and pulmonary chondromas [34]. This is an extremely rare syndrome with fewer than 30 complete cases (all three tumors diagnosed in the same individual) and 100 incomplete cases (diagnoses of two of three tumors) reported. Approximately 85% of Carney triad patients are female with onset in the majority of patients before the age of thirty. The GIST component of the triad predominately arises in the stomach and these tumors typically lack detectable KIT and PDGFRA mutations. In addition, this disorder displays a chronic yet indolent course [35]. Therefore, the similarities between sporadic pediatric GISTs and those occurring as part of the Carney triad are clear and making a distinction between the two can be quite difficult given that other components of the triad most often do not present until several years later. IHC analysis, performed in our laboratory, shows strong KIT expression in a primary GIST and a subsequent paraganglioma from a pediatric patient diagnosed with Carney's triad (Figure 1 top, left and right panels). This is in agreement with a previous report showing expression of KIT in both the GIST and paraganglioma samples from a Carney triad patient [36], although others contradict this observation [24].
Figure 1. Immunohistochemical Analysis of Tumor Tissues from a Carney Triad Patient.
Expression of IGFIR (left panels) and KIT (right panels) in GIST tissue (top panels) and paraganglioma tissue (bottom panels) from a pediatric patient diagnosed with incomplete Carney triad.
Molecular Characteristics of Pediatric GISTs
Contrary to most adult GISTs that harbor KIT or PDGFRA mutations, approximately 90% of the pediatric tumors are wild-type for both receptors. Currently, only six pediatric GIST cases with a KIT or PDGFRA mutation have been reported [17, 18, 25, 27]. For those patient in which the gender was reported 3 of the 4 were males. When combined with a higher frequency of spindled tumors in male versus female pediatric GISTs, these observations points to a potential difference in the pathogenesis between male and female pediatric tumors. The first reported KIT mutation in a pediatric GIST was a novel homozygous point mutation in exon 9 that results in an amino acid change at codon 456 [25]. Of the five remaining cases, mutations in KIT exon 11 were found in two instances, and in the other three cases PDGFRA exon 18 mutations were identified. The frequency of mutations found in the young adult population appears to be greater than in the pediatric subset [16], but lower than in the adult population, again pointing to the mixed nature of this group. A current focus in this field is to search for genetic determinants of GIST pathogenesis, other than KIT or PDGFRA, in order to gain insights into these tumors and to provide additional therapeutic options for these young patients, of which there are very few. To this end, the BRAF serine/threonine-protein kinase proto-oncogene, which has been implicated in various cancers, including non-Hodgkin lymphoma, colorectal cancer, malignant melanoma, thyroid carcinoma, nonsmall cell lung carcinoma, and adenocarcinoma of lung, was not found to be mutated in a small number of pediatric GISTs [18]. Interestingly, we have recently found that BRAF may be mutated in a low percentage of adult WT GISTs (Rink & Godwin, unpublished data).
Importantly, although the majority of pediatric GISTs lack activating mutations in either KIT or PDGFRA, they express KIT, suggesting a common precursor cell for all GISTs. Janeway and colleagues recently examined KIT signaling in WT pediatric tumors, mutant pediatric GISTs and mutant adult GISTs and found that KIT was activated in all but one WT sample. This is of interest because these tumors lack the typical gain of function mutation in KIT or PDGFRA that leads to the constitutive activation of the receptor. The authors suggest a number of potential mechanisms that may be responsible, including overexpression of the KIT ligand stem cell factor (SCF) creating an autocrine loop, impaired degradation of the receptor, a mutation in a phosphatase, or the presence of a KIT/RTK heterooligomer that could cause crossphosphorylation. These are all plausible theories that merit further investigation. Downstream effectors of KIT such as AKT and MAPK were also activated in all samples to varying degrees with the exception of one WT sample that did not show any phosphorylated MAPK, suggestion heterogeneity in signaling. However, searches for other activated RTKs found no evidence of other dominantly activated pathways. Agaram et al. recently reported similar findings on a small series of WT pediatric GIST extracts, i.e., all five tumors displayed consistent activation of KIT and its downstream signaling effectors, AKT, PDK1, mTOR, S6 kinase, and ribosomal S6 [18]. However, MAPK was only activated in three of the five samples. In addition, no activation of PDGFRA or PDGFRB was shown in any of the WT pediatric samples, pointing towards KIT as the major activated RTK in these tumors. The authors compared the five WT pediatric tumors to WT and mutant adult GISTs and ultimately discovered no significant differences in the activation of KIT or any of its downstream effectors. Agaram et al. also utilitzed a phospho-RTK array to screen for alternative RTKs activated in pediatric and young adult WT GISTs. In addition to activated KIT, the epidermal growth factor receptor (EGFR) was also found to be weakly phosphorylated. Mutational analysis of EGFR was performed in these samples and no mutations were found.
