Abstract
Language and communication deficits are among the core features of autism spectrum disorder (ASD). Reduced or reversed asymmetry of language has been found in a number of disorders, including ASD. Studies of healthy adults have found an association between language laterality and anatomical measures but this has not been systematically investigated in ASD. The goal of this study was to examine differences in gray matter volume of perisylvian language regions, connections between language regions, and language abilities in individuals with typical left lateralized language compared to those with atypical (bilateral or right) asymmetry of language functions. 14 adolescent boys with ASD and 20 typically developing adolescent boys participated, including equal numbers of left- and right-handed individuals in each group. Participants with typical left lateralized language activation had smaller frontal language region volume and higher fractional anisotropy of the arcuate fasciculus compared to the group with atypical language laterality, across both ASD and control participants. The group with typical language asymmetry included the most right-handed controls and fewest left-handers with ASD. Atypical language laterality was more prevalent in the ASD than control group. These findings support an association between laterality of language function and language region anatomy. They also suggest anatomical differences may be more associated with variation in language laterality than specifically with ASD. Language laterality therefore may provide a novel way of subdividing samples, resulting in more homogenous groups for research into genetic and neurocognitive foundations of developmental disorders.
Keywords: autism, language, asymmetry, MRI, DTI, handedness, Broca’s area, planum temporale, arcuate fasciculus
INTRODUCTION
The left hemisphere plays a predominant role relative to the right in language functions in more than 95% of right-handed healthy individuals (see Foundas, 2001; Pujol et al., 1999; Springer et al., 1999). Language laterality has been examined in autism spectrum disorder (ASD), with a number of studies showing reduced left lateralization of language functions in this population. For example, several positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) studies have demonstrated decreased left lateralization of activation in autism compared to controls during auditory language processing (Boddaert et al., 2003; 2004; Gervais et al., 2004; Müller et al., 1998; 1999; Redcay & Courchesne, 2008). Similarly, Dawson and colleagues examined averaged cortical evoked responses to linguistic auditory stimuli and found reversed (right greater than left) asymmetry in children with ASD compared to typically developing children (Dawson et al., 1986; 1989). Flagg and his colleagues used magnetoencephalography (MEG) to measure activation during auditory vowel processing. They found an increase in leftward asymmetry with age in controls. In contrast, there was an increased rightward asymmetry with age in the ASD group (Flagg et al., 2005). fMRI studies using language tasks have found similar results when frontal regions were examined, with decreased left lateralization or right hemisphere dominance in individuals with ASD relative to controls (Kleinhans et al., 2008; Knaus et al., 2008; Takeuchi et al., 2004).
Atypical asymmetry of language functions is not specific to ASD. Reduced left lateralization or rightward asymmetry of language functions have also been reported in healthy left-handers (see Geschwind & Galaburda, 1985; Jorgens et al., 2007; Knecht et al., 2000; Pujol et al., 1999; Szaflarski et al., 2002; Tzourio et al., 1998a) and in a number of other disorders, including developmental stuttering, dyslexia, specific language impairment, attention deficit hyperactivity disorder (ADHD), and schizophrenia (Blomgren et al., 2003; Geschwind & Galaburda, 1985; Pecini et al., 2005; Wehner et al., 2007). These complex neurodevelopmental disorders, which share increased rates of atypical lateralization of language, all have heterogeneous co-morbid behavioral characteristics, with overlapping subgroups often identified. For example, within specific language impairment there are subgroups with co-morbid ADHD or dyslexia (Pennington & Bishop, 2009); within ASD, subgroups with co-morbid specific language impairment have been described (Tager-Flusberg, 2006). Thus, certain behavioral characteristics are commonly found across these disorders, such as deficits in attention and impaired language. In addition, along with these behavioral problems, increased rates of left- or mixed-handedness have been reported in these neurodevelopmental disorders (Dragovic & Hammond, 2005; Geschwind & Behan, 1982).
These similarities, with overlap in behavioral characteristics, increased rates of non-right-handedness, and increased rates of atypical laterality of language functions, suggest that this group of neurodevelopmental disorders may have similar underlying partially shared etiologies. All of these disorders are known to have genetic components that are complex and likely to involve multiple genes, each conferring a small degree of risk. Genetic studies have supported overlapping underlying genetic components, some of which may be related to language asymmetry. For example, Francks and his colleagues (2007) found that the gene LRRTM1 was associated with handedness and schizophrenia. Moreover, they demonstrated that this gene was expressed during development and is likely to be involved in brain asymmetry. Smalley et al. (2004) identified seven chromosomal regions of overlap between autism, ADHD, and dyslexia. Genes associated with atypical (non-left) cerebral asymmetry for language overlapped with these regions. Based on reviews of other research, Smalley and her colleagues found that several of these regions had also emerged in genome studies of schizophrenia, bipolar disorder, specific language impairment, and handedness. They state: “We suggest that ACA [atypical functional cerebral asymmetry] may be a phenotype resulting in ‘risk’ for a wide range of neurobehavioral disorders…” (Smalley et al., 2004, p.82). These findings suggest that atypical lateralization of language may be a phenotype representative of a common underlying genetic component. Language laterality is likely related to brain development, the in utero environment, and genetic factors, suggesting that even within diagnostic groups, variation in language laterality may be related to different complex underlying etiologies, which may be important for prognosis and treatment.
There is some evidence that language laterality is associated with anatomical measures of perisylvian language regions, however, this has only been studied in normal controls most of whom were right-handers. Research using the Wada test has shown that most subjects with typical left lateralized language had leftward planum temporale (PT) asymmetry (Foundas et al., 1994) or leftward pars triangularis (PTR) asymmetry (Foundas et al., 1996). Using voxel-based morphometry, Dorsaint-Pierre et al. (2006) found a region in the posterior portion of the inferior frontal gyrus (pars opercularis, POP) that was larger in the left hemisphere in the group with left lateralized language and favored the right hemisphere in the group with right lateralized language. However, using manual PT tracings, they found no association between PT size or asymmetry and language lateralization (Dorsaint-Pierre et al., 2006). Additional studies have examined the relationship between PET activation during story listening and PT surface area measurements. One study found significant correlations between left PT size with left superior temporal gyrus (STG) activation and asymmetry of activation (Tzourio et al., 1998b) while another study found that larger PT predicted more left lateralized activation (Josse et al., 2003). However, Eckert et al. (2006), using fMRI with a single word comprehension task and PT surface area measures, did not find a significant association between PT asymmetry and language laterality. They did, however, find that individuals with smaller overall brain size had stronger left lateralized language. Another study using dichotic listening and PT area measurements in right- and left-handers, did not find a structural-functional relationship across all their participants, however, among right-handed males increased PT asymmetry was associated with increased functional lateralization (Dos Santos Sequeira et al., 2006). Taken together, these studies suggest that, in right-handed individuals, there is a relationship between anatomy and language functions, although this is likely to be a fairly complex relationship.
