Abstract
Aim
Our aim is to assess the ability of human neutrophil peptide α-defensins (HNPs) and human β-defensins (HBDs) to attenuate proinflammatory cytokine responses and enhance antibody responses to recombinant hemagglutinin B (rHagB) or recombinant fimbrillin A (rFimA) from Porphyromonas gingivalis 381 in mice.
Materials & methods
In the first study, C57BL/6 mice were given 10 μg rHagB or rFimA without and with 1 μg HNP1, HNP2, HBD1, HBD2 or HBD3. At 24 h, mice were euthanized and cytokine concentrations were determined in nasal wash fluid (NWF), bronchoalveolar lavage fluids, saliva and serum. In the second study, C57BL/6 mice were given 10 μg rHagB or rFimA without and with 1 μg HNPs or HBDs similarly on days 0, 7 and 14. At 21 days, mice were euthanized and rHagB- and rFimA-specific antibody responses were determined in NWF, bronchoalveolar lavage fluids, saliva and serum.
Results
Mice given rHagB + HNP2, rHagB + HBD1 and rHagB + HBD3 produced significantly lower (p < 0.05) IL-6 responses than mice given rHagB alone. Mice given rHagB + HNP1, rHagB + HNP2, rHagB + HBD1 and rHagB + HBD3 produced significantly lower (p < 0.05) keratinocyte-derived chemokine responses than mice given rHagB alone. Mice given rFimA produced very low levels of IL-6 and only moderate levels of keratinocyte-derived chemokine in NWF that were not attenuated by prior incubation of rFimA with any defensin. Mice given rHagB + HNP1 produced a significantly higher (p < 0.05) serum IgG antibody response than mice given rHagB alone and mice given rFimA + HNP2 produced a higher, but not significant, antibody response.
Conclusion
The ability of HNPs and HBDs to attenuate proinflammatory cytokine responses in murine NWF and enhance IgG antibody responses in serum was dependent upon both the defensin and antigen of P. gingivalis.
Keywords: defensins, fimbriae A, hemagglutinin B, immunity, Porphyromonas gingivalis
Defensins are small, host-derived peptides with broad antimicrobial activity against Gram-negative and Gram-positive bacteria, fungi and enveloped viruses [1–4]. In humans, there are human neutrophil peptide α-defensins (HNPs), human θ-defensins (HBDs) and human θdefensins. HNPs are found in neutrophil granules, macrophages, mucosal crypt cells and Paneth cells and contain 29–35 amino acids. HNP1–4 are found in azurophil granule fractions of human neutrophils and human defensins (HD)5 and 6 are found in human Paneth cells. HBDs are expressed in epithelia of many organs and in nonepithelial tissues [5–9]. The latter tissues include articular cartilage, synovial membranes and bone [10–12]. HBDs contain 36–45 amino acids. Although 28 β-defensin genes have been described in humans, HBD1–4 are among the more closely studied HBDs.
Defensins regulate innate immune mechanisms [3,13]. In addition to their antimicrobial activity, they chemoattract CD4/CD45 RA+ cells, CD8+ T cells, monocytes and immature dendritic cells, enhance phagocytosis by macrophages, activate and degranulate mast cells; regulate cytokine production, regulate complement activation and inhibit glucocorticoid production. HBD2 has a functional overlap with chemokines and one region resembles regions of MIP-3α/CCL20, which is considered to be the domain responsible for the specific interaction with CCR6. Defensins also enhance adaptive immunity. They interact with G protein-coupled receptors in immature dendritic cells, stimulate dendritic-cell maturation, have direct effects on T lymphocytes and enhance antigen-specific immune responses in vivo.
Porphyromonas gingivalis is an oral periodontal pathogen whose extracellular products are capable of inducing proinflammatory cytokines and producing intense inflammatory responses at mucosal surfaces. P. gingivalis produces a number of adhesins that are available in recombinant form, including recombinant hemagglutinin B (rHagB) and recombinant fimbrillin (rFimA) [14,15]. Defensins clearly regulate innate immune responses and regulate early inflammatory events. For example, HNPs and HBDs have powerful anti-inflammatory effects on human monocytes [16], human monocyte-derived macro phages [17] and human myeloid dendritic cells [18]. In addition, the systemic administration of HNP protects mice from a murine model of peritonitis. However, the effect of HNPs and HBDs on the attenuation of mucosal inflammation to rHagB or rFimA is not known. Likewise, defensins elicit enhanced humoral, protective and therapeutic immune responses. HNPs and HBDs both enhance ovalbumin-specific serum IgG antibody responses in mice. In this study, we assess whether HNPs and HBDs can attenuate proinflammatory cytokine responses and enhance antibody responses to rHagB and rFimA in mucosal secretions and serum of mice.
Materials & methods
rHagB & rFimA
Recombinant hemagglutinin B was produced as previously described [14,18]. The composition and purity of rHagB was verified by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), western blot, mass spectrometry and amino acid analysis (High Resolution Mass Spectrometry Facility, University of Iowa, IA, USA). rHagB had an observed matrix-assisted laser desorption/ionisation (MALDI) mass of 49,560.56 m/z. A stock solution of rHagB (2 mg/ml) was prepared in phosphate-buffered saline (PBS).
Recombinant fimbrillin A was also produced as previously described [19,20]. The purity of the rFimA preparation was assessed by SDS-PAGE, western blot, mass spectrometry, and amino acid analysis (High Resolution Mass Spectometry Facility, University of Iowa). rFimA had an observed MALDI mass of 45,359.2 m/z. A stock solution of rFimA (2 mg/ml) was prepared in PBS.
α- & β-defensins
Human neutrophil peptide α-defensins 1 and 2 were purchased from the American Peptide Company (CA, USA). Recombinant HBD1, HBD2 and HBD3 were purchased from PeproTech (Rocky Hill, NJ, USA). The purity, mass, and composition of these peptides were confirmed by MALDI and content and composition of each defensin was determined by amino acid analysis (High Resolution Mass Spectometry Facility, University of Iowa). Stock solutions of HNPs (200 μg/ml) and HBDs (200 μg/ml) were prepared in PBS.