Two additional studies have attempted to further characterize pediatric GISTs using expression profiling [16, 18]. In 2005 Prakash and colleagues performed gene expression analysis on seven gastric samples: five from two pediatric WT patients and two from different young adult patients (1 mutant, 1 WT) and compared these to ten gastric adult GISTs (9 mutant, 1 WT). Although the sample sizes were small, the authors reported that all of the pediatric and young adult samples, with the exception of one pediatric sample, clustered together and were distinct from the adult GISTs, suggesting major molecular differences between these two GIST subsets. 385 genes were determined to be differentially expressed (> 2 fold change) between the two groups. Genes upregulated in the pediatric and young adult group included neuroligin 4 (NLGN4), ankyrin 3 (ANK3), frizzled 2 (FZD2), insulin-like growth factor receptor type 1 (IGF1R) and phosphorylase kinase alpha 1 (PHKA1). Genes that were significantly downregulated included dermatopontin (DPT), PDGFRA, RAS-family member, RAB38, and G-protein-coupled receptor 88 (GPR88). These results point to a possible genotype/phenotype correlation, since the pediatric/young adult group consisted of mostly WT tumors while the adult group were mutant tumors.
More recently, Agaram and colleagues used microarray analysis to compare gene expression in thirteen tumor nodule samples from eight WT pediatric GIST patients (2 within the Carney Triad) to five adult WT GIST samples. Again, the pediatric group formed a tight cluster that segregated from the adult group. In this study a total of 1,532 genes were found to be differentially expressed (> 2 fold change) between the two groups. To help rule out the possibility that the expression profiles were biased because all samples in the pediatric group were gastric, a second analysis was performed comparing the pediatric WT group to a group of adult gastric GISTs with varying genotype (3 WT, 4 PDGFRA mutants, 12 KIT mutants). This analysis yielded 1,335 differentially expressed genes (> 2 fold change), 814 of which were in common with the first analysis. The genes with significantly different expression in the pediatric group as compared to the adult tumors included FGF4* (fibroblast growth factor 4), BAALC* (brain and acute leukemia, cytoplasmic), IGF1R*, NELL1* (NEL-like 1), CRLF1 cytokine receptor-like factor 1), PLAG1* (pleomorphic adenoma gene 1) and FGF3 (fibroblast growth factor 3). Differential expression for five of these (denoted with *). was confirmed by qPCR. The only common gene to show up in both studies was IGF1R, which has been of great interest to our research group. Although our work has not focused exclusively on pediatric GISTs, we have performed extensive studies on WT adult GISTs and were the first to report that IGF1R mRNA and protein is frequently overexpressed in WT adult and pediatric GISTs as compared with mutant GISTs [37, 38, 39]. For example, IHC analysis on a primary GIST and a paraganglioma from the Carney triad patient shows strong IGF1R expression (Figure 1 bottom, left and right panels). In addition, using FISH analysis and a genomic-based quantitative PCR assay, amplification of IGF1R was more frequently detected in WT samples (including the pediatric Carney Triad case) as compared to KIT or PDGFRA-mutated samples. With respect to IGF1R and its downstream signaling molecules, we showed that the small molecule tyrosine kinase inhibitor, NVP-AEW541 (Novartis), which has activity against IGF1R can lead to cytotoxicity in mutant GIST cell lines, via AKT and MAPK signaling that is independent from KIT signaling. Similar findings were observed when IGF1R levels were impaired using targeted siRNAs. We have observed additive effects by combining NVP-AEW541 and IM, suggesting a potential therapeutic benefit in targeting IGF1R in GISTs that are unresponsive to IM, including pediatric GISTs which overexpress IGF1R as well as combination therapies in all tumors.
