Abstract
Tumor necrosis factor-α (TNF-α), a pro-inflammatory cytokine, produces pain and hyperalgesia by activating and/or sensitizing nociceptive sensory neurons. In the present study, using whole-cell patch clamp techniques, the regulation of potassium currents by TNF-α was examined in acutely dissociated small dorsal root ganglion neurons. We found that acute application of TNF-α inhibited, in a dose-dependent manner, the non-inactivating sustained potassium current without changing the rapidly inactivating transient current or the kinetics of steady-state inactivation. The effects of TNF-α on potassium currents were similar to that of prostaglandin E2 as reported previously and also demonstrated in the current study. Furthermore, indomethacin, a potent inhibitor for both cyclo-oxygenase (COX) -1 and COX-2, completely blocked the effect of TNF-α on potassium currents. These results suggest that TNF-α may sensitize or activate sensory neurons by suppressing the sustained potassium current in nociceptive DRG neurons, possibly via stimulating the synthesis/release of endogenous prostaglandins.
Keywords: Tumor necrosis factor-α (TNF-α), dorsal root ganglion (DRG), potassium currents, prostaglandins, indomethacin
Introduction
TNF-α, a potent pro-inflammatory cytokine, can be synthesized and released by a variety of cells including monocytes, fibroblasts, endothelial, glia, macrophages Schwann cells, and neurons under normal and pathological conditions (Wagner and Myers, 1996, Creange et al., 1997, Dubovy et al., 2006). TNF-α is also found in normal nucleus pulposus (Igarashi et al., 2000) and herniated lumbar disc specimens (Ahn et al., 2002) that are adjacent to the sensory ganglia. The importance of TNF-α in inflammatory and neuropathic pain has been well-documented. TNF-α induces robust mechanical allodynia when applied to the lumbar dorsal root ganglion (DRG) in vivo (Homma et al., 2002). Neutralizing endogenous TNF-α with antiserums or soluble receptors in neuropathic pain animal models decreases both mechanical and thermal hyperalgesia (Lindenlaub et al., 2000, Sweitzer et al., 2001). Evidence obtained from in vivo and in vitro electrophysiological studies suggests that TNF-α contributes to pathological pain by enhancing neuronal excitability. Topical application of TNF-α to the whole DRG in vitro increases the excitability (Liu et al., 2002b) and induces or enhances spontaneous activity in DRG neurons of various sizes, effects that are dependent on the protein kinase A (PKA) pathway (Zhang et al., 2002). Similarly, ectopic discharges can be elicited by applying TNF-α to nerve trunks (Sorkin et al., 1997) or injecting into peripheral receptive fields in vivo (Junger and Sorkin, 2000).
TNF-α and other inflammatory cytokines may increase neuronal excitability by directly regulating ion channel activity. For example, acute application of TNF-α rapidly enhances TTX-resistant Na+ currents in isolated mouse DRG neurons (Jin and Gereau, 2006). This effect is thought to be the underlying mechanism for TNF-α-mediated mechanical hypersensitivity. TNF-α also reduces potassium conductance in Aplysia (Sawada et al., 1990), and retinal ganglion neurons in rats (Diem et al., 2001). Prostaglandin E2 (PGE2), another potent inflammatory mediator that can be synthesized and released upon exposure to TNF-α through the cyclo-oxygenase (COX) pathway (Dinarello et al., 1986, Chen et al., 1995, Pang and Knox, 1997, Mollace et al., 1998, Mark et al., 2001), also suppresses potassium current in dissociated embryonic rat DRG neurons (Nicol et al., 1997b). In the present study, using whole cell patch clamp techniques, we attempted to examine the effects of TNF-α on potassium currents in small adult DRG neurons, and to determine if such effects are related to PGE2 by manipulating the COX pathway.
Methods
Animals
Young female Sprague-Dawley rats (body weight 120-180 g) were housed one or two per cage under a controlled diurnal cycle of 12 h light and 12 h dark with free access to water and food. The ambient environment was maintained at constant temperature (22 ± 0.5°C) and relative humidity (60-70%). All the surgical procedures and the experimental protocols were approved by the institutional animal care and use committees of the University of Arkansas for Medical Sciences (Little Rock, Arkansas, USA) and University of Cincinnati (Cincinnati, OH, USA).
Cell Culture
Rats were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg). The bilateral L4 and L5 DRGs were isolated and the sheath was carefully removed in ice-cold normal Ringer solution. The connective tissue was digested by exposure to Ca2+-free solution containing 1.0% collagenase II for 30 min at 37°C followed by washout in normal Ringer solution for another 10 min. DRGs were then dissociated by trituration with fire-polished pasteur pipettes. DRG cells were plated onto poly-D-lysine coated glass coverslips in Medium199 (Sigma, St. Louis, MO, USA) containing 10% heat-inactivated FBS and 1000 U/ml each of penicillin and streptomycin. DRG cells were incubated at 37°C for 12-24 hours before recording.