Detection & control of lipopolysaccharide
Small amounts of lipopolysaccharide (LPS) can still be present in recombinant proteins such as rHagB and rFimA and this becomes important, particularly when assessing proinflammatory cytokine responses in vivo in mice. LPS content was determined using the Limulus Amebocyte Lysate assay (QCL-1000, Cambrex Bio Science, MD, USA). To limit LPS contamination, stock solutions were prepared using 0.01 M sodium phosphate buffer with 0.14 M sodium chloride made with pyrogen-free water and adjusted to pH 7.2 (PBS). LPS content was 0.0088 pg LPS/ml PBS; 1.9 ng LPS/μg rHagB; 60 pg LPS/μg rFimA; and 0.10–10.12 ng LPS/μg HNP or HBD.
Inoculation of mice
A total of 262 female mice (Mus musculus domesticus; strain C57BL/6; 5–7 months of age; Charles River Breeding Laboratories, Inc., MA, USA) were used in these studies and housed under accredited conditions in The University of Iowa Medical Laboratory rodent facility.
Inocula were prepared by combining equal amounts of stock solutions of rHagB, rFimA, HNP, HBD or PBS. Mice were slightly sedated with CO2/O2 and given 10 μl of inoculum intranasally (5 μl/nare). The final doses contained 10 μg rHagB or 10 μg rFimA without and with 1 μg HNP or 1 μg HBD per mouse, as previously described [21,22]. Control mixtures included 1 μg HNP, 1 μg HBD and PBS. Additional controls were added to assess the role of trace contaminating amounts of LPS in rHagB and rFimA. One control solution contained 19 ng LPS, equivalent in concentration to that found by QCL-1000 assays in 10 μg rHagB. Another control solution contained rHagB inactivated by heating at 100 °C for 15 min.
To demonstrate defensin-attenuated mucosal cytokine responses, 72 mice were exposed to the above inocula and then euthanized at 24 h postinoculation for collection of fluids. To demonstrate defensin-enhanced antibody responses, 190 mice were immunized intranasally with inocula on days 0, 7 and 14 and then euthanized at 21 days postinoculation for collection of fluids.
Collection of fluids
Since intranasal inoculation of rHagB and rFimA could have an impact on the production of proinflammatory cytokine concentrations and antibodies in adjacent mucosal tissues, the nasal wash fluid (NWF), bronchoalveolar lavage fluid (BALF), saliva and serum were all collected and stored at −80°C, as previously described [23].
Detection of cytokines
The concentrations (pg/ml) of proinflammatory cytokines (IL-1β, IL-6, granulocyte-macrophage colony-stimulating factor, TNF-α, and IL-12p40); keratinocyte-derived chemokine (KC), Th1 cytokines (IL-2 and IFN-γ) and Th2 cytokines (IL-4, IL-5 and IL-10) in NWF, BALF, saliva and serum were determined as recently described using a commercial multiplexed fluorescent bead-based immunoassay (Millipore, MA, USA) in the Luminex 100 IS Instrument (Luminex, TX, USA) [23].
ELISA of rHagB- or rFimA-specific antibody responses
The amount of IgA, IgM and IgG in NWF, BALF, saliva and serum was determined using an ELISA. To generate the isotype standard curve, the upper three rows of microtiter plates (Falcon Probind plates, Becton Dickinson, NJ, USA) were coated with 1.5 μg/ml antibody to the heavy and light chain of mouse immunoglobulin (Jackson ImmunoResearch Laboratories, Inc., PA, USA). To determine the rHagB- or rFimA-specific antibody isotype response, the lower five rows of each plate were coated with 100 μl of rHagB or rFimA (1 μg/ml in carbonate buffer, pH 9.6). After incubation overnight at 4°C, the plates were then incubated with 10 mM Tris buffered saline containing 1% fish gelatin and 0.05% Tween 20 for 30 min at room temperature to block nonspecific binding sites. A total of 100 μl of standard, containing 0, 4, 8, 16, 32 and 64 ng/ml of IgA, IgM or IgG (Zymed Laboratories, Inc., CA, USA) were added to the wells containing the 1.5 μg/ml antibody to the heavy and light chain of mouse immunoglobulin. NWF, BALF, saliva and serum were diluted and 100 μl of each sample was added to the wells containing bound rHagB or rFimA. After incubation at 37°C for 2 h, the plates were washed three times with blocking buffer and the appropriate horseradish peroxidase-labeled goat antimouse secondary antibody was added (Southern Biotechnology Associates, Inc., AL, USA). The plates were incubated at 37 °C for 2 h and washed with blocking buffer. A total of 100 μl of azino-diethyl-benzthi-azoline-sulfonate substrate (ABTS, Southern Biotechnology Associates, Inc.) was added and the plates were incubated at room temperature for 30 min. Optical density values were determined at 405 nm (PowerWaveX™, Bio-Tex Instruments, Inc., VT, USA).
Statistics
A log10 transformation was applied to the cytokine responses in the NWF to account for the positive skewness in the measurements and to make the assumption of normality more defensible [18]. In cases where cytokine concentrations could not be determined by the Beadview™ software (Millipore, MO, USA), a value of 1.3 pg/ml was used before the log10 transformation. This value represents 1 pg/ml less than the lowest value of the standard curve (e.g., 2.3 pg/ml). Analysis of variance (ANOVA) models were fitted to the log-transformed responses using SAS (version 9.1, SAS Institute, NC, USA). A one-way ANOVA was used to analyze the effect of HNPs or HBDs on rHagB or rFimA and a Dunnett's multiple comparison test was used to compare the group differences to that induced by rHagB alone (positive control) and by PBS alone (negative control). To verify the trends, a t-test (least significant difference) was performed on the log10 IL-6 values.
A one-way ANOVA was also applied to analyze the effect of HNPs or HBDs on the antibody concentrations induced by rHagB or rFimA. In cases where antibody concentrations were not detected, 1 ng/ml was added to each number in the data set before the log10 transformation. A Dunnett's multiple comparison test was used to compare the group differences with that induced by rHagB alone (positive control) and by PBS alone (negative control). An overall 0.05 level of statistical significance was used in conjunction with the multiple comparisons adjustment.
Results
Immunizing doses
Mice exposed to 1, 5 and 10 μg rHagB and PBS (20 mice) or 1, 5 and 10 μg rFimA and PBS (20 mice) had clear dose-related antibody responses in their serum. Mice immunized with 10 μg rHagB or rFimA had the highest serum IgG responses (p < 0.05). Therefore, 10 μg rHagB and 10 μg rFimA were used as the exposure and immunizing doses.
