Abstract
The dopamine transporter (DAT) is a primary determinant of the concentration of dopamine in the synapse and is involved in a number of psychiatric and neurological diseases. The transporter actively takes up its physiological substrate, dopamine, when it is on the surface of the plasmalemmal membrane, but the concentration of DAT in the membrane is highly regulated by substrate. Substrates initially, and very rapidly, recruit more DAT into the membrane for greater function, but continued presence of substrate downregulates the activity of DAT and even membrane DAT content. This biphasic regulation is orchestrated by numerous signal transduction mechanisms, including a palette of protein kinases. Understanding the mechanisms of rapid regulation of DAT could provide new therapeutic strategies to improve transporter function and modulate responses to its more notorious substrates, amphetamine and methamphetamine.
Keywords: amphetamine, endocytosis, PKCβ, recycling, trafficking
Dopamine (DA), a monoaminergic neurotransmitter, is involved in the control of movement, reward, motivation, emotion, learning, and pituitary and hypothalamic function. The DA transporter (DAT), which removes DA from the synapse through reuptake, is crucial for the regulation of dopaminergic signaling and homeostasis in many areas of the brain. DAT (SLC6A3) belongs to the SLC6 Na+/Cl−-dependent transporter family, which also consists of serotonin transporter (SERT) and norepinephrine transporter (NET). DAT tightly controls the duration and strength of dopaminergic neurotransmission at the synapse by taking up extraneuronal DA into presynaptic terminals, thus terminating DA action at pre- and post-synaptic sites [1]. Dysfunctional dopaminergic signaling related to DAT has been implicated in multiple neurodegenerative and psychiatric disorders, including Parkinson's disease, schizophrenia, bipolar disorder, attention-deficit/hyperactivity disorder and drug addiction [2–4]. Recently, the molecular basis of infantile parkinsonism-dystonia was traced to a homozygous loss-of-function mutation in DAT [5]. Furthermore, the expression density of DAT strongly correlates with the extent of DA cell loss in Parkinson's disease [6]. Coding variants of DAT have been identified in some patients with attention-deficit/hyperactivity disorder and bipolar disorder [7]. DAT is a target of abused psychostimulants, such as methamphetamine and cocaine, and therapeutic drugs, such as amphetamine (AMPH; Dexedrine and Adderall) and methylphenidate (Ritalin), as well as the antidepressant and antismoking drug, bupropion (Wellbutrin and Zyban).
The primary function of DAT is to remove DA from the synaptic cleft; the newly taken-up DA then re-enters the synaptic vesicle through the vesicular monoamine transporter (VMAT)-2 for storage until it is released again by exocytosis. DAT functions as a gated channel, in that it is open to either the extracellular or intracellular milieu, but not both. These conformations are delineated as outward-facing and inward-facing, respectively (see Figure 1). It is presumed that when extracellular substrate, together with sodium and chloride, bind to DAT, they promote change in the conformation of DAT from one that is primarily outward-facing to one predominantly inward-facing. Recently, a molecular model was proposed to illustrate these conformational changes of DAT [8]. In this model, a salt bridge between Arg-60 in the N-terminus close to the cytoplasmic end of transmembrane (TM)-1 and Asp-436 at the cytoplasmic end of TM8 of DAT was demonstrated to be stabilized by a cation-π interaction between Arg-60 and Tyr-335 at the cytoplasmic end of TM6. The continuous interruption and reformation of the salt bridge was hypothesized to determine the inward- and outward-facing state. However, the mechanism governing the conformational transition of DAT is not understood.
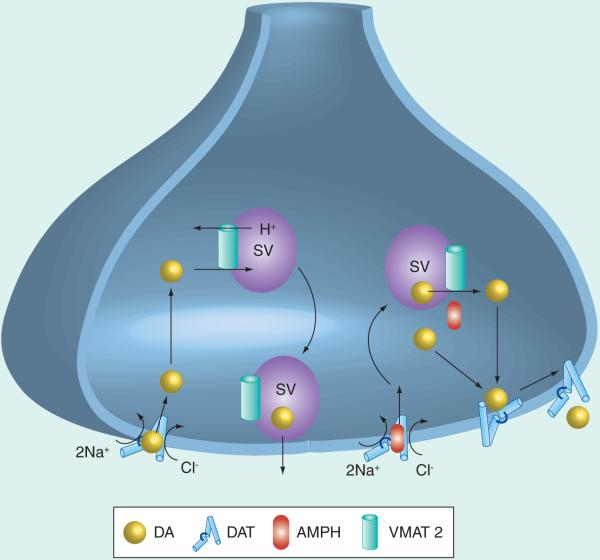
Figure 1. Transporters regulate dopamine reuptake and storage in the nerve terminal.
The plasmalemmal transporter, DAT, is a Na+, Cl−-dependent transporter that is normally outward-facing on the plasmalemmal membrane. Na+ that is transported into the terminal is transported back to the extracellular milieu by Na+, K+-ATPase. Following exocytotic release, the substrate DA diffuses to the transporter, binds to DAT and is transported into the cytosol. DA in the cytosol is transported into synaptic vesicles through the H+-ATPase-coupled VMAT2. AMPH (and methamphetamine), as a substrate, binds to DAT, is transported inside the terminal along with Na+ and Cl−, and depletes DA from the vesicle. However, upon dissociation of AMPH, cytosolic DA can rebind to the now inward-facing transporter; the transporter then reverses such that DA is transported back into the synapse. AMPH also inhibits the ability of DA to be transported by VMAT2 into the synaptic vesicle. The increased concentration of DA is then transported into the synapse, increasing the maximal amount of DA that can be effluxed in response to AMPH.
AMPH: Amphetamine; DA: Dopamine; DAT: DA transporter; SV: Synaptic vesicles; VMAT2: Vesicular monoamine transporter 2.
Both AMPH and cocaine, through distinct mechanisms at DAT, inhibit DA reuptake and increase extracellular levels of DA. Cocaine is a DAT blocker, while AMPH is a substrate of DAT and is taken up into the nerve terminal. AMPH competitively inhibits uptake of DA into the terminal at DAT and into the synaptic vesicle at VMAT2. Thus, AMPH prevents proper storage of DA. AMPH is also a weak inhibitor of monoamine oxidase activity. Once inside the terminal, AMPH promotes DA efflux through a functional reversal of the transporter (see Figure 1). While there are several models describing AMPH-stimulated DA efflux (for review see [9,10]), it is generally thought that AMPH translocation through DAT results in an altered electrochemical gradient and a DAT conformation (inward-facing), which favors DA efflux [11].
The importance of DAT in maintaining DA homeostasis is exemplified in mice lacking the DAT gene. DAT-knockout mice have increased basal locomotor activity compared with wild-type mice and high levels of extracellular DA [12]. The DAT-knockout mice also have reduced DA tissue content, reduced DA release and reduced DA receptors relative to wild-type controls, underscoring the importance of DAT in the maintenance of normal DA levels. Although DAT-knockout mice were unresponsive to the locomotor effects of cocaine and AMPH, they still exhibited cocaine- and amphetamine-induced conditioned place preference, resulting from neuroadaptation in the lifetime absence of the DAT gene. However, cocaine-induced locomotor activity and conditioned place preference were abolished in mice in which mutations on DAT residues abrogated cocaine binding to DAT [13], giving emphasis to this protein as the major site of psychostimulant reward function.