Electrophysiology
After the short-term culture, coverslips were transferred to a recording chamber and DRG cells were visualized under differential interference contrast using an inverted microscope (IX71, Olympus America Inc., Center Valley, PA). Whole cell voltage-clamp recordings of small DRG neurons (diameter less than 30 μm) were conducted at room temperature with an AxoPatch-200B amplifier (Molecular Devices Corp., Sunnyvale, CA). Patch pipettes (2-4.0 MΩ) were fabricated from borosilicate glass (Sutter Instruments, Novato, CA). The recording chamber was continuously perfused at room temperature with oxygenated bath solution at a flow rate of 2 ml/min.
Data were acquired on a Pentium IV computer with the Clampex 8.0 program. The cell capacitance artifact was canceled by the nulling circuit of the recording amplifier. Ohmic leakage currents were subtracted using the P4/±4 subtraction protocol. Voltage errors were minimized by using ≥80% series resistance compensation. The current was filtered at 5 kHz and sampled at 50 kHz.
Total potassium current and the non-inactivating current were measured by depolarizing voltage steps from -60 mV to +50 mV after a 1-s prepulse to -120 or -30 mV, respectively. The rapidly inactivating transient current was obtained by subtracting the non-inactivating current from the total. The steady-state inactivation (measuring the total current) protocol consisted of a 1-s conditioning prepulse to potentials ranging between -100 mV and -10 mV followed by depolarizing voltage steps to +20 mV.
Solutions and chemical application
The normal bath solution for recording excitability parameters contained (in mM) 130 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose. The pH was adjusted to 7.4 with NaOH, and the osmolarity adjusted to ∼300 - 310 mOsm with sucrose. The bath solution for recording K+ currents contained (in mM) 130 Choline Cl, 5 KCl, 1 MgCl2, 2 CoCl2, 10 HEPES and 10 glucose. The pH was adjusted to 7.4 with Tris-base, and the osmolarity was adjusted to ∼300 - 310 mOsm with sucrose. The pipette solution contained (in mM) 140 KCl, 1 CaCl2, 2 MgCl2, 11 EGTA, 10 HEPES, 2 Mg ATP and 1 Li GTP. The pH was adjusted to 7.2 with Tris-base, and osmolarity to ∼290 - 300 mOsm with sucrose. Voltages were not corrected for liquid junction potentials, which were estimated to be <10 mV in all cases.
Recombinant human TNF-α (R and D Systems, Minneapolis, MN) was dissolved in 0.1% bovine serum albumin (BSA) in buffered saline to a concentration of 100 ng/ml and stored at -80°C in 10 μl aliquots for later use. PGE2 and indomethacin were both dissolved in ethanol and diluted to the final concentration of 1 μM for PGE2 and 10 μM for indomethacin prior to use. Control solutions for TNF-α, PGE2 and indomethacin contained the same amount of BSA or ethanol.
After measuring K currents in control solution, TNF-α at different doses (0.001, 0.01, 0.1, 1 and 5 ng/ml) was bath applied to DRG neurons for 15 min before repeating the same protocols for K current recording. The duration of TNF-α application was based on our previously reported finding that it takes more than 15 min for TNF-α to elicit C-fiber firing in a in vitro nerve-DRG preparation (Zhang et al., 2002). PGE2 was bath applied to dissociated neurons for 5 min prior to the measurement of its effect on potassium currents. To determine if the regulation of potassium currents by TNF-α is mediated by the COX pathway, indomethacin (Sigma, St. Louis, MO), a potent blocker for both COX-1 and COX-2, was applied to neurons for 15 min followed by mixture of indomethacin and TNF-α for additional 15 min. To avoid repetitive drug application, only one cell was studied per dish.
Data Analysis
All data are expressed as means ± S.E.M. The voltage dependence of activation of the K currents was fitted with the Boltzmann function: G/Gmax = δ/[1+exp(V0.5-Vm)/κ]. G is the conductance which is calculated by G=I/(Vm-Ek) where I is the K current, Vm is the voltage step and Ek is the potassium equilibrium potential which is calculated to be -84 mV for our intra-and extra cellular potassium concentrations. Gmax is the maximal conductance obtained at the membrane potential of + 50 mV prior to drug application. δ is a factor to account for inhibition by TNF-α or PGE2. V0.5 is the membrane potential for half-activation or inactivation. κ is the slope factor. An inhibitory effect was defined as reducing the G/Gmax by greater than 5% at +50 mV. For inactivation the relation G/Gmax = c + {(1-c)/[1+exp(Vm -V0.5)/κ] was used where c is the fraction of non-inactivating current. Currents were normalized with respect to Gmax. Normalized currents (G/Gmax) were plotted as a function of membrane potential. Two-way repeated measures ANOVA (RM ANOVA) with pairwise multiple comparison (Holm-Sidak method) was used to determine at which voltage levels the differences between control and drug treatment were significant if an overall effect of drug treatment was observed. P<0.05 is considered statistically significant.