Left hemisphere dominance for language functions is considered to be the norm but few studies have examined the relationship between language asymmetry and language ability in different populations. Dawson and her colleagues examined this relationship in children with ASD using averaged event-related potentials (ERPs) to auditory speech stimuli. In two studies, they found that autistic children with more impaired language abilities were more likely to have reversed laterality than those with less impaired language (Dawson et al., 1986; 1989), although ERPs are not the ideal method for evaluating functional localization.
The purpose of this study was to examine the relationship between language laterality, anatomical language measures, language abilities and handedness in adolescents with ASD and typical controls. We examined differences between language asymmetry groups (typical, atypical), defined on the basis of a language-processing task using fMRI, in left- and right-handed adolescents with ASD and controls in language region volume, integrity of connections between language regions, and language abilities. The genetic studies summarized earlier suggest that there are common underlying components associated with language laterality in a variety of populations, including normal controls. Furthermore, differences in language asymmetry within diagnostic groups are likely to be associated with underlying etiological differences. Thus, we would expect to find that language laterality rather than diagnosis would be strongly associated with differences in anatomy and behavior. Based on these assumptions, we predicted that there would be differences in gray matter volume of critical language regions between groups defined as showing typical or atypical lateralization of language, in both ASD and controls. Furthermore, we hypothesized that in the arcuate fasciculus higher fractional anisotropy (FA), which is associated with increased integrity of white matter connections, would be found in the group with left lateralized language compared to those with atypical (mixed or right hemisphere) language laterality, in both ASD and controls. We also predicted that left lateralized language functions would be associated with higher language scores. Finally, we predicted that among the participants who showed atypical language laterality there would be more individuals with ASD and more left-handers.
MATERIALS AND METHODS
Subjects
Participants included 14 adolescent boys with ASD, 11–19 years old, and 20 typically developing adolescent boys in the same age range. They were primarily selected from a larger group who had successfully participated in an anatomical MRI study in our lab. This larger sample included right- and left-handed boys and girls, 7–19 years old, who had been recruited through, previous studies in our lab, word of mouth, flyers and brochures in the community and pediatrician offices, local afterschool programs, websites such as craigslist, Asperger’s Association of New England, Autism Speaks, and Interactive Autism Network, homeschooling websites, and our lab website. Right- and left-handed adolescent (current age of 11–19 years old) males, were recruited from this larger sample and individuals who had a difficult time with the anatomical scan, whom we did not think would be able to lie still without a movie playing, or whom we did not think would be able to understand the task were not recruited. Individuals who could understand and perform the task without training (a single practice session was done to ensure they could do the task) and who could lie still while performing the task during the MRI were included in the study. One ASD subject was excluded because he could only do the task after extensive practice with other stimuli at home with his mother. Some subjects were excluded immediately after scanning, when we could see that they were moving during the scanning and there was visible movement on the scans. Other subjects were excluded later, after pre-processing of the fMRI data when the pre-processing indicated excessive movement. The anatomical study did include some left-handers, however, we also specifically recruited more left-handers, especially controls, for this study, utilizing the same recruitment methods as above. We recruited equal numbers of right- and left-handers in the ASD and control groups to increase the likelihood of including individuals with both typical and atypical language laterality. In the ASD group, 7 participants were right-handed and 7 were left-handed; in the typically developing group, there were 10 right-handers and 10 left-handers. Handedness was based on writing hand, self-report, and a modified version of the Dean handedness inventory (Dean, 1988). The modified Dean handedness consists of 12 unimanual tasks and scores range from −24, indicating complete left-handedness, to +24, indicating complete right-handedness. For this study, individuals with a positive handedness score, who wrote with their right hand, and who considered themselves right-handed were classified as right-handed. Similarly, those with a negative handedness score, who wrote with their left hand, and who considered themselves left-handed were classified as left-handed. Based on these criteria, each participant was classified as right- or left-handed and no ambidextrous subjects were included. All participants were male and monolingual English-speakers.
Subjects were administered the Kaufman Brief Intelligence Test (K-BIT-II; Kaufman & Kaufman, 2004) to assess IQ, and the Clinical Evaluation of Language Fundamentals (CELF-3; Semel et al., 1995) to assess language abilities. Four subtests of the CELF-III were administered: Concepts and Directions, Word Classes (Receptive language subtests), Formulated Sentences, and Recalling Sentences (Expressive language subtests). For ASD subjects, diagnosis was based on DSM-IV criteria (American Psychiatric Association, 1994) using the Autism Diagnostic Interview-Revised (ADI-R; Rutter et al., 2003), the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000) and confirmed by an expert clinician. Based on the ADI-R, all the ASD participants had a history of delayed onset of language milestones. Individuals with frank neurological damage, with a known genetic disorder, who were born prematurely (less than 35 weeks), or who had experienced seizures within the last three years were excluded from the study. The typically developing subjects had no history or current diagnosis of developmental, learning, psychiatric, or neurologic disorders.
Subjects 18 years and older were informed of the procedures and gave written consent prior to participation in the study. For subjects under 18 years old, parents and subjects were informed of the procedures and parents gave written consent prior to the child’s participation in the study. Children also provided written or verbal assent, prior to participation. All data reported here were collected in compliance with the Boston University School of Medicine Institutional Review Board.
MRI Acquisition
All the participants practiced in a mock scanner prior to the actual MR scanning. Images were acquired on a Philips 3 Tesla Intera scanner. Volumetric T1-weighted images were obtained as a series of 95–110, 1.4 mm gapless axial images, aligned parallel to the intercommissural plane. The parameters used for the3D MPRage were: TR = 7.3 ms, TE = 3.4 ms, flip angle = 8 degrees, FOV = 230 mm, pixel matrix = 256 × 256. An FE-EPI axial sequence aligned parallel to the intercommissural plane was acquired for each participant. fMRI scans were acquired using Blood Oxygen Level Dependent (BOLD) contrast with the following parameters: TR = 2000 ms, TE = 35 ms, flip angle=90 degrees, FOV = 230 mm, pixel matrix = 128 × 128, 36 contiguous slices, slice thickness = 3.5 mm. Three axial diffusion-weighted images, aligned parallel to the intercommissural plane, were acquired using echo planar imaging, as a series of 73, 2 mm contiguous images. The following parameters were used: b-value = 1000 sec/mm2, 15 gradient directions plus 1 reference image (b = 0), pixel matrix = 128 × 128, FOV = 230 mm.
fMRI Task
fMRI data from the right-handers were collected as part of a previous study (Knaus et al., 2008). A block-design paradigm was used, which consisted of a reading version of a response-naming task (Bookheimer et al., 1997) and a control letter-judgment task. During the response-naming task, subjects were shown a three-word phrase (e.g. keeps hands warm) and asked to think of what word was being described (e.g. gloves). They were then asked to choose, by pressing a button, from two options displayed on the screen, the word that best matched what they had thought of. For the control task, three strings of letters were presented and subjects had to indicate, with a button press, whether the letters were in upper or lower case. This task was chosen so that areas related to primary visual processing and motor areas related to the button press could be subtracted out of the language activation.