Even though mutational activation of KIT or PDGFRA is essential to the pathogenesis of the vast majority of GISTs, other genetic and cytogenetic changes have been implicated in the progression to malignancy and metastasis. Specific chromosomal aberrations, such as losses in chromosomes 1p, 14q, and 22q, are commonly seen in GISTs regardless of tumor risk, while other changes (e.g. losses of 9p and 13q, gains in 5p, 8q, 17q, and 20q) are more commonly seen in malignant and/or metastatic GISTs. A recent study examined the cytogenetic profiles of pediatric GISTs using a low-density SNP (single nucleotide polymorphism) array [17]. In the three adult mutant cases the authors detected common chromosomal aberrations seen in GISTs, including LOH at 1p as well as losses at 14q and 22q, with various additional changes (gains/losses). Similar to the adult mutant GISTs, the pediatric mutant GIST samples showed the typical changes described previously as well as multiple additional regions of aberration. Importantly, the SNP analysis on the thirteen pediatric WT samples revealed few cytogenetic changes. None of these pediatric WT samples displayed the expected GIST aberrations, and only three of these cases displayed large chromosomal regions of LOH or copy number change. Two of these had only one area of LOH (5p and 11q) and the third pediatric WT case had multiple regions of LOH (1p, 3q, 5q, and chromosomes 13 and 18). This observation is fascinating considering these tumors were all described as high grade, tumors which would be expected to contain a number of large-scale chromosomal changes. This may be one of the first reports of a clinically aggressive solid tumor that displays genomic stability and suggests that other mechanisms such as epigenetic changes (e.g., overexpression of IGF1R) or focal regions of amplification/deletions may be involved in the pathogenesis of pediatric disease.
Treatment of Pediatric GISTs
The primary treatment for pediatric GISTs remains surgical resection of the tumor with testing and removal of involved lymph nodes, followed by postoperative testing utilizing CT and PET [18F] fluorodeoxyglucose-positron emission tomography) scans monitoring for reoccurrence. In addition, a number of recent reports have described the use of IM and sunitinib in pediatric GIST patients [16, 18, 23, 24, 27, 33, 40, 41]. Sunitinib is a small molecule multi-targeted RTK inhibitor with selectivity against FLT3, KIT, PDGFR and VEGFR that has been used to treat IM-refractory adult GISTs. A recent Children's Oncology Group (COG) phase II study of IM in children with refractory or relapsed solid tumors showed that IM as a single agent (440 mg/m2/day) demonstrated little or no activity in seventy children with refractory or relapsed Ewing sarcoma, osteosarcoma, neuroblastoma, desmoplastic small round cell tumors and GIST. Of all participants only one partial response was observed, in a child with Ewing sarcoma. Agaram and colleagues described seven (six metastatic, one as adjuvant therapy) pediatric GISTs treated with IM for 3 to 18 months (median 5 months). One patient had stable disease (9 month follow-up), four patients had no response and the last patient showed mixed response with some nodules stable and others that progressed albeit at a reduced rate. The patient in the adjuvant setting was treated for 24 months, but as soon as treatment was stopped the tumor reoccurred. In addition, the authors noted side effects of the drug in two of the seven patients, including severe muscle, joint, and/or bone pain that ultimately led to halting of the therapy. In addition, the authors report a description of four patients treated with sunitinib, who first had progressed on IM or who stopped IM treatment due to severe toxicities. Of these four, two patients developed intolerance to the drug and were discontinued from treatment, one patient showed