Considering that the primary function of DAT is the reuptake of synaptic DA, the protein will only be effective when it is inserted into the plasmalemmal membrane. Similar to most other membrane-bound proteins, DAT is not static in the membranes, but traffics between the plasmalemmal membrane and subcellular organelles in a regulated fashion. DAT trafficking is subjected to regulation by DAT substrates, inhibitors and a complicated protein network, including protein kinases, DAT-interacting proteins, oligomerization and glycosylation [14]. Numerous reviews have covered facets of DAT trafficking, particularly the internalization of DAT [1,15]. However, rapid DAT trafficking to the surface has not been fully investigated and understood. In this review, we summarize the recent findings in this field, focusing on the characterization and potential mechanisms of a time-dependent, biphasic effect of DAT substrates and PKC on DAT trafficking. Since DAT plays such a critical role in normal dopaminergic transmission, and because its location at the surface of the neuron is required for normal function, strategies to regulate the trafficking of DAT could be therapeutically useful in a number of disease states.
Constitutive DAT trafficking
The trafficking of newly synthesized DAT from the endoplasmic reticulum and Golgi through to lysosomes has been succinctly described in a recent review by Zahniser and Sorkin [1]. As shown in Figure 2, DAT undergoes constitutive internalization [16–21] and recycling to the surface [16,18,20,22], with a surface half-life of approximately 13 min when measured in heterologous systems [16,23]. Constitutive bidirectional movement of DAT labeled with fluorescent cocaine analogs has been demonstrated by fluorescence recovery after photobleaching analysis in neurites and varicosities of cultured live midbrain dopaminergic neurons [20]. These studies indicated that DAT was highly mobile in both extensions and varicosities of the neurons. DAT constitutively internalized into vesicular structures and partially colocalized with transferrin. Dynamin-dependence of DAT constitutive endocytosis has also been demonstrated in heterologous cell systems using either overexpression of a dominant-negative dynamin I mutant (K44A) or knockdown of clathrin heavy chain [18,20,24–26].
Figure 2. Dopamine transporter undergoes constitutive and substrate-induced trafficking to the surface and endocytosis.
DAT constitutively internalizes to and recycles from the recycling endosome and early endosome). The basal surface DAT endocytic rate is 3–5%/min. The maintenance of the basal surface DAT is PKCβ- and Rab11-dependent. DAT also undergoes time-dependent biphasic trafficking upon substrate exposure. Short-term exposure of substrates (DA or AMPH) stimulate an ultra-rapid recycling of DAT that is PKCβ dependent. Upon continuous substrate administration, DAT invaginates into membrane pits (probably when it is in the inward-facing form) and internalizes. CaMKII contributes to substrate-induced internalization.
AMPH: Amphetamine; DA: Dopamine; DAT: DA transporter; CaMKII: Ca2+/calmodulin-dependent protein kinase II; EE: Early endosome; RE: Recycling endosome. VMAT2: Vesicular monoamine transporter 2.
Internalized DAT colocalizes with the small GTPases Rab-5 and -11, which are markers for recycling endosomes [20,27], demonstrating that DAT internalizes into recycling endosomes. Rab11 has recently been demonstrated to be involved in constitutive DAT recycling. Overexpression of the constitutively active Rab11 mutant in N2A neuroblastoma cells stably expressing human DAT (hDAT) resulted in increased surface DAT expression, whereas the dominant-negative Rab11 mutant led to reduced surface DAT expression [28]. Although DAT lacks conventional endocytic signals, basal DAT trafficking is directed by a constitutive motif in the C-terminus of DAT (the amino acid residues 589–598), demonstrated by the observation that mutations in this motif increase the endocytic rate of DAT, and impair DAT exit from the endoplasmic reticulum and recycling to the surface [17,18,29,30]. Residues in the N-terminus of DAT (amino acids 60–65) also play an important role in constitutive DAT endocytosis because mutations in these residues or removal of the entire N-terminus of DAT results in retention of the DAT mutant in early and recycling endosomes [24]. Furthermore, the N-terminus is necessary for the trafficking of newly synthesized DATs to the plasma membrane, as demonstrated by an intracellular accumulation of a DAT mutant lacking the first 60 residues of the N-terminus [31]. The retention of the DAT mutant in the endosomes is primarily attributed to accelerated constitutive endocytosis, rather than retarded recycling since monesin, a recycling inhibitor, results in greater endosomal accumulation of the DAT mutant than that of controls [24].
DAT recycling is regulated by PKC. PKC activation by phorbol 12-myristate 13-acetate (PMA) in PC-12 cells stably expressing hDAT increases the DAT endocytic rate and decreases the transporter recycling rate, thus reducing DAT surface levels [16]. Among all PKC isoforms, PKCβ is potentially important in constitutive maintenance of the surface DAT (Figure 2). Compared with wild-type mice, PKCβ-knockout mice have a reduced surface DAT expression level and reduced [3H]DA uptake with no difference in the total DAT expression in the striatum [32]. Importantly, treatment of striatal synaptosomes of wild-type mice with a specific PKCβ inhibitor (LY379196) reduced basal surface DAT expression and DA uptake, mimicking the basal DAT trafficking pattern in PKCβ-knockout mice [32]. It remains to be determined whether PKCβ-dependent maintenance of normal surface DAT levels is due to reduced endocytosis or an enhanced recycling rate.
PKC-dependent internalization appears to be dependent upon ubiquitylation of three lysine groups in the N-terminus of DAT; mutation of these lysines resulted in a diminished internalization of DAT to PKC activation [33]. The NEDD4–2 (neural precursor cell expressed, developmentally downregulated 4–2) protein, a homologous to E6-associated protein C-terminus (HECT) domain-containing E3 ubiquitin ligase, was demonstrated to be a requisite component of PKC-dependent DAT internalization [22].
PKC-dependent regulation of constitutive DAT trafficking is readily apparent in various heterologous cell types [1,15], as well as in striatal dopaminergic nerve endings [21,34,35]. By contrast, PKC regulation of constitutive DAT trafficking was not observed in cultured live midbrain dopaminergic neurons because the PKC inhibitor (staurosporine) did not prevent constitutive DAT internalization [20]. It is unclear whether this is a characteristic of post-natal cell cultures or whether it is reflective of dopaminergic cell bodies. In addition to PKC, basal DAT surface expression is also mediated by the signaling of PKB (Akt). Overexpression of a dominant-negative mutant of Akt (K179R) or treatment with Akt inhibitors reduces the surface DAT expression [36].
Many of the studies on factors affecting constitutive or even substrate-induced trafficking of DAT have relied heavily on the use of heterol ogous cells, and caution should be used in interpretation of the data. Loder and Melikian found that internalization of DAT was insensitive to PKC in cells expressing high basal levels of DAT [16]. In addition to the effect of expression levels, the use of cell types that do not contain the normal contingent of proteins available in the DA terminal could also affect trafficking results. Therefore, findings from heterologous cells should be replicated in animal models, synaptosomes and/or cultured mesencephalic neurons.
DAT internalization induced by continuous exposure to DAT substrates
DAT shows a time-dependent, biphasic trafficking pattern in vitro and in vivo upon long- and short-term exposure to substrates such as AMPH and DA, as illustrated in Figure 2. It has been well documented that exposure to AMPH results in DAT internalization or reduced DA uptake in cultured cell lines stably expressing DAT [23,24,27,37,38] or in striatal synaptosomes [39,40]. AMPH-induced DAT internalization is blocked by DAT inhibitors, such as cocaine, nomifensine and mazindol [37], indicating that AMPH must bind to the transporter to elicit internalization. Other DAT substrates, such as DA and methamphetamine, also significantly reduce surface DAT expression or DA uptake [37,40]. Similar to the in vitro AMPH treatment, in vivo injection of AMPH also reduces DAT Vmax with no change in Km from striatal synaptosomes [40–42]. In contrast to AMPH, administration of a high dose of cocaine in vivo has no effect on DAT activity [41], although long-term treatment of heterologous cells with cocaine increases surface DAT [43]. Increases in DAT binding have been demonstrated in brains from cocaine addicts upon autopsy [44] and studies using single photon emission computed tomo graphy in cocaine addicts [45,46], which probably represent surface DAT.