Results
Potassium currents in small DRG neurons
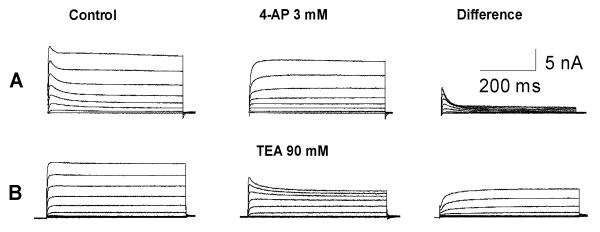
As shown in Figure 1, two types of outward potassium currents, rapidly inactivating transient current and non-inactivating sustained current, were recorded from dissociated DRG neurons. The non-inactivating current is highly sensitive to 4-AP whereas the rapidly inactivating current can be blocked by TEA. In the present study, the total potassium current and the non-inactivating current were measured by depolarizing voltage steps from -60 mV to +50 mV after a 1-s prepulse to -120 or -30 mV, respectively. The rapidly inactivating transient current was obtained by subtracting the sustained current from the total.
Figure 1.
Pharmacological isolation of the K current reveals 2 distinct classes of small DRG neurons. A: A typical neuron with a large amount of non-inactivating sustained component and a smaller component of rapidly inactivating transient component. B: A typical small neuron with both non-inactivating sustained and fast inactivating components. Note that the rapidly inactivating component is sensitive to 4-AP; and the non-inactivating sustained component is blocked by TEA.
Acute application of TNF-α suppresses sustained potassium current without affecting the rapidly inactivating component
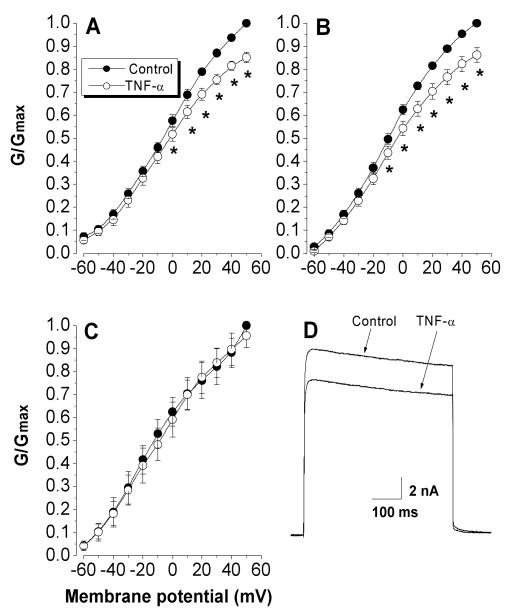
Topical TNF-α application for 15 min suppressed the total potassium current in 8 of the 11 neurons tested (P=0.006, two-way RM ANOVA) (Figure 2A and D). The 3 cells that did not show response to TNF- α had both sustained and transient components. Three of the 8 cells responded to TNF- α only had sustained component. The average reduction in G/Gmax measured at +50 mV was 15% ranging from 7% to 23%. Further analyses revealed that TNF-α suppressed the sustained non-inactivating potassium currents (P=0.003, two-way RM ANOVA) (Figure 2B) but did not affect the rapidly inactivating potassium currents (P=0.504, two-way RM ANOVA) (Figure 2C). The G/Gmax measured at +50 mV was reduced by an average of 13% (n=8, Figure 2B) for sustained non-inactivating K+ currents but less than 5% (n=8, Figure 2C) for rapidly inactivating currents. Two-way repeated measure ANOVA indicated that the effect of TNF-α was significant at individual voltages above 0 mV on the total K current (P=0.009 at 0 mV) (Figure 2A) and above -10 mV on the sustained K current (P=0.02 at -10 mV) (Figure 2B).
Figure 2.
Effects of TNF-α on potassium currents. TNF-α (1 ng/ml) suppressed the activation of total (A) and sustained K currents (B), but did not change the rapidly inactivating transient current (C). The K current was normalized by the maximum conductance, and fitted by the Boltzmann equation, and plotted as a function of membrane potential. The activation protocol consisted of 500-ms depolarizing voltage steps to potentials between −60 and 50 mV after a 1-s voltage step to either −120 (A) or −30 mV (B). Currents were normalized by the Gmax obtained from control at +50 mV.