The stimuli were presented in red lettering on a black background using E-Prime software (http://www.pstnet.com/products/e-prime/). Prior to scanning, a practice session in the mock scanner was carried out, during which each subject performed one run consisting of stimulus items different from those used in the actual scanning. There were three 28 second long blocks of the response-naming task, alternated with three blocks of the control task with each block containing 4 trials, resulting in 84 time points. A trial was presented every 6 seconds, with the three word phrase or letter strings presented for 3.5 seconds, a blank screen for 0.2 seconds, the two word choices (for the language task) or the words ‘upper’ and ‘lower’ (for the control letter task) displayed for 2 seconds, and a blank screen for 0.3 seconds. At the beginning of each block a crosshair was presented for 4 seconds.
Volume Measurements
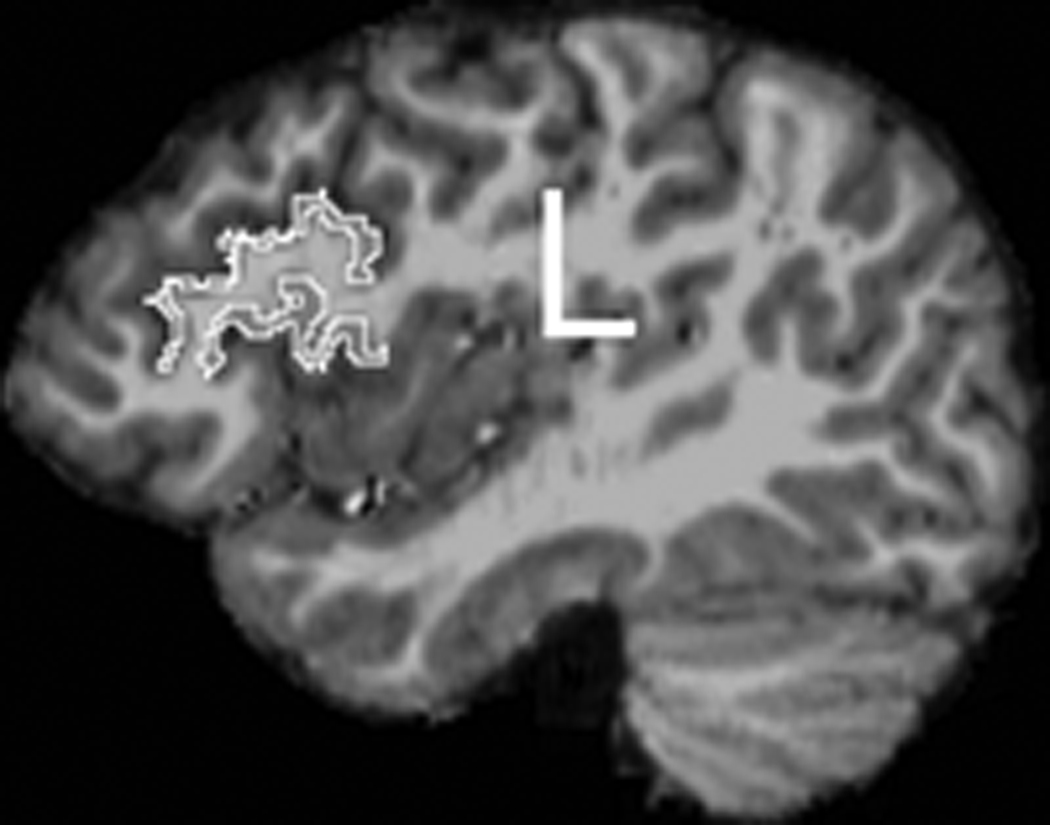
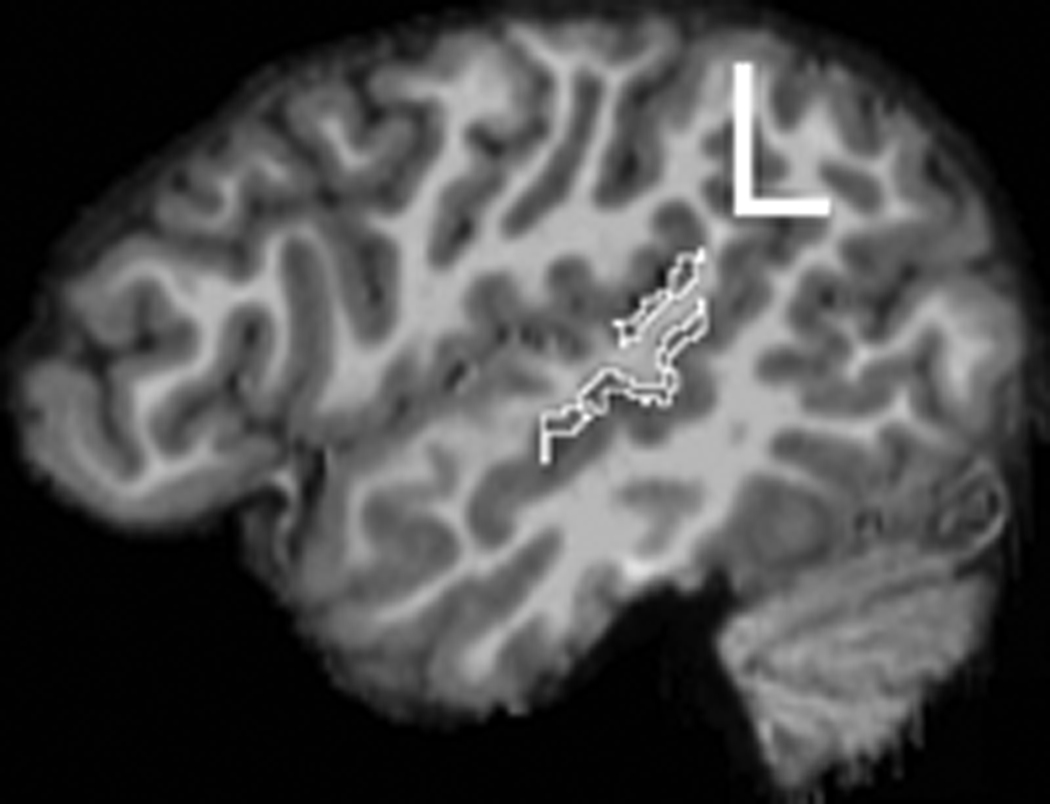
Each MRI was assigned a blind number for subject confidentiality and to ensure that all measurements were performed blind to group and subject identity. Freesurfer (surfer.nmr.mgh.harvard.edu) was used for cortical reconstruction and parcellation of regions. Detailed methods for Freesurfer have been reported in several papers (Dale et al., 1999; Fischl et al., 1999). Briefly, intensity normalization and removal of non-brain tissue was performed. Segmentation into white matter or ‘other’ was then carried out, based on voxel intensity. Hemispheres were then separated and the cerebellum and brainstem were removed. An initial white matter surface was generated for each hemisphere, corrected for topological defects using an automated algorithm (Fischl et al., 2001), and deformed outward to create the pial surface (Fischl & Dale, 2000). The cortical sulci and gyri were then automatically labeled (Desikan et al., 2006; Fischl et al., 2004). These gray matter labels were imported into 3DSlicer (www.slicer.org), where specific boundaries were edited. First, the brain and labels were aligned with the anterior and posterior commisures and rotated into alignment in the sagittal, axial, and coronal planes in order to eliminate any head rotation. For frontal language regions, the PTR and POP were combined to create a total frontal language region (Figure 1a). The STG measure from Freesurfer includes both anterior and posterior regions, as well as the PT. Heschl’s sulcus was used as the anterior boundary; any label anterior to this was deleted so that the measurement included only posterior STG (pSTG) and PT. This boundary was defined in the coronal plane as the most anterior image in which Heschl’s gyrus was clearly visible, with a small amount of white matter lateral to the gyrus. If Heschl’s gyrus was completely bifurcated, the first gyrus was used as the boundary and the second gyrus was included as part of the planum (see Knaus et al., 2004). A posterior boundary was also applied as the most posterior point of the Sylvian fissure, which was defined in the coronal plane as the most posterior slice where the Sylvian fissure was clearly visible, before it became intermixed with white matter (see Knaus et al., 2004). See Figure 1b for an example of the temporal language region measurement.