It is possible that reduction of surface DAT in response to continuous substrate exposure is a protective mechanism against the build-up of toxic levels of DA. Excessive levels of cytosolic DA and its oxidative products can be toxic to dopaminergic neurons and are postulated to contribute to the development of Parkinson's disease [47–49]. AMPH and methamphetamine both cause toxic effects on DA neurons, most likely owing to their ability to deplete synaptic vesicles of DA, thus increasing cytosolic DA. Although AMPH abuse ultimately results in depletion of brain DA, toxic effects of AMPHs are not manifested as Parkinson's disease because the deficits are more pronounced in the caudate than in the motor function-related putamen [50]. PET studies demonstrate that chronic methamphetamine use in humans leads to a reduction in the density of striatal DAT. This may not be due to permanent damage, but rather to neuroadaptative changes, since there is no change in other presynaptic markers, such as VMAT2, upon autopsy in methamphetamine abusers [51].
Potential mechanisms underlying substrate-induced DAT endocytosis
Acceleration of DAT endocytosis
The reduced surface DAT expression upon long-term substrate exposure is due to accelerated DAT endocytosis rather than impeded DAT recycling [23]. Pretreatment with concanavalin A, a stabilizer for cell surface integrity, prevents AMPH-induced loss of surface DAT [37], further supporting the notion that acceleration of DAT endocytosis underlies long-term AMPH-induced loss of surface DAT. The accelerated DAT endocytosis is dynamin-dependent. Co-expression of hDAT with a dominant-negative mutant of dynamin I (K44A) inhibits AMPH-mediated loss of surface DAT compared with co-expression with wild-type dynamin I [37].
DAT conformational change
AMPH treatment results in DA efflux through transporter-like and channel-like pathways, in which intracellular sodium is required [52–54]. Therefore, it is hypothesized that AMPH-induced DAT endocytosis is a consequence of the change to an inward-facing conformation, which is driven by an inward current and intracellular sodium. Conformation-destabilizing mutations, which promote an inward-facing state, display a similar pattern of DAT endocytosis and distribution to that of the wild-type DAT exposed to AMPH [24].
Intracellular AMPH
An increase in intracellular AMPH is important for long-term AMPH-induced DAT internalization [38]. A mutant DAT (Y355A) displaying normal substrate binding but impaired substrate transport does not internalize when exposed to extracellular AMPH. However, the mutant DAT does internalize when AMPH is applied intracellularly [38]. This mutation shifts the conformational equilibrium of DAT away from a predominantly outward-facing state. Considering the fact that DAT blockers abrogate the ability of AMPH to induce internalization [37], it would appear that AMPH must be taken up by the wild-type DAT and act intracellularly to promote endocytosis. By contrast, direct intracellular application of DA did not induce DAT internalization [38], indicating that the cellular mechanisms of intra-cellular AMPH- and DA-induced endocytosis might differ.
PKC-dependent mechanism
Numerous studies have demonstrated that direct activation of PKC leads to downregulation of DAT activity with an accompanying redistribution of DAT from the cell surface [1,15]. Similarly, there is some evidence that PKC may mediate DAT substrate-stimulated downregulation of DAT activity. Continuous exposure to AMPH reduces DA uptake in rat striatal synaptosomes, which can be blocked by pretreatment with a PKC inhibitor, bisindoylmaleimide I (BIM I) [40]. BIM I was also shown to block DA- and methamphetamine-induced downregulation of DA uptake in rat DAT (rDAT)–LLC–PK1 cells [55,56]. However, Boudanova et al. reported that the AMPH-induced reduction in surface DAT in hDAT-PC12 cells was not inhibited by BIM I [23] and did not necessitate structural determinants required for PKC-dependent internalization signals [21]. Although all three groups utilized different cells, somewhat different drugs and different incubation times, the differences among the results may partially lie in the outcome measure [1,23,55]. Inhibition of the psychostimulant action by the PKC inhibitor BIM I was detected when a reduction of DA uptake was measured [1,55], while no effect of BIM I was detected when reduction of surface DAT was measured [23]. In fact, a DAT substrate-induced reduction in DA uptake occurs long before a reduction in surface DAT in rat synaptosomes [1]. However, recent evidence strongly indicates that AMPH and PKC activation induce internalization of DAT through independent pathways [57].
AMPH treatment activates PKC, which results in N-terminal phosphorylation of DAT [55]. Although it has not been tested in vivo whether phosphorylation of DAT would induce DAT internalization, mutagenesis studies in cultured cell lines expressing mutant DAT have demonstrated that neither the N-terminus of DAT or the phosphorylation of serines within the N-terminus is required for AMPH-triggered DAT trafficking [55,58]. Recent work from Vaughan andcoworkers indicates that PKC- induced DAT regulation may depend on the precise membrane localization of the transporter [59]. DAT that can be phosphorylated by PKC resides mostly in lipid rafts, whereas those that are internalized by PKC are in nonlipid rafts, suggesting that the membrane localization determines the fate of DAT phosphorylation or sequestration by PKC in vitro. Although the relationship between DAT phosphorylation and internalization is still not definitive, PKC phosphorylation of SERT has been linked to SERT internalization and consequently reduced SERT activity [60].
Other kinase-dependent mechanisms
In addition to PKC, Ca2+/calmodulin-dependent protein kinase II (CaMKII) has also been implicated in AMPH-induced DAT internalization. AMPH increases intracellular Ca2+ [61,62] and activates CaMKII activity in rat and mouse striatal synaptosomes and in overexpressed cells [63,64]. As discussed in this review, activation of PI3K and Akt, a protein kinase in the insulin pathway immediately downstream of PI3K, increase surface DAT [26,36]. Upon examining mechanisms of AMPH-induced internalization, Wei et al. demonstrated that AMPH inhibited Akt activity via activation of CaMKII [64]. However, the role of CaMKII in constitutive DAT internalization is unclear. CaMKII inhibitors blocked AMPH-induced DAT internalization, but the effect of CaMKII inhibitors on basal DAT levels was not presented.
Although these data strongly suggest that activation of CaMKII by AMPH leads to a down-regulation of DAT, there are other data suggesting that CAMKII contributes to DAT activity. CaMKIIα binds directly to the C-terminus of DAT and facilitates in vitro phosphorylation of N-terminal serines [65]. The addition of activated CaMKIIα increased AMPH-induced DA efflux, while inhibition of CaMKII decreased this activity in hDAT-EM4 cells and mouse midbrain neuronal cultures. Furthermore, the CaMKII inhibitor, KN93, reduced AMPH-stimulated DA efflux in perfused striatal slices. Other investigators have reported no effect of the CaMKII inhibitor on AMPH-stimulated DA efflux in striatal slices [66] or microdialysis of rat striatum in naive rats [67]. In addition, intra-accumbens KN93 had no effect on self-administration of AMPH in rats that had not previously been sensitized to AMPH, although it inhibited this activity in mice previously sensitized to AMPH [68]. Therefore, more studies need to be performed in the intact animal to investigate the role of CaMKII in AMPH-stimulated DA efflux. Whether CaMKII phosphorylation of DAT governs DAT trafficking also warrants further investigation.
Rapid DAT trafficking to the surface induced by short-term exposure to DAT substrates
In contrast to DAT internalization induced by long-term exposure to DAT substrates, an extremely short-term exposure to DAT substrates results in an increase in surface DAT expression (Figure 2). A rapid upregulation in surface DAT expression elicited by AMPH was demonstrated by 30 s in rat striatal synaptosomes and in rDAT-HEK 293 cells [39]. The AMPH-induced increase in surface DAT was blocked by cocaine, demonstrating that AMPH had to interact with DAT in order to elicit the increase. Furthermore, reverse biotinylation studies suggested that AMPH was actively stimulating the recycling of DAT-containing vesicles to the surface, and not reducing endocytosis.