stable disease after eight months of treatment (25 mg/d), and the fourth patient went through five cycles of sunitinib with dose escalation (up to 50 mg/d), but eventually developed disease progression. The latter patient was switched to nilotinib which resulted in stable disease for an additional nine months, at which time nilotinib was discontinued due to unexplained necroses of the bowel. The remaining reports in the literature regarding IM treatment in pediatric GISTs are mixed: three children either progressed or had no response while on IM therapy and three children showed stable disease or partial response with ≤12 month follow-up [16, 24, 30, 33, 40]. In addition, Prakash et al. reported findings from four young adult patients with recurrent disease treated with IM. Three of these patients exhibited stable disease for five to twelve months while one patient discontinued treatment due to drug toxicities. A 2006 ASCO abstract reported the successful use of sunitinib in three IM-refractory metastatic pediatric GIST patients [42]. After 5 cycles of sunitinib treatment, all of the tumors were either stable or regressed as evaluated by CT scan, and in one patient two lung nodules had a complete regression However, it is becoming increasingly evident that these small molecule inhibitors are not having the desired clinical benefit and that additional targets, such as IGF1R, will need to be explored in the treatment of pediatric patients.
Conclusion
In summary, GISTs are the most common mesenchymal tumor of the digestive tract. Late onset tumors tend to possess mutations in either KIT or PDGFRA while the driving force in the pathogenesis of most early-onset GISTs is unknown. For patients with localized resectable GIST, cytoreductive surgery remains the primary option. This surgery seeks to remove the entire gross tumor, and frequently requires total or subtotal organ resection, depending on tumor location and size. However, surgery has limited success for locally recurrent or metastatic GIST and clinical response of young patients to systemic therapy using novel biologics such as imatinib mesylate and sunitinib is abysmal. Recent advancement in the molecular characterization of pediatric GISTs has lead to the discovery of other pathways that may be important in this disease. Clinical trials are currently being designed and implemented to target some of these pathways. Only time will tell if these new therapeutic approaches will have the type of impact seen in adult patients when first treated introduced to imatinib.
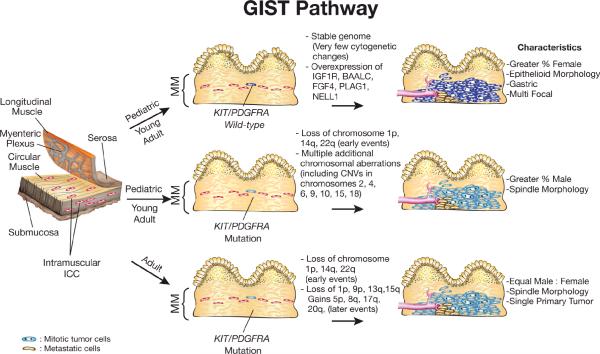
Figure 2. Genetic and Cytogenetic Changes Associated with Pathogenesis of Pediatric and Adult GISTs.
GISTs are thought to arise from the Interstitial Cells of Cajal (ICC), the pacemaker cells of the gut, or from interstitial mesenchymal precursor stem cells that also have the potential of giving rise to cells in the omentum and peritoneal surfaces. Pediatric GISTs are thought to develop independent from acquired mutations in KIT or PDGFRA. These tumors are frequently associated with increased expression of IGF1R, BAALC, FGF4, PLAG1 and NEL1. Adult GISTs and for some young adult GISTs develop as a result of an acquired mutation in KIT or PDGFRA and a series of sequential genetic and genomic abnormalities. Loss of chromosome 1p and monosomy 14 and 22 are two of the earliest cytogenetic changes that have been detected cytogenetically. MM - Muscularis Mucosae, CNVs - copy number variants. (Illustration by Karen Trush)
Acknowledgments
Grant supports: This work was supported in part by NIH grants (CA106588 and a supplement to U10 CA21661) to A.K.G., an award by the FCCC Translational Research Committee to A.K.G as part of the FCCC core grant (P30 CA006927), an NIH Training Grant (Institutional NRSA) Appointment (CA009035-31) to L.R, and by Tania Stutman and the GIST Cancer Research Fund.