Figure 1.
An example of the frontal language region (PTR+POP) measurement in the left hemisphere.
The temporal language region measurement (pSTG+PT) in the left hemisphere.
Diffusion Tensor Imaging (DTI) Measures
The edited parcellations from Freesurfer described above, which included the PTR+POP and pSTG+PT in both hemispheres, were used for probabilistic tractography. Each region for each subject was edited to include a small amount of white matter, 1–2 voxels on each side (Parker et al., 2005). Tractography was performed separately in the left and right hemispheres with the temporal language areas (pSTG+PT) used as the seeding mask and the frontal language areas (PTR+POP) as the termination region.
Analyses
fMRI data
fMRI analyses were carried out using Neurolens (www.neurolens.org). The first two volumes were discarded to allow for magnet stabilization. The functional run was motion-corrected using a volume registration algorithm in which each volume was co-registered to a target volume (Cox & Jesmanowicz, 1999). The output files from motion correction were examined to ensure that there was not significant motion. Subjects with movement in any direction 2 mm or 2 degrees or more were excluded. To test for group (ASD, control) differences in movement, the mean of the absolute value of translations and rotations across the run was calculated for each direction for each subject. A multivariate analysis of variance (MANOVA) was performed with the mean translation and rotation in each of the three directions as the dependent variables and group (ASD, control) as the independent variable. There were no significant group differences in motion for any direction. Spatial smoothing was also performed, using a 3D Gaussian kernel with 6 mm full width at half-max.
A general linear model (GLM) fitting the task block’s time vector convolved with a gamma variate estimate of hemodynamic response was performed for each functional run, resulting in an activation map (the –log probability map which corresponds to the t-statistic), a map of the effect, and a map of the standard error of the effect. The words task and a baseline plus drift were modeled. To control for multiple comparisons, Bonferroni correction was used with the activation map for each participant thresholded to p ≤ 10−7, which was overlaid on each subject’s respective high-resolution T1-image. In our previous study of right-handers with ASD and controls using this language generation task we found group differences in activation asymmetry in frontal, but not temporal language regions (Knaus et al., 2008). Therefore in this study we focused on frontal language regions. This region of interest (ROI) was anatomically defined using well-established anatomical landmarks and all measurements were done by one rater experienced in anatomically defining this region (Knaus et al., 2008). The frontal language ROI was defined in the sagittal plane and included the PTR and POP. The anterior boundary was the anterior horizontal ramus of the Sylvian fissure and the posterior boundary was the pre-central sulcus. The superior boundary was the inferior frontal sulcus. Activation in both banks of all of these gyri was included. Percent signal change was calculated in the right and left hemisphere as (mean of the modeled effect/mean of the baseline effect) *100. An asymmetry quotient (AQ) of the percent signal change was calculated as (L − R)/(L + R), such that a positive AQ indicated higher percent signal change in the left region and a negative AQ indicated higher percent signal change in the right area. Similar to other studies (Holland et al., 2001; Szaflarski et al., 2006), activation was considered to be left lateralized if the AQ was greater than 0.1 and right lateralized if the AQ was less than − 0.1. AQs between −0.1 and + 0.1 indicated no asymmetry.
Probabilistic Tractography
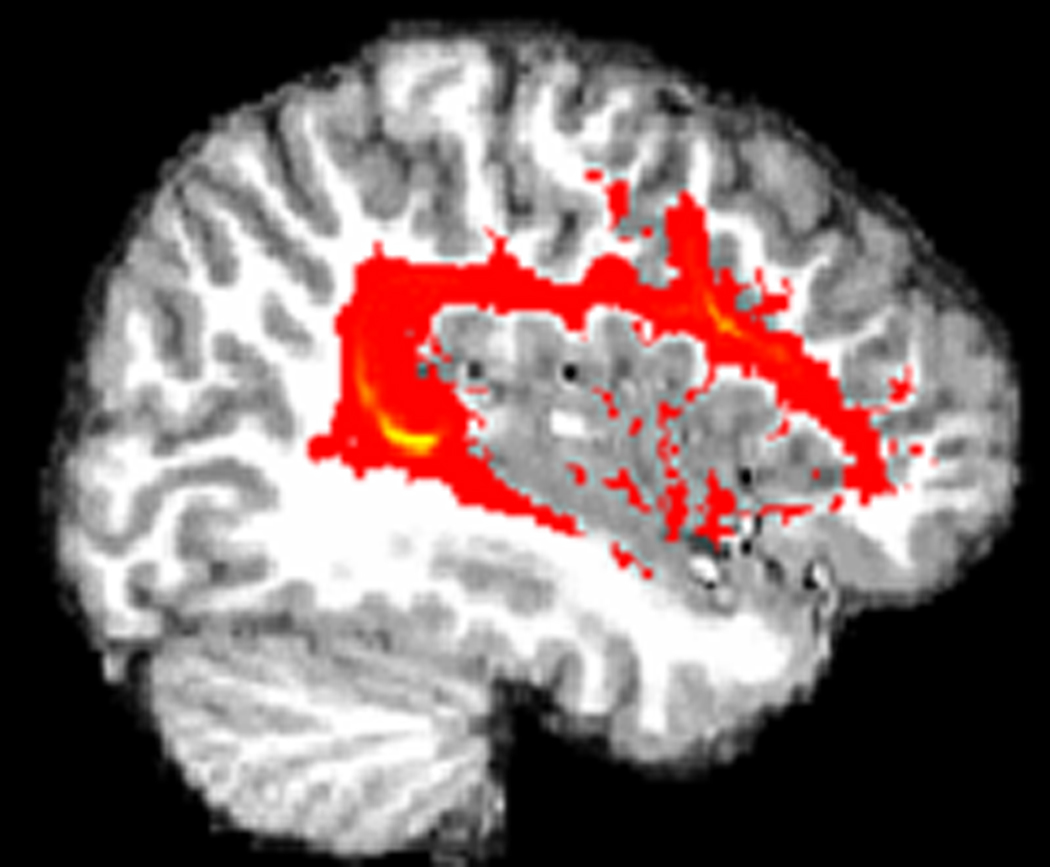
The 3 diffusion scans were averaged to improve the signal to noise ratio. FMRIB’s Diffusion Toolbox (FDT) which is part of FSL (www.fmrib.ox.ac.uk/fsl) was used for all analyses and detailed methods have been described previously (Behrens et al., 2003). Briefly, the diffusion and T1 data were first skull-stripped using the BET tool (Smith, 2002). Diffusion data were then transformed, using affine registration, to a reference volume (the first volume) to correct for eddy currents and head motion. The DTI and T1 data, along with the edited labels from Freesurfer were aligned using affine registration. Bayesian techniques were used to create a probability distribution of fiber direction for each voxel. Probability connectivity distributions between seed and termination points were created by repeatedly sampling from the distributions on voxel-wise diffusion directions. This resulted in each voxel having a value representing the probability of connection to the masks. This connection probability image was then binarized and multiplied by individual FA maps and the mean FA of the tract was calculated. See Figure 2 for tractography results in 1 subject.