Mice given 10 μg rHagB had higher IL-6 and KC cytokine responses in the NWF than mice given heated rHagB, 19 ng LPS or PBS (Table 1).
Table 1.
Mice given rHagB had higher IL-6 and keratinocyte-derived cytokine responses in the nasal wash fluid than mice given boiled rHagB, lipopolysaccharide or phosphate-buffered saline.
| Cytokine | rHagB* | rHagB(boiled)‡ | LPS§ | PBS¶ |
|---|---|---|---|---|
| IL-6 | 1143.0 (664.4) | 519.3 (435.5) | 63.0 (11.1) | 2.3 (1.9)# |
| KC | 1399.7 (370.7) | 1118.0 (291.0) | 689.3 (75.5) | 219.7 (99.4)# |
Data given are the mean (standard error of the mean).
LPS content in a 10-μl dose of PBS containing 10 μg rHagB was 19 ng LPS.
Heated at 100°C for 15 min.
LPS content in a 10-μl dose of PBS was 19 ng LPS (equivalent to that found in 10 μg rHagB).
LPS content in a 10 μl dose of 0.01 M PBS, pH 7.2 (prepared with pyrogen-free water) was 0.0001 pg LPS.
p <0.05, treated vs rHagB control (significance was based on the log 10 transformed data).
KC: Keratinocyte-derived chemokine; LPS: Lipopolysaccharide; PBS: Phosphate buffered saline; rHag: Recombinant hemagglutinin.
Mice given HBDs, HNPs and PBS did not produce high IL-6 or KC cytokine responses in the NWF or specific antibody responses in mucosal fluids or serum. Defensin control groups were included in Figure 1, but were not included in Figure 2 and Tables 2 & 3.
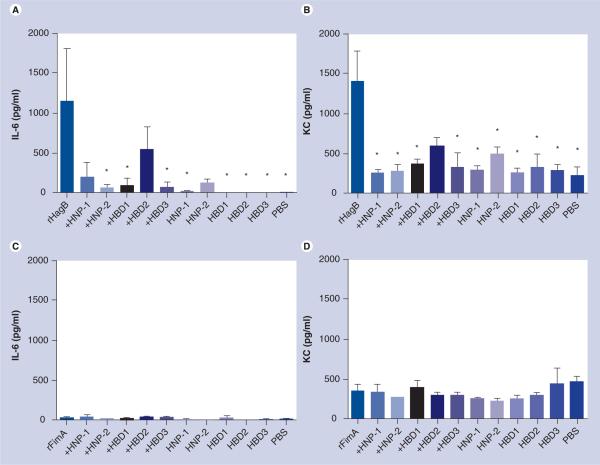
Figure 1. A proinflammatory IL-6 cytokine and keratinocyte-derived chemokine response in the nasal wash fluid of mice exposed intranasally with 10 μg rHagB (A & B) or 10 μg rFimA (C & D) without and with 1 μg HNP1 or 2, and HBD1, 2 or 3.
Mice given rHagB + HNP2, rHagB + HBD1, and rHagB + HBD3 produced significantly lower (p < 0.05) IL-6 responses than mice given rHagB alone (A) and mice given rHagB + HNP1, rHagB + HNP2, rHagB + HBD1, and rHagB + HBD3 produced significantly lower (p < 0.05) KC responses than mice given rHagB alone (B). Mice given rFimA produced very low levels of IL-6 and only moderate levels of KC in nasal wash fluid that were not attenuated by prior incubation of rFimA with any defensin (C & D). Bars represent the mean with standard errors of the mean.
*p < 0.05, treated versus rHagB control (significance was based on the log10 transformed data).
HBD: Human β-defensin; HNP: Human neutrophil peptide α-defensin; KC: Keratinocyte-derived chemokine; PBS: Phosphate-buffered saline; rFim: Recombinant fimbrillin; rHag: Recombinant hemagglutinin.
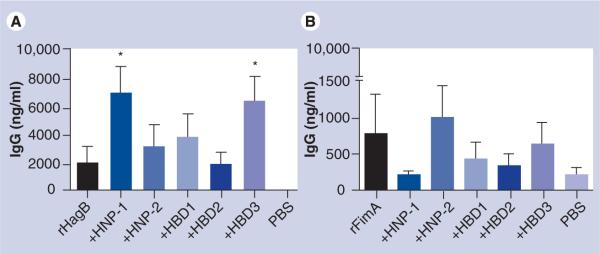
Figure 2. IgG antibody responses in the serum of mice immunized with 10 μg rHagB (A) or 10 μg rFimA (B) without and with 1 μg HNP1 or 2, and HBD1, 2 or 3.
Both rHagB and rFimA induced a serum IgG antibody response; the serum IgG antibody response induced by rHagB was higher than that induced by rFimA. Mice given rHagB + HNP1 and rHagB + HBD3 produced a significantly higher (p < 0.05) serum IgG antibody response than mice given PBS (A), and mice given rFimA + HNP2 produced a higher, but not significant, antibody response than mice given rFimA and PBS (B). Bars represent the mean with standard errors of the mean.
*p < 0.05, treated versus PBS control (significance was based on the log10 transformed data).
HBD: Human β-defensin; HNP: Human neutrophil peptide α-defensin; PBS: Phosphate-buffered saline; rFim: Recombinant fimbrillin; rHag: Recombinant hemagglutinin.
Table 2.