Temporal and spatial resolution of the AMPH-induced ultra-rapid trafficking of DAT to the surface was demonstrated by live-cell imaging using total internal reflection fluorescence microscopy (TIRFM) [69]. TIRFM provides real-time resolution coupled with the ability to selectively detect and analyze cytosol to plasma-lemmal membrane movement of vesicles and granules. TIRFM has been successfully used to discern tethering of the glucose transporter 4 (GLUT4)-containing vesicles to the rat adipocyte membrane before fusion [70], as well as the multiple tethering states and significant motion of chromaffin granules immediately preceding exocytosis [71]. In N2A neuroblastoma cells stably expressing GFP-rDAT, AMPH was shown to dose-dependently induce movement of DAT-containing vesicles toward the plasmalemmal membrane within 2 min, as demonstrated by the increased surface intensity of GFP-rDAT in TIRFM analysis. A highly novel finding is that the physiological substrate, DA, also stimulated the movement of DAT toward the plasmalemmal membrane. It has been demonstrated that DA, acting on the D2/D3 subtype of DA receptors, will elicit a rapid movement of DAT toward the surface [72–74], but Furman et al. demonstrated that substrates could also influence trafficking of DAT to the surface in the absence of D2/D3 DA receptors [69]. D2-type DA receptors were not detected in the GFP-rDATN2A cells; the D2-type antagonist, sulpiride, did not block the effect of either AMPH or DA in increasing surface DAT, and the D2/D3 agonist, quinpirole, had no effect on DA uptake in those cells. Although an interaction of DA with D2 DA receptors was not required for rapid trafficking of DAT to the surface, an interaction with DAT was required. The increased surface intensity induced by substrates was also blocked by cocaine and was reduced upon drug removal. These data suggest that the interaction of substrate with DAT triggers a further increase in surface DAT. Extracellular Na+ was required for the effect, suggesting that both binding and transport of the substrate is required.
The ultra-rapid increase in surface DAT elicited by substrates has physiological consequences. Incubation of cells or synaptosomes with AMPH or DA from 30 s to 2 min, followed by extensive washing, increases [3H]DA uptake [32,73] or AMPH-stimulated DA efflux [39,69]. Moreover, the time frame of the substrate-elicited increases in surface DAT and DAT function are within the physiological time frame of DA uptake and DAT-mediated DA release. The half-life (T50) of DA clearance in the striatum of a freely moving rat is 26–57 s [75]. The decay of the DA signal is mediated largely by reuptake. Furthermore, AMPH elicits the release of DA within seconds and efflux is maximal by 2 min [76]. This strongly suggests that the physiological substrate, DA, regulates its own uptake to limit excessive DA within the synapse.
Potential mechanisms underlying substrate-induced DAT trafficking to the plasmalemmal membrane
PKCβ-dependence
Evidence that PKCβ is involved in the regulation of DAT functional activity emerged from the finding that the specific PKCβ inhibitor, LY379196, blocked the ability of AMPH to elicit DA efflux in rat striatal synaptosomes [77]. A schematic illustrating rapid trafficking and the role of PKCβ is shown in Figure 2. Moreover, PKCβ co-immunoprecipitated with DAT, indicating that the two proteins are closely associated in the membrane. The ability of LY379196 to block AMPH-stimulated DA efflux may be due to a regulation of the ultra-rapid trafficking of DAT to the membrane following substrate stimulation. Thus, LY379196 blocked AMPH- and DA-stimulated ultra-rapid DAT exocytotic trafficking in rat and mouse striatal synaptosomes, and GFP-rDAT-N2A cells [32,69]. The role of PKCβ in rapid DAT trafficking was further explored and validated using PKCβ-knockout mice. PKCβ-knockout mice on the C56BL/6 background exhibited less surface DAT, less [3H]DA uptake, less AMPH-stimulated DA efflux and less AMPH-stimulated locomotor activity, compared with their wild-type counterparts [32]. Importantly, the regulation of DAT in response to AMPH was entirely opposite in the PKCβ-knockout mice compared with wild-type mice; incubation with AMPH in striatal synaptosomes from PKCβ-knockout mice rapidly decreased surface DAT, but after 1 h, surface DAT increased above vehicle levels [32].
Since transport of DAT to the surface is slowed, but not stopped, in the striata from PKCβ-knockout mice, it appears that PKCβ is not absolutely required for substrate-induced DAT exocytosis, but plays a major role in regulating the rate of DAT trafficking to the surface. This is consistent with the finding of Johnson et al., that the ability of AMPH to rapidly increase surface DAT reflects an increase in membrane targeting of DAT, not a slowing of internalization [39]. Taken together, these data suggest that PKCβ is intrinsically important for rapid transport of DAT to the surface and, thus, the degree of responsiveness of the transporter to the presence of substrate.
Importantly, it appears that PKCβ and CaMKII have opposite effects on DAT trafficking, such that PKCβ promotes rapid AMPH-evoked DAT trafficking to the plasma membrane [32,69] and CaMKII appears to be involved in DAT trafficking away from the plasma membrane [64]. It is of interest to know whether continuous DAT trafficking is regulated sequentially by PKCβ and CaMKII. If the evidence bears out that CaMKII is required for AMPH-stimulated DA efflux in the naive, untreated rat, then CaMKII could, similar to PKC, exhibit biphasic effects on surface DAT. This is suggested by the fact that CaMKII is required for AMPH to couple to syntaxin 1A at the cell surface [78]. Furthermore, DAT is a substrate for protein phosphatase 1 in vitro and in rat striatal tissue, but not for PP2A [79], although PP2A forms a complex with DAT [80]. Whether PKCβ-promoted rapid DAT trafficking is dampened by these DAT-associated phosphatases warrants future investigation.
SNARE protein dependence
Although the demonstration of real-time imaging of fusion of an individual DAT-containing vesicle on the membrane upon short-term substrate exposure has not yet been achieved, the rapid increase of surface DAT expression is dependent on soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins [69]. SNARE proteins, such as syntaxin 1A and the vesicle-associated membrane protein (VAMP)/synaptobrevin, participate in the process of membrane fusion and exocytosis. Multiple neurotransmitter transporters, including DAT, NET and SERT, form a complex with the SNARE protein, syntaxin 1A, through direct physical interaction [81–83]. The DA-stimulated ultra-rapid increase of surface DAT in GFP–rDAT-N2A cells is blocked by overexpression of the light chain of botulinum neurotoxin C (BoNT C) or tetanus neurotoxin (TeNT), both of which inhibit vesicle fusion [69]. BoNT C proteolyzes the plasmalemmal membrane SNARE protein, syntaxin 1A, while TeNT proteolyzes VAMP/synaptobrevin. Presumably, the VAMP/synaptobrevin is located on the DAT-containing vesicle. Although DAT has not been directly demonstrated to be present in vesicles, the homolog GAT1 has been localized in small vesicles with a diameter of approximately 50 nm [84]. Overexpression of syntaxin 1A increases AMPH-stimulated DA efflux in hDAT-HEK293 cells, and treatment with 10 μM AMPH for 5 min increased a syntaxin 1A–DAT interaction [78]. Syntaxin 1A is crucial for docking of vesicles to the plasmalemmal membrane [85] and may function similarly for DAT-containing vesicles. These data suggest that the substrate-induced rapid increase in surface DAT is an exocytotic event that is regulated by SNARE proteins. The effect of syntaxin 1A on surface DAT has not yet been reported, but our laboratory has found that cleavage of syntaxin 1A in rat brain synaptosomes by BoNT C reduces basal DAT expression [KIM ET AL., UNPUBLISHED OBSERVATIONS]. It has been reported that cleavage of syntaxin 1A in mouse brain synaptosomes by BoNT C reduces basal NET surface expression [82]. Furthermore, NET and syntaxin 1A interaction mediates NET sequestration in response to AMPH [86]. Although syntaxin 1A facilitates AMPH-induced DA efflux [78], it remains unknown whether the association of syntaxin 1A and DAT modulates basal and AMPH-stimulated DAT internalization or recycling.