References
- 1.Corless CL, Heinrich MC. Molecular Pathobiology of Gastrointestinal Stromal Sarcomas. Annu Rev Pathol. 2007;3:557–586. doi: 10.1146/annurev.pathmechdis.3.121806.151538. [DOI] [PubMed] [Google Scholar]
- 2.Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7(6):507–19. doi: 10.1097/00000478-198309000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Rubin BP. Gastrointestinal stromal tumours: an update. Histopathology. 2006;48(1):83–96. doi: 10.1111/j.1365-2559.2005.02291.x. [DOI] [PubMed] [Google Scholar]
- 4.Sircar K, Hewlett BR, Huizinga JD, et al. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999;23(4):377–89. doi: 10.1097/00000478-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152(5):1259–69. [PMC free article] [PubMed] [Google Scholar]
- 6.El-Rifai W, Sarlomo-Rikala M, Andersson LC, et al. DNA sequence copy number changes in gastrointestinal stromal tumors: tumor progression and prognostic significance. Cancer research. 2000;60(14):3899–903. [PubMed] [Google Scholar]
- 7.Tworek JA, Appelman HD, Singleton TP, et al. Stromal tumors of the jejunum and ileum. Mod Pathol. 1997;10(3):200–9. [PubMed] [Google Scholar]
- 8.Tworek JA, Goldblum JR, Weiss SW, et al. Stromal tumors of the anorectum: a clinicopathologic study of 22 cases. Am J Surg Pathol. 1999;23(8):946–54. doi: 10.1097/00000478-199908000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Miettinen M, Monihan JM, Sarlomo-Rikala M, et al. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol. 1999;23(9):1109–18. doi: 10.1097/00000478-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Fang FC, Tzao C, Cheng YL, et al. Surgical treatment of gastrointestinal stromal tumor in the esophagus: report of three cases. Zeitschrift fur Gastroenterologie. 2007;45(12):1252–6. doi: 10.1055/s-2007-963428. [DOI] [PubMed] [Google Scholar]
- 11.Tarn C, Merkel E, Canutescu AA, et al. Analysis of KIT mutations in sporadic and familial gastrointestinal stromal tumors: therapeutic implications through protein modeling. Clin Cancer Res. 2005;11(10):3668–77. doi: 10.1158/1078-0432.CCR-04-2515. [DOI] [PubMed] [Google Scholar]
- 12.DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231(1):51–8. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 14.Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42(8):1093–103. doi: 10.1016/j.ejca.2006.01.030. [DOI] [PubMed] [Google Scholar]
- λ15.Miettinen M, Lasota J, Sobin LH. Gastrointestinal stromal tumors of the stomach in children and young adults: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases with long-term follow-up and review of the literature. Am J Surg Pathol. 2005;29(10):1373–81. doi: 10.1097/01.pas.0000172190.79552.8b. [DOI] [PubMed] [Google Scholar]; A study of 44 pediatric and young adult GIST cases describing the following distinct characteristics in the majority of these tumors: female-bias, gastric antrum location, epithelioid cytology, no mutations in KIT or PDGFRA, and unpredictable but slow rate of progression
- 16.Prakash S, Sarran L, Socci N, et al. Gastrointestinal stromal tumors in children and young adults: a clinicopathologic, molecular, and genomic study of 15 cases and review of the literature. J Pediatr Hematol Oncol. 2005;27(4):179–87. doi: 10.1097/01.mph.0000157790.81329.47. [DOI] [PubMed] [Google Scholar]