Descriptive statistics of the antibody response in nasal wash fluid, bronchoalveolar lavage fluid, saliva and serum of mice immunized intranasally with rHagB from Porphyromonas gingivalis without and with HNPs and HBDs.
| Antibody | Fluid | rHagB | rHagB + HNP1 | rHagB + HNP2 | rHagB + HBD1 | rHagB + HBD2 | rHagB + HBD3 | PBS |
|---|---|---|---|---|---|---|---|---|
| IgA | NWF | 0.0–136.3 (0.0) | 0.0–1344.0 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–206.0 (0.0) | 0.0–0.0 (0.0) |
| BALF | 0.0–3.2 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | |
| Saliva | 0.0–271.1 (0.0) | 0.0–197.8 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–9.2 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | |
| Serum | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–406.3 (0.0) | 0.0–0.0 (0.0) | 0.0–132.3 (0.0) | 0.0–48.7 (0.0) | 0.0–286.8 (0.0) | |
| IgM | NWF | 0.0–2.6 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–14.8 (0.3) |
| BALF | 0.0–651.0 (2.4) | 0.0–43.6 (0.1) | 0.0–2.8 (0.1) | 0.0–1.4 (0.0) | 0.0–0.8 (0.0) | 0.0–1.2 (0.0) | 0.0–37.4 (0.1) | |
| Saliva | 0.0–29.1 (0.1) | 0.0–0.7 (0.0) | 0.0–17.2 (0.0) | 0.0–1.7 (0.0) | 0.0–0.0 (0.0) | 0.0–6.7 (0.0) | 0.0–20.3 (1.8) | |
| Serum | 41.6–651.0 (367.5) | 46.5–651.0 (553.2) | 21.8–651.0 (618.4) | 60.4–651.0 (519.6) | 72.7–651.0 (442.1) | 76.4–651.0 (401.7) | 57.0–651.0 (262.8) | |
| IgG | NWF | 0.0–0.8 (0.0) | 0.0–3.3 (0.2) | 0.0–68.2 (0.0) | 0.0–15.4 (0.0) | 0.0–0.5 (0.0) | 0.0–219.6 (0.0) | 0.0–0.0 (0.0) |
| BALF | 0.0–88.8 (0.0) | 0.0–7.4 (0.0) | 0.0–65.9 (0.0) | 0.0–59.9 (0.0) | 0.0–28.2 (0.0) | 0.0–32.4 (0.3) | 0.0–0.0 (0.0) | |
| Saliva | 0.0–32.3 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | |
| Serum | 0.0–12,800.0 (270.6) | 12.8–12,800.0 (9129.8) | 1.8–12,800.0 (330.0) | 0.0–12,800.0 (1372.6) | 0.0–7248.1 (171.1) | 2.3–12,800.0 (6763.9) | 0.0–24.6 (0.0) | |
Data shown are the minimum value–maximum value (median value).
BALF: Bronchoalveolar lavage fluid; HBD: Human β-defensin; HNP: Human neutrophil peptide α-defensin; NWF: Nasal wash fluid; PBS: Phosphate-buffered saline; rHag: Recombinant hemagglutinin.
Table 3.
Descriptive statistics of the antibody response in nasal wash fluid, bronchoalveolar lavage fluid, saliva and serum of mice immunized intranasally with rFimA from Porphyromonas gingivalis without and with HNPs and HBDs.
| Antibody | Fluid | rFimA | rFimA + HNP1 | rFimA + HNP2 | rFimA + HBD1 | rFimA + HBD2 | rFimA + HBD3 | PBS |
|---|---|---|---|---|---|---|---|---|
| IgA | NWF | 4.0–253.7 (6.0) | 5.8–50.4 (7.3) | 7.5–61.3 (22.6) | 4.6–18.5 (7.7) | 4.6–73.0 (6.7) | 5.6–120.6 (9.6) | 3.8–8.4 (5.0) |
| BALF | 1.2–22.5 (5.0) | 1.2–8.3 (3.8) | 2.5–10.4 (5.3) | 0.9–7.7 (3.8) | 0.0–10.1 (2.8) | 2.2–21.6 (4.7) | 1.6–3.8 (2.2) | |
| Saliva | 12.1–215.8 (12.8) | 8.4–38.6 (10.0) | 10.1–57.4 (23.0) | 8.4–22.8 (10.1) | 9.2–32.7 (9.8) | 8.5–77.8 (11.5) | 8.5–11.0 (10.6) | |
| Serum | 13.4–94.2 (58.4) | 11.9–217.7 (26.3) | 17.2–147.1 (105.8) | 10.0–91.2 (33.3) | 31.2–128.4 (79.5) | 29.9–338.9 (76.7) | 23.9–175.9 (29.9) | |
| IgM | NWF | 10.0–22.0 (11.6) | 10.0–15.5 (12.4) | 10.8–22.0 (14.1) | 11.6–13.3 (12.0) | 10.4–12.8 (12.0) | 11.2–14.1 (11.2) | 11.6–15.5 (12.4) |
| BALF | 10.9–13.5 (11.9) | 11.2–13.9 (11.9) | 11.2–13.5 (11.6) | 11.2–14.7 (12.7) | 10.5–13.1 (12.3) | 10.5–16.7 (13.9) | 10.9–13.5 (11.9) | |
| Saliva | 14.7–57.7 (20.6) | 14.7–28.1 (21.5) | 10.0–78.0 (18.9) | 11.5–47.1 (15.5) | 9.2–13.1 (10.7) | 7.7–13.1 (11.5) | 10.0–27.2 (25.2) | |
| Serum | 373.6–661.0 (504.8) | 287.8–509.2 (356.0) | 281.8–429.9 (303.2) | 205.0–1090.2 (332.2) | 156.1–478.9 (275.8) | 284.8–1634.1 (639.7) | 300.1–751.9 (433.8) | |
| IgG | NWF | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) | 0.0–0.0 (0.0) |
| BALF | 0.0–88.8 (0.0) | 0.0–7.4 (0.0) | 0.0–65.9 (0.0) | 0.0–59.9 (0.0) | 0.0–28.2 (0.0) | 0.0–32.4 (0.3) | 0.0–0.0 (0.0) | |
| Saliva | 13.4–19.0 (14.7) | 13.0–19.0 (14.7) | 13.9–18.1 (14.3) | 13.4–19.9 (14.7) | 13.0–17.3 (14.7) | 12.2–18.6 (15.1) | 13.0–15.6 (13.9) | |
| Serum | 89.0–3021.6 (204.5) | 117.0–353.6 (208.4) | 113.0–2442.3 (436.1) | 87.0–1385.5 (154.1) | 93.0–995.7 (181.3) | 200.6–1906.1 (288.8) | 109.1–622.6 (111.0) | |
Data given are the minimum value–maximum value (median value).
BALF: Bronchoalveolar lavage fluid; HBD: Human β-defensin; HNP: Human neutrophil peptide α-defensin; NWF: Nasal wash fluid; PBS: Phosphate-buffered saline; rHag: Recombinant hemagglutinin.