Presynaptic dopamine D2/D3-receptor dependence
DAT function and trafficking is regulated by D2 DA receptors, presumably the short form of the D2 DA receptor (D2SR), which is probably the autoreceptor [87]. Both D2 and D3 DA receptors are found on DA neurons and both regulate presynaptic function [88]. D2/D3 DA-receptor agonists increase the Vmax value of DAT substrates and surface DAT expression [72–74,89]. Quinpirole, a D2/D3 DA-receptor agonist, increases surface DAT expression within the first 5 min of exposure in cells overexpressing either D2 or D3 DA receptors [73,89].
Modulation of DAT trafficking by D2SR is Gi/Go-dependent and requires ERK1/2 but not PI3K activation [73]. However, D2 receptor-independent DAT trafficking has also been reported in N2A cells stably expressing hDAT [69], suggesting that DA can modulate DAT trafficking both as a substrate for DAT and as an agonist for D2/D3 DA receptors. DAT is regulated by D2SR through a direct physical interaction involving the DAT N-terminus and the third intracellular loop of D2SR [74], which probably facilities intracellular DAT trafficking to the surface. Disruption of D2SR–DAT interaction in the mouse brain in vivo results in decreased striatal synaptosomal DA uptake and increased locomotor activity, mimicking DAT-knockout mice [74]. The strong relationship between D2 DA receptors and DAT is also expressed at the genetic level. There is significant D2 receptor/DAT interaction in prefrontal cortex and striatum during both working memory and encoding of recognition memory as assessed by patients undergoing blood oxygenation level-dependent functional magnetic resonance imaging [90].
Insulin-stimulated trafficking
Insulin signaling has been demonstrated to increase surface DAT in heterologous cells [36]. Furthermore, the ability of AMPH to elicit behaviors and DA efflux is reduced in hypoinsulinemic rats [91,92]. Insulin can also reverse AMPH-induced DAT internalization through the action of PI3K, suggesting a hormonal regulation of DA homeostasis [64]. Activation of Akt via insulin signaling or overexpression of a dominant-negative Akt mutant counteracts AMPH-evoked DAT internalization, while Akt inhibition, similar to AMPH treatment, induces DAT trafficking away from the plasma membrane [52]. The earliest time point at which insulin significantly increased [3H]DA uptake in FLAG–hDAT-EM4 cells was 5 min, although there was a trend to an increase at 2 min, suggesting that the effect of insulin on surface DAT is relatively rapid [26]. Similarly, the reduction of [3H]DA uptake following treatment of hDAT overexpressed cells in response to 100 μM of the Akt inhibitor ML9 was significant within 5 min. The latter experiment, which was performed in the absence of insulin, suggests that Akt is important for constitutive surface DAT, similar to the effect of PKCβ. A time course of the effect of insulin on surface DAT would clarify whether insulin elicits ultra-rapid trafficking of DAT to the surface.
Future perspective
DAT is one of the most important regulators of dopaminergic homeostasis, and is potentially involved in several neurodegenerative and psychiatric diseases. Since DAT is active when situated at the plasmalemmal membrane, the concentration of DAT at the plasmalemmal membrane is crucial to proper dopaminergic function. Research in the past few years has led to an explosion of knowledge concerning the modulation of DAT trafficking by substrates, protein kinases, second messengers and interacting proteins. Continuous exposure to DAT substrates elicits a downregulation of DAT at the plasmalemmal membrane, which could serve as a protection mechanism against a build-up of toxic substrates in the terminal. However, recent studies have demonstrated that substrates also elicit, within seconds, trafficking of DAT to the plasmalemmal membrane. This ultra-rapid trafficking takes place during the physiological time frame of DA uptake in response to exocytotically-released DA and DA efflux in response to the psychostimulant AMPH. One can imagine that blockade of the ultra-rapid response would reduce the response to AMPH but permit sufficient uptake to maintain normal function. Thus, blockade of the rapid AMPH-induced trafficking of DAT to the surface, especially by locally targeting PKCβ, could be a worthwhile approach to treat drug abuse.
Executive summary.
Background
-
■
The dopamine transporter (DAT) is a primary determinant of the concentration of dopamine in the synapse.
-
■
DAT is involved in neurological diseases, including Parkinson's disease, and psychiatric diseases, such as psychosis, attention-deficit/hyperactivity disorder and substance abuse.
Role of DAT
-
■
Synaptic dopamine released through exocytosis is taken up through the DAT back into the nerve terminal.
-
■
Ligands for DAT include dopamine, amphetamine, methamphetamine (substrates), and cocaine and methylphenidate (blockers).
Substrate-regulated trafficking of DAT
-
■
Substrates initially stimulate trafficking toward the plasmalemmal membrane, increasing reuptake.
-
■
When the substrate is amphetamine, the rapid trafficking also increases the reverse transport of dopamine.
-
■
The continuous presence of substrate results in downregulation of the transporter activity, followed by internalization of the transporter.
Strategies for altering DAT trafficking
-
■
Ultra-rapid substrate-induced trafficking of the transporter to the surface is mediated, at least partially, by PKCβ.
-
■
Blockade of PKCβ could prove useful to reduce the effectiveness of methamphetamine, yet permit enough normal uptake to keep the neuron functional.
-
■
The endocytic pathway could be modulated to keep more DAT on the surface, thus reducing synaptic dopamine, which would limit reinforcement to rewarding stimuli, or to reduce surface transporter, to protect the neuron from having too much intracellular dopamine.
Future perspective
-
■
Delineation of the molecular mechanisms involved in the exocytic/endocytic pathway of DAT will provide therapeutic opportunities for regulating synaptic and neuronal dopamine that will be useful in treating neurologic and psychiatric diseases.
-
■
Since the pathways are probably regulated separately by various signal transduction mechanisms, drugs altering the pathway can be tailored to the specific pathology.