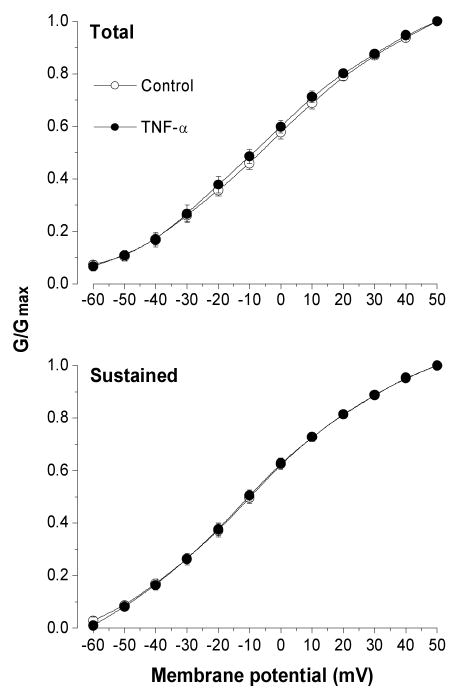
When the potassium currents obtained from control and TNF-α were independently normalized by its own Gmax at +50 mV, it revealed that TNF-α-induced suppression of potassium current was not associated with a shift in the voltage dependence for activation (Figure 3).
Figure 3.
The potassium current suppression by TNF-α is not associated with a shift in the voltage dependence for activation. Currents from control and TNF-α treatment were independently normalized by its own Gmax obtained at +50 mV and plotted as a function of membrane potential.
Potassium current reduction by 1 ng/ml TNF began at 4-5 min after TNF-α application. The inhibitory effects increased over time but the current reduction by TNF-α seemed to peak after 15-16 min of the drug application (data not shown).
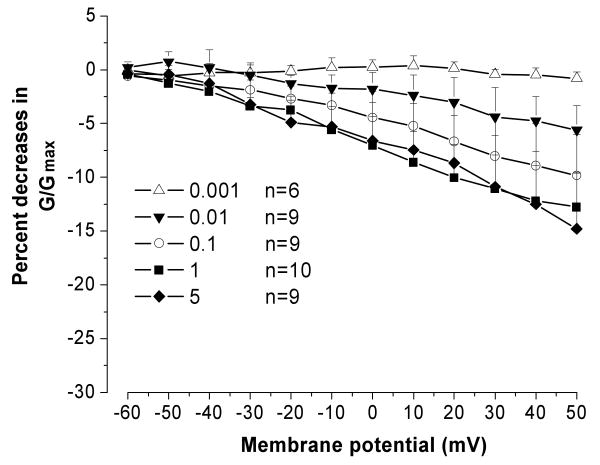
In addition to 1 ng/ml TNF-α, four other doses (0.001, 0.01, 0.1, and 5 ng/ml) were tested in different neurons. Because the rapidly inactivating transient current was not affected by 1 ng/ml TNF-α, in the following studies only the non-inactivating sustained potassium current was examined. As shown in Figure 4, the inhibitory effect of TNF-α on sustained potassium current was dose-dependent. TNF-α at 0.001 ng/ml was not able to change the activation of potassium currents (P=0.788, compared with control). Significant reduction of potassium currents was observed at 0.01 ng/ml (P=0.04, compared to 0.001 ng/ml). A similar maximal effect of TNF-α was observed at 1 and 5 ng/ml (P>0.05, compared with each other).
Figure 4.
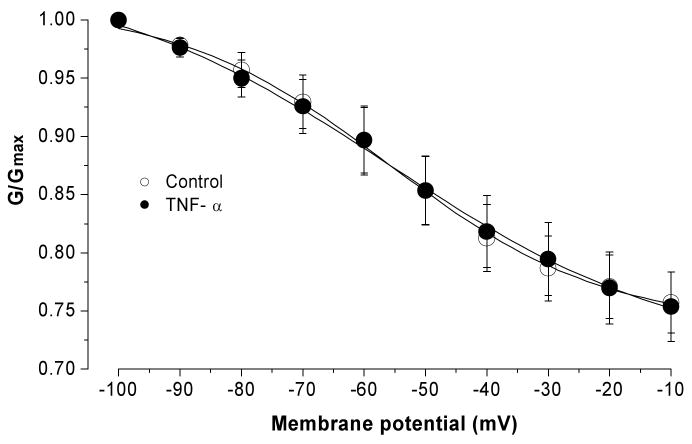
Acute bath application of TNF-α did not change the steady-state inactivation of the total K current. Current was normalized by the maximum conductance and fitted by the Boltzmann equation, and plotted as a function of membrane potential. The inactivation protocol consisted of a 1-s voltage step to potentials from -100 to -10 mV followed by depolarizing voltage step to +50 mV.
The effect of TNF-α on the voltage dependence of steady-state inactivation was also determined for the total voltage-activated potassium currents. The protocol consisted of a 1-s conditioning prepulse to potentials ranging between -100 and -10 mV followed by a depolarizing voltage step to a +20 mV test pulse for 1 second. Two-way RM ANOVA used to compare before and after TNF treatment failed to detect significant changes at any voltage levels (P=0.947, n=7) (Figure 5).
Figure 5.