Figure 2.
The results of probabilistic tractography, showing the arcuate fasciculus in the left hemisphere of a typically developing subject.
RESULTS
fMRI Behavioral Data
All individuals were able to do the task in the scanner easily after a single practice session. Behavioral data were collected during scanning for all but one subject. Data for this subject were not available due to a button box error, however, behavioral data were collected during the practice run and he made no errors. Accuracy was high with no individual subject making more than 2 errors out of the 12 trials.
Group Characteristics
Functional activation in frontal language regions was used to divide the sample into typical (leftward) and atypical (rightward or bilateral) language laterality groups. This resulted in 22 individuals with typical, leftward, asymmetry of activation (7 with ASD and 15 controls) and 12 with atypical, right lateralized or bilateral, frontal activation (7 with ASD and 5 controls). Table 1 presents the demographic information for these groups. Differences in age, IQ, and handedness were investigated with a MANOVA, with language laterality (typical, atypical activation) and diagnosis (ASD, control) as the between-subjects variables and age, KBIT verbal and non-verbal IQs, and Dean handedness score as the dependent variables. We found no significant differences between language laterality groups in age, IQ, or degree of handedness. There was a significant effect of diagnosis (F4,27 = 4.01, p = .011), which revealed a significant effect for age (F1,30 = 7.34, p = .011) and non-verbal IQ (F1,30 = 8.07, p = .008) indicating that the ASD group was older and had lower non-verbal IQ scores than controls.
Table 1.
Subject Characteristics for Typical and Atypical Language Activation Groups
| Frontal Activation Asymmetry | ||||
|---|---|---|---|---|
| Typical (L>R) (n=22) | Atypical (R>L or R=L) (n=12) | |||
| ASD (n=7) | CON (n=15) | ASD (n=7) | CON (n=5) | |
| Age | 16.83 (2.35) | 14.43 (2.47) | 15.35 (2.29) | 13.09 (1.66) |
| Handedness | +3.29 (13.80) | +4.80 (17.25) | +.57 (18.31) | −3.80 (14.50) |
| KBIT VIQ | 101.57 (16.00) | 121.87 (14.11) | 105.00 (25.46) | 110.40 (15.24) |
| KBIT NVIQ | 103.43 (11.82) | 114.53 (5.62) | 101.71 (8.69) | 108.40 (9.96) |
Gray Matter Volumes of Language Regions
To examine differences in gray matter volume between the ASD and control groups and between language laterality groups, a MANOVA was computed with hemisphere as the repeated measures variable, diagnosis (ASD, control) and language laterality (typical, atypical activation asymmetry) as the between-subjects variables, and PTR+POP (frontal) and pSTG+PT (temporal) gray matter volume as the dependent variables. Table 2 presents mean anatomical measures and language scores for each group. Supplemental Figures 1 and 2 present individual subject data for PTR+POP and pSTG+PT volumes by language laterality and diagnostic groups. Gray matter volume measurements were not available for one control subject who had typical left lateralized language activation, due to movement during the anatomical scan. The MANOVA revealed significant effects of hemisphere (F2,28 = 5.66, p = .009) and language laterality (F2,28 = 4.88, p = .015). There was no significant effect of diagnosis (F2,28 = .38, p = .690) and no significant interactions (diagnosis by language laterality, F2,28 = .34, p = .718; hemisphere by diagnosis, F2,28 = 1.12, p = .341; hemisphere by language laterality, F2,28 = .19, p = .828; hemisphere by diagnosis by language laterality, F2,28 = 2.10, p = .141). The univariate analyses indicated that the hemisphere effect was significant for the pSTG+PT (F1,29 = 7.19, p = .012), with the left volume significantly larger than the right volume. The language laterality difference was significant for the frontal area (F1,29 = 7.69, p = .010); the group with atypical language laterality had significantly larger PTR+POP gray matter volume than the group with typical language laterality. There was a non-significant trend for the pSTG+PT volume (F1,29 = 3.36, p = .077) with the atypical group having a somewhat larger volume than the typical group.
Table 2.
Anatomical and language measures for each group based on frontal activation AQ.