Acknowledgments
Financial & competing interests disclosure Funding support was provided by NIH Grants DA011697 (Margaret E Gnegy) and DA025954 (Rong Chen) in the production of this manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
- 1.Zahniser NR, Sorkin A. Trafficking of dopamine transporters in psychostimulant actions. Semin. Cell. Dev. Biol. 2009;20(4):411–417. doi: 10.1016/j.semcdb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazei-Robison MS, Bowton E, Holy M, et al. Anomalous dopamine release associated with a human dopamine transporter coding variant. J. Neurosci. 2008;28(28):7040–7046. doi: 10.1523/JNEUROSCI.0473-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehler-Wex C, Riederer P, Gerlach M. Dopaminergic dysbalance in distinct basal ganglia neurocircuits: implications for the pathophysiology of Parkinson's disease, schizophrenia and attention deficit hyperactivity disorder. Neurotox. Res. 2006;10(3–4):167–179. doi: 10.1007/BF03033354. [DOI] [PubMed] [Google Scholar]
- 4.Serretti A, Mandelli L. The genetics of bipolar disorder: genome `hot regions,' genes, new potential candidates and future directions. Mol. Psychiatry. 2008;13(8):742–771. doi: 10.1038/mp.2008.29. [DOI] [PubMed] [Google Scholar]
- 5.Kurian MA, Zhen J, Cheng SY, et al. Homozygous loss-of-function mutations in the gene encoding the dopamine transporter are associated with infantile parkinsonismdystonia. J. Clin. Invest. 2009;119(6):1595–1603. doi: 10.1172/JCI39060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Storch A, Ludolph AC, Schwarz J. Dopamine transporter: involvement in selective dopaminergic neurotoxicity and degeneration. J. Neural Transm. 2004;111(10–11):1267–1286. doi: 10.1007/s00702-004-0203-2. [DOI] [PubMed] [Google Scholar]
- 7.Mazei-Robison MS, Couch RS, Shelton RC, Stein MA, Blakely RD. Sequence variation in the human dopamine transporter gene in children with attention deficit hyperactivity disorder. Neuropharmacology. 2005;49(6):724–736. doi: 10.1016/j.neuropharm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Kniazeff J, Shi L, Loland CJ, Javitch JA, Weinstein H, Gether U. An intracellular interaction network regulates conformational transitions in the dopamine transporter. J. Biol. Chem. 2008;283(25):17691–17701. doi: 10.1074/jbc.M800475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson SD, Matthies HJ, Galli A. A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Mol. Neurobiol. 2009;39(2):73–80. doi: 10.1007/s12035-009-8053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog. Neurobiol. 2005;75(6):406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Guptaroy B, Zhang M, Bowton E, et al. A juxtamembrane mutation in the N terminus of the dopamine transporter induces preference for an inward-facing conformation. Mol. Pharmacol. 2009;75(3):514–524. doi: 10.1124/mol.108.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379(6566):606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 13.Chen R, Tilley MR, Wei H, et al. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc. Natl Acad. Sci. USA. 2006;103(24):9333–9338. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres GE. The dopamine transporter proteome. J. Neurochem. 2006;97(Suppl 1):3–10. doi: 10.1111/j.1471-4159.2006.03719.x. [DOI] [PubMed] [Google Scholar]
- 15.Melikian HE. Neurotransmitter transporter trafficking: endocytosis, recycling, and regulation. Pharmacol. Ther. 2004;104(1):17–27. doi: 10.1016/j.pharmthera.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Loder MK, Melikian HE. The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J. Biol. Chem. 2003;278(24):22168–22174. doi: 10.1074/jbc.M301845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holton KL, Loder MK, Melikian HE. Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization. Nat. Neurosci. 2005;8(7):881–888. doi: 10.1038/nn1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorkina T, Hoover BR, Zahniser NR, Sorkin A. Constitutive and protein kinase C-induced internalization of the dopamine transporter is mediated by a clathrin-dependent mechanism. Traffic. 2005;6(2):157–170. doi: 10.1111/j.1600-0854.2005.00259.x. [DOI] [PubMed] [Google Scholar]; ■ Demonstrates that constitutive and PKC-induced dopamine transporter (DAT) endocytic trafficking utilize the clathrin-dependent endocytic pathway.
- 19.Li LB, Chen N, Ramamoorthy S, et al. The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J. Biol. Chem. 2004;279(20):21012–21020. doi: 10.1074/jbc.M311972200. [DOI] [PubMed] [Google Scholar]
- 20.Eriksen J, Rasmussen SG, Rasmussen TN, et al. Visualization of dopamine transporter trafficking in live neurons by use of fluorescent cocaine analogs. J. Neurosci. 2009;29(21):6794–6808. doi: 10.1523/JNEUROSCI.4177-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Uses novel fluorescently tagged cocaine analogs to visualize DAT and DAT trafficking in cultured live midbrain dopaminergic neurons.
- 21.Chi L, Reith ME. Substrate-induced trafficking of the dopamine transporter in heterologously expressing cells and in rat striatal synaptosomal preparations. J. Pharmacol. Exp. Ther. 2003;307(2):729–736. doi: 10.1124/jpet.103.055095. [DOI] [PubMed] [Google Scholar]
- 22.Sorkina T, Miranda M, Dionne KR, Hoover BR, Zahniser NR, Sorkin A. RNA interference screen reveals an essential role of NEDD4–2 in dopamine transporter ubiquitination and endocytosis. J. Neurosci. 2006;26(31):8195–8205. doi: 10.1523/JNEUROSCI.1301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boudanova E, Navaroli DM, Melikian HE. Amphetamine-induced decreases in dopamine transporter surface expression are protein kinase C-independent. Neuropharmacology. 2008;54(3):605–612. doi: 10.1016/j.neuropharm.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorkina T, Richards TL, Rao A, Zahniser NR, Sorkin A. Negative regulation of dopamine transporter endocytosis by membrane-proximal N-terminal residues. J. Neurosci. 2009;29(5):1361–1374. doi: 10.1523/JNEUROSCI.3250-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniels GM, Amara SG. Regulated trafficking of the human dopamine transporter. Clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J. Biol. Chem. 1999;274(50):35794–35801. doi: 10.1074/jbc.274.50.35794. [DOI] [PubMed] [Google Scholar]
- 26.Carvelli L, Moron JA, Kahlig KM, et al. PI 3-kinase regulation of dopamine uptake. J. Neurochem. 2002;81(4):859–869. doi: 10.1046/j.1471-4159.2002.00892.x. [DOI] [PubMed] [Google Scholar]
- 27.Sorkina T, Doolen S, Galperin E, Zahniser NR, Sorkin A. Oligomerization of dopamine transporters visualized in living cells by fluorescence resonance energy transfer microscopy. J. Biol. Chem. 2003;278(30):28274–28283. doi: 10.1074/jbc.M210652200. [DOI] [PubMed] [Google Scholar]
- 28.Furman CA, Lo CB, Stokes S, Esteban JA, Gnegy ME. Rab 11 regulates constitutive dopamine transporter trafficking and function in N2A neuroblastoma cells. Neurosci. Lett. 2009;463:78–81. doi: 10.1016/j.neulet.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miranda M, Sorkina T, Grammatopoulos TN, Zawada WM, Sorkin A. Multiple molecular determinants in the carboxyl terminus regulate dopamine transporter export from endoplasmic reticulum. J. Biol. Chem. 2004;279(29):30760–30770. doi: 10.1074/jbc.M312774200. [DOI] [PubMed] [Google Scholar]
- 30.Boudanova E, Navaroli DM, Stevens Z, Melikian HE. Dopamine transporter endocytic determinants: carboxy terminal residues critical for basal and PKC-stimulated internalization. Mol. Cell. Neurosci. 2008;39(2):211–217. doi: 10.1016/j.mcn.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Delineates that basal DAT endocytosis and PKC-stimulated DAT internalization are governed by an unconventional motif within the C-terminus of DAT.
- 31.Torres GE, Carneiro A, Seamans K, et al. Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. J. Biol. Chem. 2003;278(4):2731–2739. doi: 10.1074/jbc.M201926200. [DOI] [PubMed] [Google Scholar]
- 32.Chen R, Furman CA, Zhang M, et al. Protein kinase Cβ is a critical regulator of dopamine transporter trafficking and regulates the behavioral response to amphetamine in mice. J. Pharmacol. Exp. Ther. 2009;328(3):912–920. doi: 10.1124/jpet.108.147959. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Demonstrates that PKCβ regulates constitutive and amphetamine-induced rapid DAT trafficking to the surface in striatal synaptosomes using PKCβ wild-type and knockout mice.