Defensins attenuate proinflammatory cytokine responses to rHagB
Mice were found to have high levels of IL-6 and KC in NWF 24 h after intranasal administration of rHagB (Figure 1). Mice given rHagB + HNP2, rHagB + HBD1, rHagB + HBD3, HNP1, HBD1, HBD2, HBD3 and PBS produced significantly lower (p < 0.05) IL-6 responses than mice given rHagB alone (Figure 1A). Mice given rHagB + HNP1, rHagB + HBD2 and HNP2 did not produce significantly lower (p < 0.05) IL-6 responses than mice given rHagB alone. Mice given HNP1, HBD1, HBD2 and HBD3 did not produce significantly higher (p < 0.05) IL-6 responses than mice given PBS.
Mice given rHagB + HNP1, rHagB + HNP2, rHagB + HBD1, rHagB + HBD3, HNP1, HNP2, HBD1, HBD2, HBD3 or PBS produced significantly lower (p < 0.05) KC responses than mice given rHagB alone (Figure 1B). Mice given rHagB + HBD2 did not produce significantly lower (p < 0.05) IL-6 responses than mice given rHagB alone. Mice given HNP2 produced significantly higher (p < 0.05) IL-6 response than mice given PBS, and mice given HNP1, HBD1, HBD2 and HBD3 did not produce significantly higher (p < 0.05) IL-6 responses than mice given PBS.
Other proinflammatory cytokines, Th1 cytokines and Th2 cytokines were not induced by rHagB. Levels of these cytokines in the serum were similar to the baseline levels reported previously [23].
Defensins do not attenuate proinflammatory cytokine responses to rFimA
Mice were found to have very low levels of IL-6 and only moderate levels of KC in NWF 24 h after intranasal administration of rFimA. These cytokine responses were not dampened by prior incubation of rFimA with any defensin used and there were no differences among IL-6 levels (Figure 1C) and KC levels (Figure 1D). Other proinflammatory cytokines, Th1 cytokines and Th2 cytokines were not induced by rFimA, and levels of these cytokines in the serum were similar to the baseline levels reported previously [23].
Defensins enhance antibody responses to rHagB
A total of 21 days after intranasal administration of rHagB without and with HNPs or HBDs, mice did not have consistent or significant IgA, IgM or IgG antibody responses in NWF, BALF or saliva or IgA antibody responses in serum. The descriptive statistics are shown in Table 2. Columns contain the minimum value–maximum value and the median value, and show that IgA, IgM or IgG antibody responses occurred only in one or two mice per group.
Intranasal administration of rHagB induced IgM antibody responses in serum that were similar among groups (Table 2). Intranasal administration of rHagB induced a high IgG antibody response in serum (Figure 2A). There was some variability with some interesting trends.
Mice given rHagB + HNP1 produced significantly higher (p < 0.05) serum IgG antibody responses than mice given rHagB alone. Mice given rHagB + HNP1 and rHagB + HBD3 produced a serum IgG antibody response that was significantly higher (p < 0.05) than that induced in mice by PBS. This trend was lost after transformation of the data. However, this trend was confirmed by a similar IgG1 antibody response. Mice given rHagB + HBD3 produced a serum IgG1 antibody response that was significantly higher (p < 0.05) than that in mice given rHagB and PBS. After log10 transformation of the data, rHagB + HNP1, rHagB + HNP2, rHagB + HBD2 and rHagB + HBD3 were all significantly higher (p < 0.05) than that in mice given PBS.
Defensins enhance antibody responses to rFimA
A total of 21 days after intranasal administration of rFimA without and with HNPs or HBDs, mice did not have a consistent or significant IgA, IgM or IgG antibody response in NWF, BALF or saliva. The descriptive statistics are shown in Table 3. Again, columns contain the minimum value–maximum value and the median value, and show that elevated IgA or IgM concentrations occur in one or two mice per group. Mice given rFimA induced some IgA and IgM antibody responses in serum (Table 3).
Mice given rFimA produced a variable serum IgG antibody response with some interesting trends (Figure 2B). Mice given rFimA + HNP2 produced a high, but not significant, response. However, mice given rFimA + HBD3 produced a significantly higher (p < 0.05) serum IgG1 response, mice given rFimA, rFimA + HNP2 and rFimA + HBD3 produced a significantly higher serum IgG2b response, and mice given rFimA produced a significantly higher (p < 0.05) serum IgG3 response than mice given PBS.
Discussion
Human neutrophil peptide α-defensin 2, HBD1 and HBD3 attenuate proinflammatory IL-6 cytokine responses in NWF to rHagB, HNP1, HNP2, HBD1, HBD2 and HBD3 attenuate KC chemokine responses in NWF to rHagB, and HNP1 and HBD3 enhance antibody responses to rHagB. Interestingly, no HNP or HBD used in this study attenuate the cytokine response to rFimA. HNP1 and HBD3 enhance a serum IgG response to rHagB, and HNP2 and HBD3 enhance the IgG antibody responses in BALF and serum to rFimA. These results confirm and extend our previous results and those of others demonstrating that HBD3 and HNP1 and 2 can bind to rHagB and rFimA, HBD3 can attenuate proinflammatory cytokine responses of human myeloid dendritic cells exposed to rHagB, and HNP1–3 and HBD1 and 2 can enhance antibody responses to coadministered antigens [18,19,21,22,24]. We noted that intranasal administration of HNPs, HBDs or PBS alone to mice did not induce significant proinflammatory cytokine responses in mucosal fluids or serum. We also noted that mice given 10 μg rHagB had higher IL-6 and KC cytokine responses in the NWF than mice given heated rHagB, 19 ng LPS or PBS (Table 1). The IL-6 response of LPS was dramatically, although not significantly, lower (p = 0.0581), than the IL-6 response of rHagB. This suggests that trace LPS content could become problematic in mouse studies using higher doses of rHagB, a situation that will be monitored closely in subsequent studies.
Interestingly, only IL-6 and KC were induced in mice by rHagB and to a lesser extent by rFimA. This cytokine profile is consistent with a Th17 response. To what extent rHagB induces a Th17 response, and whether HNPs and HBDs attenuate via this mechanism, needs to be determined.