| Frontal Activation Asymmetry | ||||
|---|---|---|---|---|
| Typical (L>R) | Atypical (R>L or R=L) | |||
| ASD | CON | ASD | CON | |
| L PTR+POP | 8.48 (1.51) | 9.10 (.98) | 9.56 (1.20) | 10.26 (1.05) |
| R PTR+POP | 8.62 (1.11) | 9.39 (1.73) | 10.82 (1.80) | 9.99 (.84) |
| L pSTG+PT | 6.69 (1.09) | 6.76 (1.22) | 7.59 (.84) | 7.26 (1.85) |
| R pSTG+PT | 6.21 (.99) | 5.86 (1.01) | 6.81 (1.20) | 6.69 (2.08) |
| L Arc Fasc FA | .321 (.017) | .308 (.025) | .288 (.032) | .288 (.007) |
| R Arc Fasc FA | .282 (.012) | .305 (.022) | .281 (.024) | .289 (.018) |
| CELF Receptive | 101.14 (16.80) | 119.20 (9.91) | 101.71 (24.99) | 104.40 (12.01) |
| CELF Expressive | 96.00 (23.09) | 109.67 (8.16) | 94.57 (22.35) | 108.80 (8.41) |
Fractional Anisotropy in the Arcuate Fasciculus
Group differences in FA were examined using analysis of variance (ANOVA) with hemisphere as the repeated measures variable, diagnosis (ASD, control) and language laterality (typical, atypical) as the between-subjects variables, and FA of the arcuate fasciculus as the dependent variable, shown in Table 2. In addition to the subject on whom we could not obtain volume measures, two additional control subjects were excluded from the DTI analysis because we were not able to collect all 3 DTI scans; one had typical and one had atypical frontal language activation. There was a significant effect of language laterality (F1,27 = 7.09, p = .013), indicating significantly higher FA in the group with typically lateralized frontal activation compared to those with atypical asymmetry of frontal activation. There was also a significant effect of hemisphere (F1,27 = 4.38, p = .046) with higher FA in the left than the right hemisphere. There were no significant effects of diagnosis (F1,27 = .49, p = .489). The hemisphere by diagnosis interaction was close to significant (F1,27 = 4.00, p = .056), with a slightly larger hemisphere difference in the ASD group. The hemisphere by language laterality (F1,27 = 2.68, p = .113), hemisphere by diagnosis by language laterality (Fs = 1.61, p = .216), and diagnosis by language laterality (F1,27 = .01, p = .922) interactions were not significant. See Supplemental Figure 3 for individual FA measures for the language laterality group and the diagnostic groups.
Language Scores
Group differences in CELF scores were investigated with MANOVA with language laterality (typical, atypical activation) and diagnosis (ASD, controls) as the between subject variables and CELF receptive and expressive scores as the dependent variables using the data shown in Table 2. There were no significant effects for language laterality or diagnostic group or laterality by diagnostic group interactions in either receptive or expressive language scores.
Language laterality, diagnosis and handedness
Table 3 shows mean frontal percent signal AQs for each diagnosis and handedness group and Table 4 presents the number of individuals in each diagnostic and handedness group in the typical and atypical language activation groups. To examine differences in the degree of frontal activation asymmetry, an ANOVA was performed with diagnosis (ASD, controls) and handedness (left, right) as the between-subjects independent variables and frontal activation percent signal AQ as the dependent variable. The ANOVA revealed no significant differences in frontal activation asymmetry between diagnosis or handedness groups or any interactions. A chi-square test was used to investigate differences in the number of individuals in each diagnosis and handedness group with typical or atypical language laterality, which did not reveal statistically significant differences. However, 75% of the controls, compared to only 50% of the ASD subjects had typical language laterality scores. Among right-handers, 70% had typical language laterality compared to 59% of left-handers. Out of the four diagnosis/handedness combinations, right-handed controls were the most prevalent and left-handers with ASD were the least prevalent in the group with typical left lateralized language activation. Stated another way, right-handers with ASD included fewer individuals with leftward asymmetry, 57%, than in the right-handed control group, 80%. The atypical activation group had more left-handers with ASD (33%) than the other diagnostic or handedness groups and the fewest right-handed controls (17%).
Table 3.
Mean (standard deviation) frontal activation percent signal change asymmetryquotient for each handedness and diagnostic group.
| Frontal Activation AQ | |
|---|---|
| RH Controls (n=10) | .488 (.448) |
| LH Controls (n=10) | .441 (.505) |
| RH ASD (n=7) | .204 (.363) |
| LH ASD (n=7) | .129 (.702) |
Table 4.
Number of individuals in each diagnostic and handedness group with typical and atypical activation.
| Typical Frontal Activation (L>R) (n=22) |
Atypical Frontal Activation (R>L or R=L) (n=12) |
|
|---|---|---|
| RH Controls (n=10) | 8 | 2 |
| LH Controls (n=10) | 7 | 3 |
| RH ASD (n=7) | 4 | 3 |
| LH ASD (n=7) | 3 | 4 |
DISCUSSION
This study examined the relationship between language laterality, the anatomy of language regions, and language abilities in right- and left-handed adolescents with ASD and typically developing adolescents. We were particularly interested in whether differences between typical and atypical lateralization of language functions are driving some of the reported differences in the anatomy of language areas in individuals with ASD, who are known to have impairments in language ability. There were three major findings. First, the group with typical lateralization of language had smaller gray matter volume of frontal and temporal language regions compared to those with atypical asymmetry of language, though only the frontal volume reached statistical significance. Second, the typical language laterality group had higher FA values for the arcuate fasciculus compared to the atypical language laterality group. And third, while there were no statistically significant differences in the number of individuals in diagnostic and handedness groups, right-handed controls were the most prevalent among the typical laterality group and left-handers with ASD were the most prevalent among those with atypical lateralization of language.
The differences in gray matter volume between language laterality groups were consistent with our initial hypothesis. This finding suggests an association between language laterality and language region anatomy, which is consistent with what has been found in typically developing subjects (e.g., Foundas et al., 1994; 1996). In a previous study, we found increased gray matter volume of frontal language regions in right-handed adolescents with ASD (Knaus et al., 2009), however, we had not evaluated functional language asymmetry. Results from this study suggest that differences in anatomical volumes for language areas may be more associated with differences in language laterality than specifically to the diagnosis of ASD. It would be important for future studies to examine anatomical differences in language asymmetry groups in other developmental and psychiatric disorders. It is interesting that smaller volumes of language regions were associated with typical language asymmetry, which is consistent with the findings reported by Eckert and colleagues (2006) of an association between smaller brain size with stronger left lateralized language functions.
Our second major finding of higher FA values in the arcuate fasciculus for individuals with typical left lateralized language also supported our hypothesis. Since FA is an indirect measure of the integrity of white matter connections, this finding suggests that language processing may be more efficient in the group with typical, compared to atypical language asymmetry. It was somewhat surprising, however, that there were not hemisphere differences in FA between the two groups (Parker et al., 2005). Contrary to our predictions, we did not find behavioral differences in language measures between the language laterality groups and no differences between diagnostic groups. Given that the average language scores for the ASD group were well within the normal range, the relationship between asymmetry and impaired language abilities could not be evaluated in this study. Nevertheless, these findings suggest that differences in arcuate fasciculus connectivity may be more related to language laterality than to ASD. Future studies should further examine language processing in relation to these connections in language asymmetry groups in groups with more impaired language.
Consistent with previous studies (Jorgens et al., 2007; Kleinhans et al., 2008; Knaus et al., 2008; Knecht et al., 2000; Pujol et al., 1999; Szaflarski et al., 2002; Takeuchi et al., 2004; Tzourio et al., 1998a), we found a trend for more left-handers and individuals with ASD to have atypical language laterality than typically developing right-handers. It is, however, important to note that there were no statistical differences in the representation of handedness or diagnostic group between the typical and atypical language laterality groups. Instead, it seems that there is considerable heterogeneity, suggesting that there is a complex set of factors that contribute to the localization of language functions. The lateralization of language functions is related to the influence of genetic and prenatal environmental interactions on brain development, which when disrupted, may result in atypical lateralization. Disruptions in this process may occur more frequently in individuals with developmental and psychiatric disorders, including ASD. Our findings suggest the possibility that there may be similar developmental alterations underlying atypical language lateralization, associated with specific anatomical differences, which may lay the foundation for a variety of developmental or psychiatric disorders. This also suggests that within specific disorders there may be differences between those with typical and atypical language asymmetry. Language laterality may therefore provide a novel way of subdividing different populations, creating more homogenous groups, which could be important for future genetic and treatment studies.