- 33.Miranda M, Wu CC, Sorkina T, Korstjens DR, Sorkin A. Enhanced ubiquitylation and accelerated degradation of the dopamine transporter mediated by protein kinase C. J. Biol. Chem. 2005;280(42):35617–35624. doi: 10.1074/jbc.M506618200. [DOI] [PubMed] [Google Scholar]
- 34.Huff RA, Vaughan RA, Kuhar MJ, Uhl GR. Phorbol esters increase dopamine transporter phosphorylation and decrease transport Vmax. J. Neurochem. 1997;68(1):225–232. doi: 10.1046/j.1471-4159.1997.68010225.x. [DOI] [PubMed] [Google Scholar]
- 35.Vaughan RA, Huff RA, Uhl GR, Kuhar MJ. Protein kinase C-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. J. Biol. Chem. 1997;272(24):15541–15546. doi: 10.1074/jbc.272.24.15541. [DOI] [PubMed] [Google Scholar]
- 36.Garcia BG, Wei Y, Moron JA, Lin RZ, Javitch JA, Galli A. Akt is essential for insulin modulation of amphetamine-induced human dopamine transporter cell-surface redistribution. Mol. Pharmacol. 2005;68(1):102–109. doi: 10.1124/mol.104.009092. [DOI] [PubMed] [Google Scholar]
- 37.Saunders C, Ferrer JV, Shi L, et al. Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc. Natl Acad. Sci. USA. 2000;97(12):6850–6855. doi: 10.1073/pnas.110035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kahlig KM, Lute BJ, Wei Y, et al. Regulation of dopamine transporter trafficking by intracellular amphetamine. Mol. Pharmacol. 2006;70(2):542–548. doi: 10.1124/mol.106.023952. [DOI] [PubMed] [Google Scholar]
- 39.Johnson LA, Furman CA, Zhang M, Guptaroy B, Gnegy ME. Rapid delivery of the dopamine transporter to the plasmalemmal membrane upon amphetamine stimulation. Neuropharmacology. 2005;49(6):750–758. doi: 10.1016/j.neuropharm.2005.08.018. [DOI] [PubMed] [Google Scholar]; ■ Demonstrates an ultra-rapid DAT trafficking to the surface upon a brief exposure (30 s) of amphetamine to DAT in rat striatal synaptosomes, which is in contrast to long exposure (≥10 min) of amphetamine-induced internalization.
- 40.Richards TL, Zahniser NR. Rapid substrate-induced down-regulation in function and surface localization of dopamine transporters: rat dorsal striatum versus nucleus accumbens. J. Neurochem. 2009;108(6):1575–1584. doi: 10.1111/j.1471-4159.2009.05910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fleckenstein AE, Haughey HM, Metzger RR, et al. Differential effects of psychostimulants and related agents on dopaminergic and serotonergic transporter function. Eur. J. Pharmacol. 1999;382(1):45–49. doi: 10.1016/s0014-2999(99)00588-9. [DOI] [PubMed] [Google Scholar]
- 42.Fleckenstein AE, Metzger RR, Wilkins DG, Gibb JW, Hanson GR. Rapid and reversible effects of methamphetamine on dopamine transporters. J. Pharmacol. Exp. Ther. 1997;282(2):834–838. [PubMed] [Google Scholar]
- 43.Little KY, Elmer LW, Zhong H, Scheys JO, Zhang L. Cocaine induction of dopamine transporter trafficking to the plasma membrane. Mol. Pharmacol. 2002;61(2):436–445. doi: 10.1124/mol.61.2.436. [DOI] [PubMed] [Google Scholar]
- 44.Little KY, Zhang L, Desmond T, Frey KA, Dalack GW, Cassin BJ. Striatal dopaminergic abnormalities in human cocaine users. Am. J. Psychiatry. 1999;156(2):238–245. doi: 10.1176/ajp.156.2.238. [DOI] [PubMed] [Google Scholar]
- 45.Crits-Christoph P, Newberg A, Wintering N, et al. Dopamine transporter levels in cocaine dependent subjects. Drug Alcohol Depend. 2008;98(1–2):70–76. doi: 10.1016/j.drugalcdep.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malison RT, Best SE, van Dyck CH, et al. Elevated striatal dopamine transporters during acute cocaine abstinence as measured by [123I] β-CIT SPECT. Am. J. Psychiatry. 1998;155(6):832–834. doi: 10.1176/ajp.155.6.832. [DOI] [PubMed] [Google Scholar]
- 47.Mosharov EV, Larsen KE, Kanter E, et al. Interplay between cytosolic dopamine, calcium, and α-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62(2):218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galvin JE. Interaction of α-synuclein and dopamine metabolites in the pathogenesis of Parkinson's disease: a case for the selective vulnerability of the substantia nigra. Acta Neuropathol. 2006;112(2):115–126. doi: 10.1007/s00401-006-0096-2. [DOI] [PubMed] [Google Scholar]
- 49.Lotharius J, Brundin P. Impaired dopamine storage resulting from α-synuclein mutations may contribute to the pathogenesis of Parkinson's disease. Hum. Mol. Genet. 2002;11(20):2395–2407. doi: 10.1093/hmg/11.20.2395. [DOI] [PubMed] [Google Scholar]
- 50.Moszczynska A, Fitzmaurice P, Ang L, et al. Why is parkinsonism not a feature of human methamphetamine users? Brain. 2004;127(Pt 2):363–370. doi: 10.1093/brain/awh046. [DOI] [PubMed] [Google Scholar]
- 51.Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 52.Kahlig KM, Binda F, Khoshbouei H, et al. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc. Natl Acad. Sci. USA. 2005;102(9):3495–3500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khoshbouei H, Wang H, Lechleiter JD, Javitch JA, Galli A. Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism. J. Biol. Chem. 2003;278(14):12070–12077. doi: 10.1074/jbc.M212815200. [DOI] [PubMed] [Google Scholar]
- 54.Sitte HH, Huck S, Reither H, Boehm S, Singer EA, Pifl C. Carrier-mediated release, transport rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. J. Neurochem. 1998;71(3):1289–1297. doi: 10.1046/j.1471-4159.1998.71031289.x. [DOI] [PubMed] [Google Scholar]
- 55.Cervinski MA, Foster JD, Vaughan RA. Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase C-dependent mechanisms. J. Biol. Chem. 2005;280(49):40442–40449. doi: 10.1074/jbc.M501969200. [DOI] [PubMed] [Google Scholar]
- 56.Gorentla BK, Vaughan RA. Differential effects of dopamine and psychoactive drugs on dopamine transporter phosphorylation and regulation. Neuropharmacology. 2005;49(6):759–768. doi: 10.1016/j.neuropharm.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 57.Wheeler DS, Hong CW, Underhill SM, Miller MP, Amara SG. Society for Neurosciences Meeting Planner. Chicago, IL, USA: 2009. Amphetamine and PMA induce internalization of DAT via distinct pathways. Program No. 618.621/D613. [Google Scholar]
- 58.Granas C, Ferrer J, Loland CJ, Javitch JA, Gether U. N-terminal truncation of the dopamine transporter abolishes phorbol ester- and substance P receptor-stimulated phosphorylation without impairing transporter internalization. J. Biol. Chem. 2003;278(7):4990–5000. doi: 10.1074/jbc.M205058200. [DOI] [PubMed] [Google Scholar]
- 59.Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J. Neurochem. 2008;105(5):1683–1699. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jayanthi LD, Samuvel DJ, Blakely RD, Ramamoorthy S. Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis, and phosphorylation. Mol. Pharmacol. 2005;67(6):2077–2087. doi: 10.1124/mol.104.009555. [DOI] [PubMed] [Google Scholar]
- 61.Gnegy ME, Khoshbouei H, Berg KA, et al. Intracellular Ca2+ regulates amphetamine-induced dopamine efflux and currents mediated by the human dopamine transporter. Mol. Pharmacol. 2004;66(1):137–143. doi: 10.1124/mol.66.1.137. [DOI] [PubMed] [Google Scholar]
- 62.Kantor L, Zhang M, Guptaroy B, Park YH, Gnegy ME. Repeated amphetamine couples norepinephrine transporter and calcium channel activities in PC12 cells. J. Pharmacol. Exp. Ther. 2004;311(3):1044–1051. doi: 10.1124/jpet.104.071068. [DOI] [PubMed] [Google Scholar]
- 63.Iwata S, Hewlett GH, Gnegy ME. Amphetamine increases the phosphorylation of neuromodulin and synapsin I in rat striatal synaptosomes. Synapse. 1997;26(3):281–291. doi: 10.1002/(SICI)1098-2396(199707)26:3<281::AID-SYN9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 64.Wei Y, Williams JM, Dipace C, et al. Dopamine transporter activity mediates amphetamine-induced inhibition of Akt through a Ca2+/calmodulin-dependent kinase II-dependent mechanism. Mol. Pharmacol. 2007;71(3):835–842. doi: 10.1124/mol.106.026351. [DOI] [PubMed] [Google Scholar]
- 65.Fog JU, Khoshbouei H, Holy M, et al. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51(4):417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 66.Kantor L, Hewlett GH, Gnegy ME. Enhanced amphetamine- and K+-mediated dopamine release in rat striatum after repeated amphetamine: differential requirements for Ca2+- and calmodulin-dependent phosphorylation and synaptic vesicles. J. Neurosci. 1999;19(10):3801–3808. doi: 10.1523/JNEUROSCI.19-10-03801.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pierce RC, Quick EA, Reeder DC, Morgan ZR, Kalivas PW. Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. J. Pharmacol. Exp. Ther. 1998;286(3):1171–1176. [PubMed] [Google Scholar]
- 68.Loweth JA, Baker LK, Guptaa T, Guillory AM, Vezina P. Inhibition of CaMKII in the nucleus accumbens shell decreases enhanced amphetamine intake in sensitized rats. Neurosci. Lett. 2008;444(2):157–160. doi: 10.1016/j.neulet.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Furman CA, Chen R, Guptaroy B, Zhang M, Holz RW, Gnegy M. Dopamine and amphetamine rapidly increase dopamine transporter trafficking to the surface: live-cell imaging using total internal reflection fluorescence microscopy. J. Neurosci. 2009;29(10):3328–3336. doi: 10.1523/JNEUROSCI.5386-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Using total internal reflection fluorescence microscopy, the temporal and spatial trafficking of DAT to the surface upon brief exposure to amphetamine and dopamine is delineated in live cells.