A mouse model to assess defensin attenuation of proinflammatory cytokine responses in nasal lavage fluid and enhanced antibody serum responses to P. gingivalis rHagB and rFimA depends, of course, upon whether HNPs and HBDs match mouse homologs closely enough to give accurate, correlated results. These results and those of others suggest that HNPs and HBDs do work well in mouse models of inflammation [17]. Schutte and colleagues, using a genomics approach, identified 28 HBDs and 43 mouse β-defensin genes in five syntenic chromosomal regions. Within each syntenic cluster, the gene sequences and organization were similar, suggesting each cluster pair arose from a common ancestor and was retained because of conserved functions [25]. Since the residues important for the structure and function of these proteins are conserved [26], it is rational to suggest that HBD with similar structure and function can be used in a mouse model. HNPs have greater than 60% homology and 40% identity with murine crypt cell defensins [26–28] and have previously been shown to enhance adaptive immunity in mice to ovalbumin [22]. Mouse β-defensin 1 is the ortholog of HBD1 [29,30], mouse β-defensin 3 is the ortholog of HBD2 [31,32] and mouse β-defensin 14 is the ortholog of HBD3 [33–35].
Clearly, the ability of defensins to attenuate proinflammatory cytokines was more dramatic than their ability to enhance the antibody responses to these microbial adhesins. The latter may be owing to the fact that the rHagB or rFimA and defensins were given as simple admixtures intranasally to mice. The outcome may have been more dramatic if the defensins were emulsified or conjugated to these microbial adhesins and injected systemically. Finally, these results suggest that mice respond differently to rHagB than rFimA. rHagB is more inflammatory in mice than rFimA and perhaps a better immunogen. In single or repeated intranasal administrations, rHagB induced both a higher proinflammatory cytokine response and a serum IgG antibody response. Historically, rHagB is known to be an antigen that responds well to adjuvants. Subcutaneous injection or intranasal exposure of mice with rHagB and 1% aluminum potassium sulfate (alum), a semisynthetic saponin analog (GPI-0100 containing a dodectlamide), monophosphoryl lipid A or Escherichia coli heat-labile enterotoxin all induced significantly higher serum IgG responses [36,37]. rHagB-specific IgG antibody responses were all significantly higher in mice immunized with rHagB and adjuvant than IgG antibodies in mice immunized with rHagB alone.
Our results suggest that the composition of the antigen may also be important. Previous studies assessed the ability of HNPs and HBDs to enhance the antibody responses to intranasal administration of ovalbumin [18,19,21,22,24] and this study assessed the ability of HNPs and HBDs to attenuate cytokine and chemokine responses and to enhance the antibody responses to intranasal administration of rHagB and rFimA. CD4+ Th cells are classified as Th1, Th2 and Th17 based on their cytokine expression profile and, in our first study, rHagB induced the production of IL-6 and KC in nasal lavage fluid of mice, a cytokine expression profile characteristic of a Th17 response. HNP2, HBD1 and HBD3 attenuated the IL-6 responses and HNP1, HNP2, HBD1 and HBD3 attenuated the KC responses to rHagB.
In our second study, HNP1 and HBD3 enhanced the antibody responses to intranasal administration of rHagB and, to a lesser extent, HNP2 and HBD3 enhanced the IgG antibody responses in BALF and serum to rFimA. Although the cytokine profiles of rHagB- and rFimA-stimulated CD4+ Th cells from these immunized mice were not determined, they may be similar to that previously reported for studies done similarly and characterized by a Th1/2 response. Lillard et al. reported that intranasal delivery of ovalbumin + HNPs enhanced ovalbumin-specific serum IgG antibody responses, and CD4+ Th cells of intranasally immunized mice displayed higher ovalbumin-specific proliferative responses and elevated production of the CD4+ Th1 and Th2 cytokines; IFN-γ, IL-5, IL-6 and IL-10 were elevated when compared with control groups receiving ovalbumin alone [22]. In our earlier work, we reported that intranasal delivery of ovalbumin + HBD1 enhanced ovalbumin-specific serum IgG and IgM antibody responses and CD4+ Th cells of intranasally immunized mice displayed elevated production of IL-10 [21]. Intranasal delivery of ovalbumin + HBD2 also enhanced the production of ovalbumin-specific serum IgG but not ovalbumin-specific serum IgM. CD4+ Th cells of intranasally immunized mice also produced lower amounts of IFN-γ. Clearly, more work needs to be done to firmly establish the role of the composition of the antigen in defensin-mediated responses.
These results also suggest that the interaction among HNPs or HBDs with rHagB and rFimA may be important. Previously we found that HBD3, HNP2 and HNP1 (in decreasing order) bind to rHagB, and HNP2, HNP1 and HBD3 (in decreasing order) bind to rFimA by surface plasmon resonance spectroscopy [18,19]. Surface plasmon resonance spectroscopy response units were higher with these defensins than for HBD1 and HBD2, which did not bind as strongly. It is possible that the strong binding of defensins to antigens would attenuate their ability to induce proinflammatory cytokines yet facilitate delivery of bound antigen to antigen-presenting cells with defensin receptors.
Conclusion
The oronasal cavity is rich in HNPs and HBDs [3,38,39] and is a prime site for initiating defensin-triggered adaptive immune responses to microbial antigens, including those of the periodontal pathogen, P. gingivalis. Our results suggest that HNP1, HNP2 and HBD3 may attenuate proinflammatory cytokine responses and enhance antibody responses to select microbial antigens. The responses were unique in that they were directed towards a particular P. gingivalis adhesin, rHagB, and involved the HNPs and HBD3. Further studies will assess whether this response is seen with other bacterial antigens and to determine if there is a potential for manipulation of this regulated response to treat or prevent periodontal disease and other oral infections.
Executive summary.
-
■
Recombinant hemagglutinin B (rHagB), but not recombinant fimbrillin A (rFimA), induced a high IL-6 cytokine and keratinocyte-derived chemokine response in the nasal wash fluid (NWF), but not in the bronchoalveolar lavage fluid (BALF), saliva or serum of mice 24 h after intranasal exposure.