There were several limitations to this study. First, as noted earlier there were very few individuals included with language impairment, with most subjects in both the ASD and control groups scoring in the normal or above normal range on the standardized language test. It would be interesting to examine differences in language ability between language laterality groups with a sample with more heterogeneous language abilities. A second limitation is the small sample size within individual groups, (e.g. left-handed controls, left-handers with ASD). More studies with larger samples are needed to further examine handedness, language laterality, and ASD. Another potential limitation is that language laterality was based on activation in frontal language regions. Since the task was a language generation task, which relies more on frontal language areas, we chose to focus on activation of these regions. It would be interesting to utilize an auditory language comprehension task, which relies more on posterior language areas to explore whether similar results are found. Finally, it would be important to extend this line of research to other neurodevelopmental disorders to investigate more systematically the relationships between language anatomy and laterality of function.
Supplementary Material
Figure 1a. Frontal language region volume of each subject in the atypical and typical language asymmetry groups.
Figure 1b. Frontal language region volume of each subject in the ASD and typically developing groups.
Figure 2a. Volume of the temporal language region of each subject broken down by language laterality groups.
Figure 2b. Temporal language area volume of each subject in the ASD and control groups.
Figure 3a. FA of the arcuate fasciculus for each subject in the atypical and typical language laterality groups.
Figure 3b. Arcuate fasciculus FA of each subject for ASD and typically developing groups.
Acknowledgments
This study was supported by a program project grant from the National Institute on Deafness and Other Communication Disorders (U19 DC 03610), which is part of the NICHD/NIDCD funded Collaborative Programs on Excellence in Autism, as well as funding for the GCRC at Boston University School of Medicine (M01-RR0533). We thank all of our research assistants for help in collecting the data. We also extend our sincere gratitude to the children and families who participated in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association Press; 1994. [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith S. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance in Medicine. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Blomgren M, Nagarajan SS, Lee JN, Li T, Alvord L. Preliminary results of a functional MRI study of brain activation patterns in stuttering and nonstuttering speakers during a lexical access task. Journal of Fluency Disorders. 2003;28:337–355. doi: 10.1016/j.jfludis.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Belin P, Chabane N, Poline J-B, Barthélémy C, Mouren-Simeoni M-C, Brunelle F, Samson Y, Zilbovicius M. Perception of complex sounds: Abnormal pattern of cortical activation in autism. American Journal of Psychiatry. 2003;160:2057–2060. doi: 10.1176/appi.ajp.160.11.2057. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Belin P, Bourgeois M, Royer V, Barthélémy C, Mouren-Simeoni M-C, Philippe A, Brunelle F, Samson Y, Zilbovicius M. Perception of complex sounds in autism: Abnormal auditory cortical processing in children. American Journal of Psychiatry. 2004;161:2117–2120. doi: 10.1176/appi.ajp.161.11.2117. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton T, Malow BA, Gaillard WD, Sato S, Kufta C, Fedio P, Theodore WH. A direct comparison of PET activation and electrocortical stimulation mapping for language localization. Neurology. 1997;48:1056–1065. doi: 10.1212/wnl.48.4.1056. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic Resonance in Medicine. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dawson G, Finley C, Philips S, Galpert L. Hemispheric specialization and the language abilities of autistic children. Child Development. 1986;57:1440–1453. [PubMed] [Google Scholar]
- Dawson G, Finley C, Philips S, Lewy A. A comparison of hemispheric asymmetries in speech-related brain potentials of autistic and dysphasic children. Brain and Language. 1989;37:26–41. doi: 10.1016/0093-934x(89)90099-0. [DOI] [PubMed] [Google Scholar]
- Dean RS. Lateral Preference Schedule. Odessa, FL: Psychological Assessment Resources, Inc; 1988. [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dorsaint-Pierre R, Penhune VB, Watkins KE, Neelin P, Lerch JP, Bouffard M, Zatorre RJ. Asymmetries of the planum temporale and Heschl's gyrus: Relationship to language lateralization. Brain. 2006;129:1164–1176. doi: 10.1093/brain/awl055. [DOI] [PubMed] [Google Scholar]
- Dos Santos Sequeira S, Woerner W, Walter C, Kreuder F, Lueken U, Westerhausen R, Wittling RA, Schweiger E, Wittling W. Handedness, dichotic-listening ear advantage, and gender effects on planum temporale asymmetry - A volumetric investigation using structural magnetic resonance imaging. Neuropsychologia. 2006;44:622–636. doi: 10.1016/j.neuropsychologia.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Dragovic M, Hammond G. Handedness in schizophrenia: A quantitative review of evidence. Acta Psychiatrica Scandinavica. 2005;111:410–419. doi: 10.1111/j.1600-0447.2005.00519.x. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Possing ET, Binder JR. Uncoupled leftward asymmetries for planum morphology and functional language processing. Brain and Language. 2006;98:102–111. doi: 10.1016/j.bandl.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences. 2000;97:11044–11049. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Flagg EJ, Cardy JE, Roberts W, Roberts TP. Language lateralization development in children with autism: Insights from the late field magnetoencephalogram. Neuroscience Letters. 2005;386:82–87. doi: 10.1016/j.neulet.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Foundas AL. The anatomical basis of language. Topics in Language Disorders. 2001;21:1–19. [Google Scholar]
