Abstract
Cells are ion conductive gels surrounded by a ~5-nm-thick insulating membrane, and molecular ionic pumps in the membrane establish an internal potential of approximately −90 mV. This electrical energy store is used for high-speed communication in nerve and muscle and other cells. Nature also has used this electric field for high-speed motor activity, most notably in the ear, where transduction and detection can function as high as 120 kHz. In the ear, there are two sets of sensory cells: the “inner hair cells” that generate an electrical output to the nervous system and the more numerous “outer hair cells” that use electromotility to counteract viscosity and thus sharpen resonance to improve frequency resolution. Nature, in a remarkable exhibition of nanomechanics, has made out of soft, aqueous materials a microphone and high-speed decoder capable of functioning at 120 kHz, limited only by thermal noise. Both physics and biology are only now becoming aware of the material properties of biomembranes and their ability to perform work and sense the environment. We anticipate new examples of this biopiezoelectricity will be forthcoming.
Biological Membranes
Biological membranes are floppy 3-nm-thick lipid bilayers composed mostly of phospholipids doped with about 20% protein, such as receptors and enzymes (Figure 1). There are no “typical” cells, and cellular structures are elaborate, nonrandom, and their properties cannot be averaged over useful dimensions. Life straddles a domain of medium entropy where complexity and flexibility are key. There are big cells such as bird eggs, 1-µm diameter cylindrical nerve cells that reach from the spine to the toe, muscle cells that reach from the hip to the knee, and bacterial cells that are less than 1 µm in diameter. All of these cells expend metabolic energy to generate an electrochemical gradient across the cell membrane of ~0.1–0.2 V, storing energy for high-demand processing. The electric field in the cell membrane is about 10 MV/m compared to 3 MV/m for lightning.
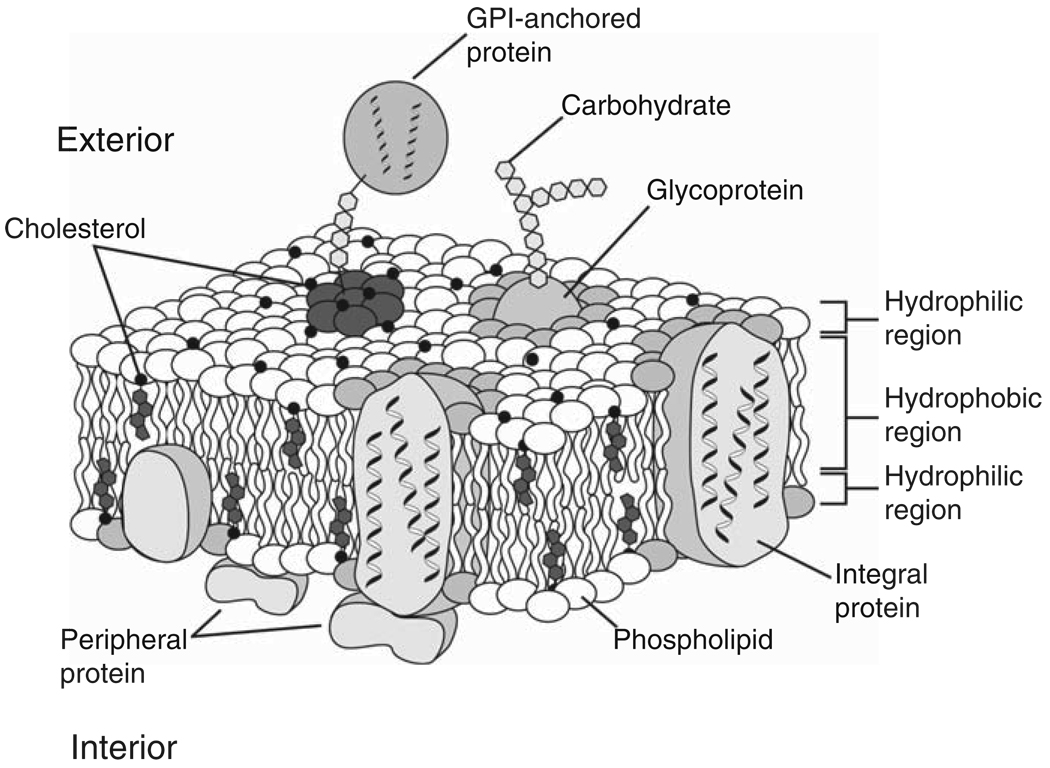
Figure 1.
Cartoon of a biomembrane after Singer & Nicolson from Dowhan et al.41 A variety of proteins, including ion channels, receptors, and enzymes, are in or near the membrane. Glycosylphosphatidylinositol (GPI)-anchored proteins are those anchored to the membrane by the glycolipid GPI.