-
■
The IL-6 response was significantly attenuated if human neutrophil peptide α-defensin (HNP)2, human β-defensin (HBD)1 and HBD3 were added to rHagB prior to intranasal exposure and the keratinocyte-derived chemokine response was significantly attenuated if HNP1, HNP2, HBD1 and HBD3 were added to rHagB prior to intranasal exposure.
-
■
At 21 days, mice given rHagB without or with HNPs or HBDs did not produce significant IgA or IgM antibody responses in saliva, NWF or BALF. Mice given rHagB + HNP1 and rHagB + HBD3 produced a significantly higher (p < 0.05) serum IgG antibody response than mice given phosphate-buffered saline (PBS). Mice given rHagB + HBD3 produced a serum IgG1 antibody response that was significantly higher (p < 0.05) than that in mice given rHagB and PBS.
-
■
At 21 days, mice given rFimA without or with HNPs or HBDs did not produce significant IgA or IgM antibody responses in saliva, NWF, BALF or serum. Mice given rFimA + HNP2 produced a high but not significant antibody response. Mice given rFimA + HBD3 produced a significantly higher (p < 0.05) serum IgG1 response and mice given rFimA, rFimA + HNP2 and rFimA + HBD3 produced a significantly higher serum IgG2b response than mice given PBS.
-
■
HNP1, HNP2 or HBD3, but not HBD1 or HBD2, attenuate proinflammatory cytokine responses in NWF to rHagB and enhance antibody responses to rHagB, but not rFimA, of Porphyromonas gingivalis in mice.
Acknowledgments
Financial & competing interests disclosure The authors were supported by funds from NIH, NIDCR R01 DE014390 and T32 DE014678. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■ of considerable interest
- 1.Menendez A, Finlay B. Defensins in the immunology of bacterial infections. Curr. Opin. Immunol. 2007;19:385–391. doi: 10.1016/j.coi.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Radek K, Gallo R. Antimicrobial peptides: natural effectors of the innate immune system. Semin. Immunopathol. 2007;29:27–43. doi: 10.1007/s00281-007-0064-5. [DOI] [PubMed] [Google Scholar]
- 3.Diamond G, Beckloff N, Ryan LK. Host defense peptides in the oral cavity and the lung: similarities and differences. J. Dent. Res. 2008;87:915–927. doi: 10.1177/154405910808701011. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Detailed and specific review on the characteristics of defensins.
- 4.Rehaume LM, Hancock RE. Neutrophil-derived defensins as modulators of innate immune function. Crit. Rev. Immunol. 2008;28:185–200. doi: 10.1615/critrevimmunol.v28.i3.10. [DOI] [PubMed] [Google Scholar]; ■■ Comprehensive review of the innate immune functions of defensins.
- 5.Garcia JR, Jaumann F, Schulz S, et al. Identification of a novel, multifunctional β-defensin (human β-defensin 3) with specific antimicrobial activity. Its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res. 2001;306:257–264. doi: 10.1007/s004410100433. [DOI] [PubMed] [Google Scholar]
- 6.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 7.Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature. 1997;387:861–862. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 8.Joly S, Organ CC, Johnson GK, McCray PB, Jr, Guthmiller JM. Correlation between β-defensin expression and induction profiles in gingival keratinocytes. Mol. Immunol. 2005;42:1073–1084. doi: 10.1016/j.molimm.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Dunsche A, Acil Y, Dommisch H, Siebert R, Schroder JM, Jepsen S. The novel human β-defensin-3 is widely expressed in oral tissues. Eur. J. Oral Sci. 2002;110:121–124. doi: 10.1034/j.1600-0722.2002.11186.x. [DOI] [PubMed] [Google Scholar]
- 10.Varoga D, Klostermeier E, Paulsen F, et al. The antimicrobial peptide HBD-2 and the Toll-like receptors-2 and -4 are induced in synovial membranes in case of septic arthritis. Virchows Arch. 2009;454:685–694. doi: 10.1007/s00428-009-0780-4. [DOI] [PubMed] [Google Scholar]
- 11.Varoga D, Pufe T, Harder J, et al. Production of endogenous antibiotics in articular cartilage. Arthritis Rheum. 2004;50:3526–3534. doi: 10.1002/art.20605. [DOI] [PubMed] [Google Scholar]
- 12.Varoga D, Wruck CJ, Tohidnezhad M, et al. Osteoblasts participate in the innate immunity of the bone by producing human β defensin-3. Histochem. Cell Biol. 2009;131:207–218. doi: 10.1007/s00418-008-0522-8. [DOI] [PubMed] [Google Scholar]
- 13.Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu. Rev. Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]; ■ Comprehensive review of the innate and adaptive immune functions of defensins.
- 14.Song H, Belanger M, Whitlock J, Kozarov E, Progulske-Fox A. Hemagglutinin B is involved in the adherence of Porphyromonas gingivalis to human coronary artery endothelial cells. Infect. Immun. 2005;73:7267–7273. doi: 10.1128/IAI.73.11.7267-7273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park Y, Xie H, Lamont RJ. Transcriptional organization of the Porphyromonas gingivalis fimA locus. FEMS Microbiol. Lett. 2007;273:103–108. doi: 10.1111/j.1574-6968.2007.00782.x. [DOI] [PubMed] [Google Scholar]
- 16.Shi J, Aono S, Lu W, et al. A novel role for defensins in intestinal homeostasis: regulation of IL-1β secretion. J. Immunol. 2007;179:1245–1253. doi: 10.4049/jimmunol.179.2.1245. [DOI] [PubMed] [Google Scholar]; ■■ Recent report showing defensins have powerful anti-inflammatory effects on human monocytes.
- 17.Miles K, Clarke DJ, Lu W, et al. Dying and necrotic neutrophils are anti-inflammatory secondary to the release of α-defensins. J. Immunol. 2009;183:2122–2132. doi: 10.4049/jimmunol.0804187. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ Recent report showing human neutrophil peptide α-defensins (HNPs) have powerful anti-inflammatory effects on human monocyte-derived macrophages.
- 18.Pingel LC, Kohlgraf KG, Hansen CJ, et al. Human β-defensin 3 binds to hemagglutinin B (rHagB), a non-fimbrial adhesin from Porphyromonas gingivalis, and attenuates a pro-inflammatory cytokine response. Immunol. Cell Biol. 2008;86:643–649. doi: 10.1038/icb.2008.56. [DOI] [PubMed] [Google Scholar]; ■■ Human β-defensin (HBD)3 serves as an upstream suppressor of cytokine signaling that binds and attenuates proinflammatory cytokine responses to recombinant hemagglutinin B from Porphyromonas gingivalis strain 381.