- Foundas AL, Leonard CM, Gilmore R, Fennell E, Heilman KM. Planum temporale asymmetry and language dominance. Neuropsychologia. 1994;32:1225–1231. doi: 10.1016/0028-3932(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Gilmore RL, Fennell EB, Heilman KM. Pars triangularis asymmetry and language dominance. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:719–722. doi: 10.1073/pnas.93.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francks C, Maegawa S, Lauren J, Abrahams BS, Velayos-Baeza A, Medland SE, Colella S, Groszer M, McAuley EZ, Caffrey TM, Timmusk T, Pruunsild P, Koppel I, Lind PA, Matsumoto-Itaba N, Nicod J, Xiong L, Joober R, Enard W, Krinsky B, Nanba E, Richardson AJ, Riley BP, Martin NG, Strittmatter SM, Moller HJ, Rujescu D, St Clair D, Muglia P, Roos JL, Fisher SE, Wade-Martins R, Rouleau GA, Stein JF, Karayiorgou M, Geschwind DH, Ragoussis J, Kendler KS, Airaksinen MS, Oshimura M, DeLisi LE, Monaco AP. LRRTM1 on chromosome 2p12 is a maternally suppressed gene that is associated paternally with handedness and schizophrenia. Molecular Psychiatry. 2007;12:1129–1139. doi: 10.1038/sj.mp.4002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais H, Belin P, Boddaert N, Leboyer M, Coez A, Sfaello I, Barthélémy C, Brunelle F, Samson Y, Zilbovicius M. Abnormal cortical voice processing in autism. Nature Neuroscience. 2004;7:801–802. doi: 10.1038/nn1291. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Behan P. Left-handedness: Association with immune disease, migraine, and developmental learning disorder. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:5097–5100. doi: 10.1073/pnas.79.16.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: I., II, III. A hypothesis and a program for research. Archives of Neurology. 1985;42:428–459. 521–552, 634–654. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Byars AW, Strawsburg RH, Schmithorst VJ, Ball WS., Jr Normal fMRI brain activation patterns in children performing a verb generation task. NeuroImage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Jorgens S, Kleiser R, Indefrey P, Seitz RJ. Handedness and functional MRI-activation patterns in sentence processing. NeuroReport. 2007;18:1339–1343. doi: 10.1097/WNR.0b013e32825a67db. [DOI] [PubMed] [Google Scholar]
- Josse G, Mazoyer B, Crivello F, Tzourio-Mazoyer N. Left planum temporale: An anatomical marker of left hemispheric specialization for language comprehension. Cognitive Brain Research. 2003;18:1–14. doi: 10.1016/j.cogbrainres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. 2nd ed. Circle Pines, MN: AGS Publishing; 2004. [Google Scholar]
- Kleinhans NM, Müller R-A, Cohen DN, Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Research. 2008;1221:115–125. doi: 10.1016/j.brainres.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus TA, Bollich AM, Corey DM, Lemen LC, Foundas AL. Sex-linked differences in the anatomy of perisylvian language cortex: A volumetric MRI study of gray-matter volumes. Neuropsychology. 2004;18:738–747. doi: 10.1037/0894-4105.18.4.738. [DOI] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Dominick KC, Schuring MD, Shaffer N, Lindgren KA, Joseph RM, Tager-Flusberg H. Age-related changes in the anatomy of language regions in autism spectrum disorder. Brain Imaging and Behavior. 2009;3:51–63. doi: 10.1007/s11682-008-9048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Lindgren KA, Hadjikhani N, Tager-Flusberg H. fMRI activation during a language task in adolescents with ASD. Journal of the International Neuropsychological Society. 2008;14:967–979. doi: 10.1017/S1355617708081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Dräger M, Deppe M, Bobe L, Lohmann H, Flöel A, Ringelstein E-B, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Lenventhal BL, DiLavore PS, Pickles A, Rutter M. The autism diagnostic observation schedule - generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Müller R-A, Behen ME, Rothermel RD, Chugani DC, Muzik O, Mangner TJ, Chugani HT. Brain mapping of language and auditory perception in high-functioning autistic adults: A PET study. Journal of Autism and Developmental Disorders. 1999;29:19–31. doi: 10.1023/a:1025914515203. [DOI] [PubMed] [Google Scholar]
- Müller R-A, Chugani DC, Behen ME, Rothermel RD, Muzik O, Chakraborty PK, Chugani HT. Impairment of dentato-thalamo-cortical pathway in autistic men: Language activation data from positron emission tomography. Neuroscience Letters. 1998;245:1–4. doi: 10.1016/s0304-3940(98)00151-7. [DOI] [PubMed] [Google Scholar]
- Parker GJM, Luzzi S, Alexander DC, Wheeler-Kingshott CAM, Ciccarelli O, Ralph MAL. Lateralization of ventral and dorsal auditory-language pathways in the human brain. NeuroImage. 2005;24:656–666. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Pecini C, Casalini C, Brizzolara D, Cipriani P, Pfanner L, Chilosi A. Hemispheric specialization for language in children with different types of specific language impairment. Cortex. 2005;41:157–167. doi: 10.1016/s0010-9452(08)70890-6. [DOI] [PubMed] [Google Scholar]
- Pennington B, Bishop DVM. Relations among speech, language and reading disorders. Annual Review of Psychology. 2009;60:283–306. doi: 10.1146/annurev.psych.60.110707.163548. [DOI] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038–1043. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2-3-year-old children with autism spectrum disorder. Biological Psychiatry. 2008;64:589–598. doi: 10.1016/j.biopsych.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview - Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals. 3rd ed. San Antonio, TX: The Psychological Corporation, Harcourt Brace and Co; 1995. [Google Scholar]
- Smalley SL, Loo SK, Yang MH, Cantor RM. Toward localizing genes underlying cerebral asymmetry and mental health. American Journal of Medical Genetics.Part B, Neuropsychiatric Genetics. 2004;135B:79–84. doi: 10.1002/ajmg.b.30141. [DOI] [PubMed] [Google Scholar]
- Smith S. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, Brewer CC, Perry HM, Morris GL, Mueller WM. Language dominance in neurologically normal and epilepsy subjects: A functional MRI study. Brain. 1999;122:2033–2046. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. fMRI study of language lateralization in children and adults. Human Brain Mapping. 2006;27:202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H. Defining language phenotypes in autism. Neuroscience Research. 2006;6:219–224. [Google Scholar]
- Takeuchi M, Harada M, Matsuzaki K, Nishitani H, Mori K. Difference of signal change by a language task on autistic patients using functional MRI. Journal of Medical Investigation. 2004;51:59–62. doi: 10.2152/jmi.51.59. [DOI] [PubMed] [Google Scholar]
- Tzourio N, Crivello F, Mellet E, Nkanga-Ngila B, Mazoyer B. Functional anatomy of dominance for speech comprehension in left handers vs right handers. NeuroImage. 1998a;8:1–16. doi: 10.1006/nimg.1998.0343. [DOI] [PubMed] [Google Scholar]
- Tzourio N, Nkanga-Ngila B, Mazoyer B. Left planum temporale surface correlates with functional dominance during story listening. NeuroReport. 1998b;9:829–833. doi: 10.1097/00001756-199803300-00012. [DOI] [PubMed] [Google Scholar]
- Wehner DT, Ahlfors SP, Mody M. Effects of phonological contrast on auditory word discrimination in children with and without reading disability: A megnetoencephalography (MEG) study. Neuropsychologia. 2007;45:3251–3262. doi: 10.1016/j.neuropsychologia.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1a. Frontal language region volume of each subject in the atypical and typical language asymmetry groups.
Figure 1b. Frontal language region volume of each subject in the ASD and typically developing groups.
Figure 2a. Volume of the temporal language region of each subject broken down by language laterality groups.
Figure 2b. Temporal language area volume of each subject in the ASD and control groups.
Figure 3a. FA of the arcuate fasciculus for each subject in the atypical and typical language laterality groups.
Figure 3b. Arcuate fasciculus FA of each subject for ASD and typically developing groups.