The dose-dependence of the inhibitory effect of TNF-α on non-inactivating sustained K current. TNF-α-induced K current suppression was tested at different concentrations (0.001-5 ng/ml). Each data point represents the percent decrease of the normalized current (G/Gmax) over control after TNF-α application.
The inhibitory effects of TNF-α on potassium current are similar to that of PGE2 and are blocked by indomethacin
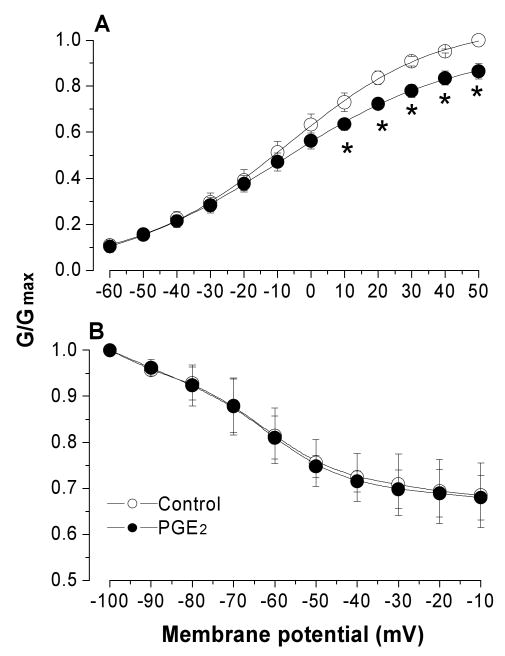
Because PGE2 has also been found, in a previous study, to reduce potassium currents in DRG neurons {Nicol, 1997 #218}, and TNF-α may stimulate release of endogenous PGE2, we tested the possibility that potassium inhibition by TNF-α may have been caused by synthesis/release of PGE2. We first tested the effect of PGE2 on the activation and inactivation of the sustained potassium currents. As shown in Figure 6, similar to TNF-α, 5 min after application, PGE2 (1 μM) inhibited the sustained non-inactivating potassium currents (Figure 6A, p=0.009) without altering the steady state voltage dependence of potassium inactivation (Figure 6B, P=0.176). After PGE2 application, the G/Gmax measured at + 50 mV decreased by 14% (n=9).
Figure 6.
Acute application of PGE2 for 5 minute inhibited the non-inactivating K current without affecting steady-state inactivation.
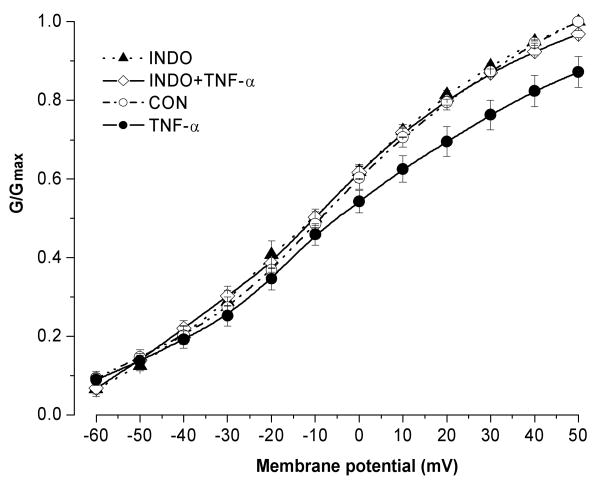
We then attempted to further examine the inhibitory effect of TNF-α on potassium current by blocking PGE2 synthesis/release using indomethacin, one of the potent cyclooxygenase inhibitors that blocks both COX1 and COX2 pathways. Indomethacin (10 μM) alone was applied the dissociated cells for 15 min followed by indomethacin plus TNF-α for another 15 min. Pretreatment of the sensory neurons with indomethacin completely blocked the inhibitory effects of TNF-α on sustained potassium currents (P=0.557, compared between INDO and TNF-α + INDO, n=10) (Figure 7). Compared to the control value (G/Gmax=1), the G/Gmax measured at +50 mV was 0.87 ± 0.038 in TNF-α treated cells (n=8) and 0.96 ± 0.008 in cells pretreated with TNF-α plus indomethacin. The normalized non-inactivating currents at +50 mV was decreased by only 3.14 ± 0.8 % when applying TNF-α together with indomethacin, which is markedly lower than 12.78 ± 3.2% when TNF-α was applied alone. This result indicates that indomethacin may block the inhibition of TNF-α on sustained potassium current by decreasing the synthesis/release of PGE2. In cells treated with indomethacin alone, no change in the activation of potassium currents was observed.
Figure 7.
Pretreatment of the DRG cells with indomethacin completely blocked TNF-α-induced K current inhibition. Indomethacin alone had no effect on the potassium currents.