- 70.Lizunov VA, Matsumoto H, Zimmerberg J, Cushman SW, Frolov VA. Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells. J. Cell. Biol. 2005;169(3):481–489. doi: 10.1083/jcb.200412069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holz RW, Axelrod D. Secretory granule behaviour adjacent to the plasma membrane before and during exocytosis: total internal reflection fluorescence microscopy studies. Acta Physiol. (Oxf.) 2008;192(2):303–307. doi: 10.1111/j.1748-1716.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 72.Meiergerd SM, Patterson TA, Schenk JO. D2 receptors may modulate the function of the striatal transporter for dopamine: kinetic evidence from studies in vitro and in vivo. J. Neurochem. 1993;61(2):764–767. doi: 10.1111/j.1471-4159.1993.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 73.Bolan EA, Kivell B, Jaligam V, et al. D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol. Pharmacol. 2007;71(5):1222–1232. doi: 10.1124/mol.106.027763. [DOI] [PubMed] [Google Scholar]; ■ Demonstrates that dopamine and D2 dopamine receptor agonists elicit rapid trafficking of the DAT to the membrane in an ERK-dependent manner
- 74.Lee FJ, Pei L, Moszczynska A, Vukusic B, Fletcher PJ, Liu F. Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. Embo J. 2007;26(8):2127–2136. doi: 10.1038/sj.emboj.7601656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sabeti J, Adams CE, Burmeister J, Gerhardt GA, Zahniser NR. Kinetic analysis of striatal clearance of exogenous dopamine recorded by chronoamperometry in freely-moving rats. J. Neurosci. Methods. 2002;121(1):41–52. doi: 10.1016/s0165-0270(02)00229-7. [DOI] [PubMed] [Google Scholar]
- 76.Chen N, Justice JB. Differential effect of structural modification of human dopamine transporter on the inward and outward transport of dopamine. Brain Res. Mol. Brain Res. 2000;75(2):208–215. doi: 10.1016/s0169-328x(99)00288-0. [DOI] [PubMed] [Google Scholar]
- 77.Johnson LA, Guptaroy B, Lund D, Shamban S, Gnegy ME. Regulation of amphetamine-stimulated dopamine efflux by protein kinase C β. J. Biol. Chem. 2005b;280(12):10914–10919. doi: 10.1074/jbc.M413887200. [DOI] [PubMed] [Google Scholar]
- 78.Binda F, Dipace C, Bowton E, et al. Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux. Mol. Pharmacol. 2008;74(4):1101–1108. doi: 10.1124/mol.108.048447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Foster JD, Pananusorn B, Cervinski MA, Holden HE, Vaughan RA. Dopamine transporters are dephosphorylated in striatal homogenates and in vitro by protein phosphatase 1. Brain Res. Mol. Brain Res. 2003;110(1):100–108. doi: 10.1016/s0169-328x(02)00645-9. [DOI] [PubMed] [Google Scholar]
- 80.Bauman AL, Apparsundaram S, Ramamoorthy S, Wadzinski BE, Vaughan RA, Blakely RD. Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. J. Neurosci. 2000;20(20):7571–7578. doi: 10.1523/JNEUROSCI.20-20-07571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee KH, Kim MY, Kim DH, Lee YS. Syntaxin 1A and receptor for activated C kinase interact with the N-terminal region of human dopamine transporter. Neurochem. Res. 2004;29(7):1405–1409. doi: 10.1023/b:nere.0000026404.08779.43. [DOI] [PubMed] [Google Scholar]
- 82.Sung U, Apparsundaram S, Galli A, et al. A regulated interaction of syntaxin 1A with the antidepressant-sensitive norepinephrine transporter establishes catecholamine clearance capacity. J. Neurosci. 2003;23(5):1697–1709. doi: 10.1523/JNEUROSCI.23-05-01697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haase J, Killian AM, Magnani F, Williams C. Regulation of the serotonin transporter by interacting proteins. Biochem. Soc. Trans. 2001;29(Pt 6):722–728. doi: 10.1042/0300-5127:0290722. [DOI] [PubMed] [Google Scholar]
- 84.Deken SL, Wang D, Quick MW. Plasma membrane GABA transporters reside on distinct vesicles and undergo rapid regulated recycling. J. Neurosci. 2003;23(5):1563–1568. doi: 10.1523/JNEUROSCI.23-05-01563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000;80(2):717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- 86.Dipace C, Sung U, Binda F, Blakely RD, Galli A. Amphetamine induces a calcium/calmodulin-dependent protein kinase II-dependent reduction in norepinephrine transporter surface expression linked to changes in syntaxin 1A/transporter complexes. Mol. Pharmacol. 2007;71(1):230–239. doi: 10.1124/mol.106.026690. [DOI] [PubMed] [Google Scholar]
- 87.Usiello A, Baik JH, Rouge-Pont F, et al. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408(6809):199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- 88.Levesque D, Diaz J, Pilon C, et al. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-npropyl-2-aminotetralin. Proc. Natl Acad. Sci. USA. 1992;89(17):8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zapata A, Kivell B, Han Y, et al. Regulation of dopamine transporter function and cell surface expression by D3 dopamine receptors. J. Biol. Chem. 2007;282(49):35842–35854. doi: 10.1074/jbc.M611758200. [DOI] [PubMed] [Google Scholar]
- 90.Bertolino A, Fazio L, Di Giorgio A, et al. Genetically determined interaction between the dopamine transporter and the D2 receptor on prefronto-striatal activity and volume in humans. J. Neurosci. 2009;29(4):1224–1234. doi: 10.1523/JNEUROSCI.4858-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sevak RJ, Koek W, Daws LC, Owens WA, Galli A, France CP. Behavioral effects of amphetamine in streptozotocin-treated rats. Eur. J. Pharmacol. 2008;581(1–2):105–112. doi: 10.1016/j.ejphar.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Williams JM, Owens WA, Turner GH, et al. Hypoinsulinemia regulates amphetamine-induced reverse transport of dopamine. PLoS Biol. 2007;5(10):E274. doi: 10.1371/journal.pbio.0050274. [DOI] [PMC free article] [PubMed] [Google Scholar]