- 19.Dietrich DE, Xiao X, Dawson DV, et al. Human α- and β-defensins bind to immobilized adhesins from Porphyromonas gingivalis. Infect. Immun. 2008;76:5714–5720. doi: 10.1128/IAI.00997-08. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ HNPs and HBDs bind to immobilized recombinant hemagglutinin B and recombinant fimbrillin of P. gingivalis strain 381, by surface plasmon resonance spectroscopy.
- 20.Xie H, Chung WO, Park Y, Lamont RJ. Regulation of the Porphyromonas gingivalisfimA (fimbrillin) gene. Infect. Immun. 2000;68:6574–6579. doi: 10.1128/iai.68.12.6574-6579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brogden KA, Heidari M, Sacco RE, et al. Defensin-induced adaptive immunity in mice and its potential in preventing periodontal disease. Oral Microbiol. Immunol. 2003;18:95–99. doi: 10.1034/j.1399-302x.2003.00047.x. [DOI] [PubMed] [Google Scholar]; ■■ Reports that HBDs can serve as adjuvants to potentiate the immune response to ovalbumin in mice.
- 22.Lillard JW, Jr, Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc. Natl Acad. Sci. USA. 1999;96:651–656. doi: 10.1073/pnas.96.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ HNP defensins plus ovalbumin enhance ovalbumin-specific serum IgG antibody responses.
- 23.Lu X, Pingel LC, Burnell KK, Cavanaugh JE, Brogden KA. Carbamoylcholine chloride induces a rapid increase in IL6 in the nasal cavity of C57BL/6 mice. Comp. Med. 2007;57:349–354. [PubMed] [Google Scholar]
- 24.Pingel L, Lu X, Brogden KA. Antimicrobial peptides as mucosal adjuvants. In: Brogden KA, Stanton TB, Cornick N, et al., editors. Virulence Mechanisms of Bacterial Pathogens. ASM Press; DC, USA: 2007. pp. 281–295. [Google Scholar]; ■■ Recent overview on the field of adjuvants.
- 25.Schutte BC, Mitros JP, Bartlett JA, et al. Discovery of five conserved β-defensin gene clusters using a computational search strategy. Proc. Natl Acad. Sci. USA. 2002;99:2129–2133. doi: 10.1073/pnas.042692699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones DE, Bevins CL. Paneth cells of the human small intestine express an antimicrobial peptide gene. J. Biol. Chem. 1992;267:23216–23225. [PubMed] [Google Scholar]
- 27.Eisenhauer PB, Harwig SSL, Lehrer RI. Cryptdins: antimicrobial defensins of the murine small intestine. Infect. Immun. 1992;60:3556–3565. doi: 10.1128/iai.60.9.3556-3565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huttner KM, Selsted ME, Ouellette AJ. Structure and diversity of the murine cryptdin gene family. Genomics. 1994;19:448–453. doi: 10.1006/geno.1994.1093. [DOI] [PubMed] [Google Scholar]
- 29.Bals R, Goldman MJ, Wilson JM. Mouse β-defensin 1 is a salt-sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract. Infect. Immun. 1998;66:1225–1232. doi: 10.1128/iai.66.3.1225-1232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison GM, Davidson DJ, Kilanowski FM, et al. Mouse β defensin-1 is a functional homolog of human β defensin-1. Mamm. Genome. 1998;9:453–457. doi: 10.1007/s003359900795. [DOI] [PubMed] [Google Scholar]
- 31.Bals R, Wang X, Meegalla RL, et al. Mouse β-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect. Immun. 1999;67:3542–3547. doi: 10.1128/iai.67.7.3542-3547.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burd RS, Furrer JL, Sullivan J, Smith AL. Murine β-defensin-3 is an inducible peptide with limited tissue expression and broad-spectrum antimicrobial activity. Shock. 2002;18:461–464. doi: 10.1097/00024382-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Rohrl J, Yang D, Oppenheim JJ, Hehlgans T. Identification and biological characterization of mouse β-defensin 14 – the orthologue of human β-defensin 3. J. Biol. Chem. 2008;283:5414–5419. doi: 10.1074/jbc.M709103200. [DOI] [PubMed] [Google Scholar]
- 34.Hinrichsen K, Podschun R, Schubert S, Schroder JM, Harder J, Proksch E. Mouse β-defensin-14, an antimicrobial ortholog of human β-defensin-3. Antimicrob. Agents Chemother. 2008;52:1876–1879. doi: 10.1128/AAC.01308-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor K, Clarke DJ, McCullough B, et al. Analysis and separation of residues important for the chemoattractant and antimicrobial activities of β-defensin 3. J. Biol. Chem. 2008;283:6631–6639. doi: 10.1074/jbc.M709238200. [DOI] [PubMed] [Google Scholar]
- 36.Yang QB, Martin M, Michalek SM, Katz J. Mechanisms of monophosphoryl lipid A augmentation of host responses to recombinant HagB from Porphyromonas gingivalis. Infect. Immun. 2002;70:3557–3565. doi: 10.1128/IAI.70.7.3557-3565.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang P, Yang QB, Marciani DJ, et al. Effectiveness of the quillaja saponin semi-synthetic analog GPI-0100 in potentiating mucosal and systemic responses to recombinant HagB from Porphyromonas gingivalis. Vaccine. 2003;21:4459–4471. doi: 10.1016/s0264-410x(03)00438-9. [DOI] [PubMed] [Google Scholar]
- 38.Davison G, Allgrove J, Gleeson M. Salivary antimicrobial peptides (LL-37 and α-defensins HNP1-3), antimicrobial and IgA responses to prolonged exercise. Eur. J. Appl. Physiol. 2009;106:277–284. doi: 10.1007/s00421-009-1020-y. [DOI] [PubMed] [Google Scholar]
- 39.Dale BA, Tao R, Kimball JR, Jurevic RJ. Oral antimicrobial peptides and biological control of caries. BMC Oral Health. 2006;6:S13. doi: 10.1186/1472-6831-6-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]