Discussion
In the present study, we found that TNF-α, a potent pro-inflammatory cytokine, inhibits the non-inactivating sustained potassium current in small DRG neurons. This inhibition was mimicked by PGE2 application and blocked by pretreatment of the cells with indomethacin, a potent blocker of the COX pathway. The rapidly inactivating potassium current and the steady-state inactivation were not affected by acute TNF-α application, a result which is similar to that with PGE2.
Potassium currents have an important role in modulating neuronal excitability, and potassium channel reduction is thought to contribute to the increased excitability and generation/patterning of spontaneous activity in sensory neurons following peripheral nerve injury (Devor, 1983, Amir and Devor, 1997, Everill and Kocsis, 1999). Potassium currents are not always reduced under pathological conditions; they may be upregulated to compensate increased sodium currents as recently described by our group in an inflammatory pain model (Wang et al., 2007). In that model, both potassium currents and sodium currents are increased in small DRG neurons after a localized inflammation.
There are three major components of voltage-gated potassium current in mammalian sensory neurons: non-inactivating current, fast and slow transient currents (McFarlane and Cooper, 1991). Gold et al (Gold et al., 1996) further classified voltage-gated potassium currents into six subtypes in adult DRG neurons based on distinct biophysical and pharmacological properties. In general, both sustained and transient components are capable of regulating action potentials of neuron. Non-inactivating current is important in limiting repetitive firing by holding the membrane potential close to the potassium equilibrium potential. The transient potassium current modulates the repolarization of single action potential, the time required to reach the threshold to fire an action potential, and repetitive firing (Kocsis et al., 1987, Numann et al., 1987, Budde et al., 1992). Our finding that TNF-α only inhibits sustained non-inactivating current suggests that TNF-α-evoked firing or enhancement of spontaneous activity, as reported in our previous study (Liu et al., 2002a, Zhang et al., 2002), may be caused by regulating the potassium channel at resting membrane potential levels, possibly related to generation of membrane potential oscillations.
The effects of TNF-α reported here and in our previous publications are most likely due to the endogenous synthesis/release of PGE2. The reasons are: 1) pro-inflammatory cytokines such as TNF-α and IL-1β induce functional expression of COX-2 in cultured DRG neurons (Fehrenbacher et al., 2005); 2) the reduction of sustained potassium current by TNF-α is blocked by the COX inhibitor indomethacin; 3) effects of TNF-α on the activation of potassium current are similar to those of PGE2: inhibition of sustained current without altering the activation of transient potassium current or the steady-state inactivation; 4) the relatively longer time (e.g., 15 min) required for TNF-α to reduce potassium current or to evoke firing; and 5) the involvement of PKA pathway in both TNF-α evoked firing and in PGE2-induced suppression of potassium currents in sensory neurons (Evans et al., 1999, Zhang et al., 2002). In addition, Nicol et al. (Nicol et al., 1997a) reported in an earlier study that enhancement of the capsaicin-evoked current in nociceptive DRG neurons by TNF-α also was blocked by a specific COX-2 inhibitor, which provides further evidence that TNF-α can enhance the sensitivity of sensory neurons to chemical stimulation by the neuronal production of prostaglandins.
Although not tested, it is possible that effects of TNF-α on potassium current may involve prostaglandins other than PGE2 because prostaglandins such as PGF2 are also regulated by COX pathway and their expression may be reduced by COX inhibitors (Mark et al., 2001). Finally, we do not believe the effects reported here are due to an indirect effect on satellite glia or other non-neuronal cells because results were performed in low density, acute cultures, in which non-neuronal cells were very sparse and intercellular mediators would be diluted by the culture medium.
In addition to indirectly regulating potassium currents in sensory neurons, TNF-α may directly act on some ion channels such as sodium channels. Jin and Gereau (Jin and Gereau, 2006) recently tested the effects of TNF-α on TTX-resistant sodium channels in isolated mouse DRG neurons and found that acute application of TNF-α rapidly enhanced TTX-resistant Na+ currents. This potentiation of TTX-resistant currents by TNF-α is mediated by the p38 mitogen-activated protein kinase pathway through TNFR1 receptor. Further study is needed to determine which TNF receptor mediates the effects of TNF- α on potassium currents.
The inhibitory effect of TNF-α on potassium current in sensory neurons may play an important role in abnormal neuronal activity under various pathological conditions. Sensitization of sensory neurons by exposure to TNF-α released from the ruptured nucleus pulposus is thought to contribute to pathogenesis of low back pain. In nerve-injury neuropathic pain models, immunohistochemical staining demonstrated increased expression of TNF-α and TNFR1 receptor in DRG neurons, satellite glia, and macrophage ipsilateral and contralateral to the nerve injury (Dubovy et al., 2006). Although our recent studies using the multiplex technique failed to detect changes in TNF-α protein levels in the inflamed or nerve injured DRG (Xie et al., 2006, Li et al., 2007), the receptor upregulation may account for the important contribution of TNF-α to abnormal neuronal firing and development of pain.