- λλ17.Janeway KA, Liegl B, Harlow A, et al. Pediatric KIT wild-type and plateletderived growth factor receptor alpha-wild-type gastrointestinal stromal tumors share KIT activation but not mechanisms of genetic progression with adult gastrointestinal stromal tumors. Cancer research. 2007;67(19):9084–8. doi: 10.1158/0008-5472.CAN-07-1938. [DOI] [PubMed] [Google Scholar]; The majority of pediatric WT GISTs progress to malignancy without acquiring large-scale chromosomal aberrations commonly seen in KIT/PDGFRA mutant GISTs
- λλ18.Agaram NP, Laquaglia MP, Ustun B, et al. Molecular characterization of pediatric gastrointestinal stromal tumors. Clin Cancer Res. 2008;14(10):3204–15. doi: 10.1158/1078-0432.CCR-07-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pediatric GISTs show distinct transcriptional signatures, suggesting a unique biologic origin as compared to adult GISTs
- 19.Orosz Z, Tornoczky T, Sapi Z. Gastrointestinal stromal tumors: a clinicopathologic and immunohistochemical study of 136 cases. Pathol Oncol Res. 2005;11(1):11–21. doi: 10.1007/BF03032400. [DOI] [PubMed] [Google Scholar]
- 20.Rauf F, Bhurgri Y, Pervez S. Gastrointestinal stromal tumors: a demographic, morphologic and immunohistochemical study. Indian J Gastroenterol. 2007;26(5):214–6. [PubMed] [Google Scholar]
- 21.Koay MH, Goh YW, Iacopetta B, et al. Gastrointestinal stromal tumours (GISTs): a clinicopathological and molecular study of 66 cases. Pathology. 2005;37(1):22–31. doi: 10.1080/00313020400023628. [DOI] [PubMed] [Google Scholar]
- 22.Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22(18):3813–25. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 23.Cypriano MS, Jenkins JJ, Pappo AS, et al. Pediatric gastrointestinal stromal tumors and leiomyosarcoma. Cancer. 2004;101(1):39–50. doi: 10.1002/cncr.20352. [DOI] [PubMed] [Google Scholar]
- 24.Sauseng W, Benesch M, Lackner H, et al. Clinical, radiological, and pathological findings in four children with gastrointestinal stromal tumors of the stomach. Pediatric hematology and oncology. 2007;24(3):209–19. doi: 10.1080/08880010601104687. [DOI] [PubMed] [Google Scholar]
- 25.Price VE, Zielenska M, Chilton-MacNeill S, et al. Clinical and molecular characteristics of pediatric gastrointestinal stromal tumors (GISTs) Pediatric blood & cancer. 2005;45(1):20–4. doi: 10.1002/pbc.20377. [DOI] [PubMed] [Google Scholar]
- 26.Liegl B, Hornick JL, Corless CL, et al. Monoclonal Antibody DOG1.1 Shows Higher Sensitivity Than KIT in the Diagnosis of Gastrointestinal Stromal Tumors, Including Unusual Subtypes. Am J Surg Pathol. 2008 Nov 13; doi: 10.1097/PAS.0b013e318186b158. [DOI] [PubMed] [Google Scholar]
- 27.Kuroiwa M, Hiwatari M, Hirato J, et al. Advanced-stage gastrointestinal stromal tumor treated with imatinib in a 12-year-old girl with a unique mutation of PDGFRA. Journal of pediatric surgery. 2005;40(11):1798–801. doi: 10.1016/j.jpedsurg.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 28.O'Sullivan MJ, McCabe A, Gillett P, et al. Multiple gastric stromal tumors in a child without syndromic association lacks common KIT or PDGFRalpha mutations. Pediatr Dev Pathol. 2005;8(6):685–9. doi: 10.1007/s10024-005-0083-y. [DOI] [PubMed] [Google Scholar]
- 29.Li P, Wei J, West AB, et al. Epithelioid gastrointestinal stromal tumor of the stomach with liver metastases in a 12-year-old girl: aspiration cytology and molecular study. Pediatr Dev Pathol. 2002;5(4):386–94. doi: 10.1007/s10024-001-0250-8. [DOI] [PubMed] [Google Scholar]