In summary, the pro-inflammatory cytokine TNF-α may sensitize sensory neurons by suppressing the non-inactivating sustained potassium current, which may be secondary to the neuronal production of endogenous prostaglandins.
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke (NINDS) Grants NS39568, NS55860, and NS45594 (JZ).
References
- Ahn SH, Cho YW, Ahn MW, Jang SH, Sohn YK, Kim HS. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002;27:911–917. doi: 10.1097/00007632-200205010-00005. [DOI] [PubMed] [Google Scholar]
- Amir R, Devor M. Spike-evoked suppression and burst patterning in dorsal root ganglion neurons of the rat. J Physiol (Lond) 1997;501:183–196. doi: 10.1111/j.1469-7793.1997.183bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde T, Mager R, Pape HC. Different Types of Potassium Outward Current in Relay Neurons Acutely Isolated from the Rat Lateral Geniculate Nucleus. Eur J Neurosci. 1992;4:708–722. doi: 10.1111/j.1460-9568.1992.tb00180.x. [DOI] [PubMed] [Google Scholar]
- Chen DB, Yang ZM, Hilsenrath R, Le SP, Harper MJ. Stimulation of prostaglandin (PG) F2 alpha and PGE2 release by tumour necrosis factor-alpha and interleukin-1 alpha in cultured human luteal phase endometrial cells. Hum Reprod. 1995;10:2773–2780. doi: 10.1093/oxfordjournals.humrep.a135790. [DOI] [PubMed] [Google Scholar]
- Creange A, Barlovatz-Meimon G, Gherardi RK. Cytokines and peripheral nerve disorders. Eur Cytokine Netw. 1997;8:145–151. [PubMed] [Google Scholar]
- Devor M. Potassium channels moderate ectopic excitability of nerve-end neuromas in rats. Neurosci Lett. 1983;40:181–186. doi: 10.1016/0304-3940(83)90299-9. [DOI] [PubMed] [Google Scholar]
- Diem R, Meyer R, Weishaupt JH, Bahr M. Reduction of potassium currents and phosphatidylinositol 3-kinase-dependent Akt phosphorylation by tumor necrosis factor-alpha rescues axotomized retinal ganglion cells from retrograde cell death in vivo. J Neurosci. 2001;21:2058–2066. doi: 10.1523/JNEUROSCI.21-06-02058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA, Cannon JG, Wolff SM, Bernheim HA, Beutler B, Cerami A, Figari IS, Palladino MA, O'Connor JV. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986;163:1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovy P, Jancalek R, Klusakova I, Svizenska I, Pejchalova K. Intra- and extraneuronal changes of immunofluorescence staining for TNF-alpha and TNFR1 in the dorsal root ganglia of rat peripheral neuropathic pain models. Cell Mol Neurobiol. 2006;26:1205–1217. doi: 10.1007/s10571-006-9006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AR, Vasko MR, Nicol GD. The cAMP transduction cascade mediates the PGE2-induced inhibition of potassium currents in rat sensory neurones. J Physiol (Lond) 1999;516:163–178. doi: 10.1111/j.1469-7793.1999.163aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everill B, Kocsis JD. Reduction in potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomy. J Neurophysiol. 1999;82:700–708. doi: 10.1152/jn.1999.82.2.700. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher JC, Burkey TH, Nicol GD, Vasko MR. Tumor necrosis factor alpha and interleukin-1beta stimulate the expression of cyclooxygenase II but do not alter prostaglandin E2 receptor mRNA levels in cultured dorsal root ganglia cells. Pain. 2005;113:113–122. doi: 10.1016/j.pain.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Gold MS, Shuster MJ, Levine JD. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J Neurophysiol. 1996;75:2629–2646. doi: 10.1152/jn.1996.75.6.2629. [DOI] [PubMed] [Google Scholar]
- Homma Y, Brull SJ, Zhang JM. A comparison of chronic pain behavior following local application of tumor necrosis factor alpha to the normal and mechanically compressed lumbar ganglia in the rat. Pain. 2002;95:235–246. doi: 10.1016/S0304-3959(01)00404-3. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Kikuchi S, Shubayev V, Myers RR. 2000 Volvo Award winner in basic science studies: Exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine. 2000;25:2975–2980. doi: 10.1097/00007632-200012010-00003. [DOI] [PubMed] [Google Scholar]
- Jin X, Gereau RWt. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci. 2006;26:246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger H, Sorkin LS. Nociceptive and inflammatory effects of subcutaneous TNF alpha. Pain. 2000;85:145–151. doi: 10.1016/s0304-3959(99)00262-6. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Eng DL, Gordon TR, Waxman SG. Functional differences between 4-aminopyridine and tetraethylammonium-sensitive potassium channels in myelinated axons. Neurosci Lett. 1987;75:193–198. doi: 10.1016/0304-3940(87)90296-5. [DOI] [PubMed] [Google Scholar]
- Li H, Xie W, Strong JA, Zhang JM. Systemic antiinflammatory corticosteroid reduces mechanical pain behavior, sympathetic sprouting, and elevation of proinflammatory cytokines in a rat model of neuropathic pain. Anesthesiology. 2007;107:469–477. doi: 10.1097/01.anes.0000278907.37774.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenlaub T, Teuteberg P, Hartung T, Sommer C. Effects of neutralizing antibodies to TNF-alpha on pain-related behavior and nerve regeneration in mice with chronic constriction injury. Brain Res. 2000;866:15–22. doi: 10.1016/s0006-8993(00)02190-9. [DOI] [PubMed] [Google Scholar]
- Liu B, Li H, Brull SJ, Zhang JM. Increased sensitivity of sensory neurons to tumor necrosis factor alpha in rats with chronic compression of the lumbar ganglia. J Neurophysiol. 2002a;88:1393–1399. doi: 10.1152/jn.2002.88.3.1393. [DOI] [PubMed] [Google Scholar]
- Liu B, Li HQ, Brull SJ, Zhang JM. Increased sensitivity of sensory neurons to tumor necrosis factor alpha in rats with chronic compression of the lumbar ganglia. J Neurophysiol. 2002b;88:1393–1399. doi: 10.1152/jn.2002.88.3.1393. [DOI] [PubMed] [Google Scholar]
- Mark KS, Trickler WJ, Miller DW. Tumor necrosis factor-alpha induces cyclooxygenase-2 expression and prostaglandin release in brain microvessel endothelial cells. J Pharmacol Exp Ther. 2001;297:1051–1058. [PubMed] [Google Scholar]
- McFarlane S, Cooper E. Kinetics and voltage dependence of A-type currents on neonatal rat sensory neurons. J Neurophysiol. 1991;66:1380–1391. doi: 10.1152/jn.1991.66.4.1380. [DOI] [PubMed] [Google Scholar]
- Mollace V, Colasanti M, Muscoli C, Lauro GM, Iannone M, Rotiroti D, Nistico G. The effect of nitric oxide on cytokine-induced release of PGE2 by human cultured astroglial cells. Br J Pharmacol. 1998;124:742–746. doi: 10.1038/sj.bjp.0701852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol GD, Lopshire JC, Pafford CM. Tumor necrosis factor enhances the capsaicin sensitivity of rat sensory neurons. J Neurosci. 1997a;17:975–982. doi: 10.1523/JNEUROSCI.17-03-00975.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol GD, Vasko MR, Evans AR. Prostaglandins suppress an outward potassium current in embryonic rat sensory neurons. J Neurophysiol. 1997b;77:167–176. doi: 10.1152/jn.1997.77.1.167. [DOI] [PubMed] [Google Scholar]
- Numann RE, Wadman WJ, Wong RK. Outward currents of single hippocampal cells obtained from the adult guinea-pig. J Physiol. 1987;393:331–353. doi: 10.1113/jphysiol.1987.sp016826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang L, Knox AJ. Effect of interleukin-1 beta, tumour necrosis factor-alpha and interferon-gamma on the induction of cyclo-oxygenase-2 in cultured human airway smooth muscle cells. Br J Pharmacol. 1997;121:579–587. doi: 10.1038/sj.bjp.0701152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Hara N, Maeno T. Extracellular tumor necrosis factor induces a decreased K+ conductance in an identified neuron of Aplysia kurodai. Neurosci Lett. 1990;115:219–225. doi: 10.1016/0304-3940(90)90458-l. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience. 1997;81:255–262. doi: 10.1016/s0306-4522(97)00147-4. [DOI] [PubMed] [Google Scholar]
- Sweitzer S, Martin D, Deleo JA. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience. 2001;103:529–539. doi: 10.1016/s0306-4522(00)00574-1. [DOI] [PubMed] [Google Scholar]
- Wagner R, Myers RR. Schwann cells produce tumor necrosis factor alpha: expression in injured and non-injured nerves. Neuroscience. 1996;73:625–629. doi: 10.1016/0306-4522(96)00127-3. [DOI] [PubMed] [Google Scholar]
- Wang JG, Strong JA, Xie W, Zhang JM. Local inflammation in rat dorsal root ganglion alters excitability and ion currents in small-diameter sensory neurons. Anesthesiology. 2007;107:322–332. doi: 10.1097/01.anes.0000270761.99469.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang JM. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142:809–822. doi: 10.1016/j.neuroscience.2006.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Li HQ, Liu B, Brull SJ. Acute topical application of tumor necrosis factor alpha evokes protein kinase A-dependent responses in rat sensory neurons. J Neurophysiol. 2002;88:1387–1392. doi: 10.1152/jn.2002.88.3.1387. [DOI] [PubMed] [Google Scholar]