- 30.Muniyappa P, Kay M, Feinberg L, et al. The endoscopic appearance of a gastrointestinal stromal tumor in a pediatric patient. Journal of pediatric surgery. 2007;42(7):1302–5. doi: 10.1016/j.jpedsurg.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 31.Kerr JZ, Hicks MJ, Nuchtern JG, et al. Gastrointestinal autonomic nerve tumors in the pediatric population: a report of four cases and a review of the literature. Cancer. 1999;85(1):220–30. doi: 10.1002/(sici)1097-0142(19990101)85:1<220::aid-cncr30>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 32.Andersson J, Sihto H, Meis-Kindblom JM, et al. NF1-associated gastrointestinal stromal tumors have unique clinical, phenotypic, and genotypic characteristics. Am J Surg Pathol. 2005;29(9):1170–6. doi: 10.1097/01.pas.0000159775.77912.15. [DOI] [PubMed] [Google Scholar]
- 33.Delemarre L, Aronson D, van Rijn R, et al. Respiratory symptoms in a boy revealing Carney triad. Pediatric blood & cancer. 2008;50(2):399–401. doi: 10.1002/pbc.21071. [DOI] [PubMed] [Google Scholar]
- 34.Carney JA, Sheps SG, Go VL, et al. The triad of gastric leiomyosarcoma, functioning extra-adrenal paraganglioma and pulmonary chondroma. N Engl J Med. 1977;296(26):1517–8. doi: 10.1056/NEJM197706302962609. [DOI] [PubMed] [Google Scholar]
- 35.Carney JA. Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney Triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clinic proceedings. 1999;74(6):543–52. doi: 10.4065/74.6.543. [DOI] [PubMed] [Google Scholar]
- 36.Horenstein MG, Hitchcock TA, Tucker JA. Dual CD117 expression in gastrointestinal stromal tumor (GIST) and paraganglioma of Carney triad: a case report. International journal of surgical pathology. 2005;13(1):87–92. doi: 10.1177/106689690501300113. [DOI] [PubMed] [Google Scholar]
- λλ37.Tarn C, Rink L, Merkel E, et al. Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(24):8387–92. doi: 10.1073/pnas.0803383105. [DOI] [PMC free article] [PubMed] [Google Scholar]; IGF1R was found to be amplified and the protein overexpressed in WT adult and pediatric GISTs. Aberrent expression of IGF1R may be associated with oncogenesis in WT GISTs and suggests an alternative and/or complementary therapeutic regimen in the clinical management of all GISTs, especially in a subset of tumors that respond less favorably to imatinib-based therapies
- 38.Belinsky MG, Rink L, Cai KQ, et al. The insulin-like growth factor system as a potential therapeutic target in gastrointestinal stromal tumors. Cell cycle. 2008;7(19):2949–55. doi: 10.4161/cc.7.19.6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- λλ39.Godwin AK, Rink L, Chi T, et al. Insulin-like growth factor 1 receptor (IGF-1R): A potential therapeutic target for gastrointestinal stromal tumors (GIST) [ASCO abstract] J Clin Oncol. 2008;26:10507. [Google Scholar]; First report of aberrant IGF1R expressed in WT adult and pediatric GISTs
- 40.Bond M, Bernstein ML, Pappo A, et al. A phase II study of imatinib mesylate in children with refractory or relapsed solid tumors: a Children's Oncology Group study. Pediatric blood & cancer. 2008;50(2):254–8. doi: 10.1002/pbc.21132. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi Y, Okazaki T, Yamataka A, et al. Gastrointestinal stromal tumor in a child and review of the literature. Pediatric surgery international. 2005;21(11):914–7. doi: 10.1007/s00383-005-1511-9. [DOI] [PubMed] [Google Scholar]
- 42.Janeway KA, Matthews DC, Butrynski JE, et al. Sunitinib treatment of pediatric metastatic GIST after failure of imatinib [ASCO abstract] J. Clin Oncol. 2006;24(18S suppl):9519. [Google Scholar]