Research in bioelectromechanics stems from studies of the electrical properties of membranes. The principle model for distributed electrical amplification in nerve cells was established by Hodgkin and Huxley in the early 1950s.1 Since then, molecular biology has allowed us to extract the molecular entities of that amplifier and many other nanomachines. 2,3 The membrane itself is not formed from a discrete genetic code but exists as an ensemble influenced by the outputs of multiple genes. Much of our understanding of membrane mechanics came from the early work of Wolfgang Helfrich4 on lipid bilayers. Since then, measurement technology has greatly improved, incorporating video microscopy, atomic force microscopy (AFM), and optical tweezers. The earliest suggestion that biological membranes were capable of an electromechanical response came from observations of changes of membrane birefringence associated with changes in potential.5 Electromotility was subsequently demonstrated in nerve fibers,6 outer hair cells (OHCs) in the mammalian ear,7 and cultured cells, such as human embryonic kidney and Chinese hamster ovary cells.8–11 In addition, experiments on pure lipid bilayers (black lipid membranes) showed that they polarize with curvature,12 curve with a potential,13 and polarize along the membrane surface under shear.14
Membrane Flexoelectricity: Curvature and Polarization
Liquid crystals display flexoelectricity as a mechanoelectric property similar to the piezoelectric effect in solid crystals.15 In most liquid crystals, an applied electric field induces an orientational distortion of the local directors. Conversely, any distortion of the director field will induce macroscopic polarization. Flexoelectricity is well understood in liquid crystal physics,16 and in the special case of a two-dimensional liquid crystal, flexoelectricity refers to a curvature-induced membrane polarization or, equivalently, an electric field-induced curvature. In the first case,15,17
| (1) |
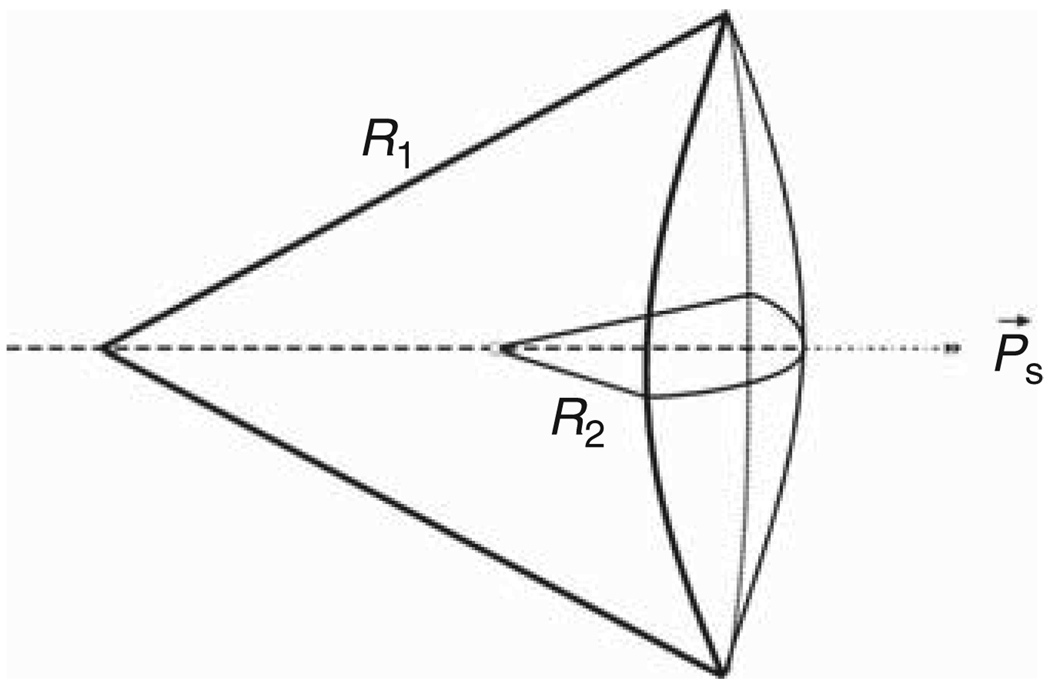
where Ps is the electric polarization per unit area in C/m, c1 and c2 are the two principal membrane curvatures in m−1 (where c1 = 1/R1 and c2 = 1/R2, and R1 and R2 are the principal radii of membrane curvature as shown in Figure 2), and f is the area flexoelectric coefficient in C (Coulombs), typically a few units of electron charge. The flexocoefficient is defined positive if polarization points outward from the center of curvature (Figure 2). Curvature of the membrane leads to a splay orientation of the lipids that would otherwise lie parallel to the membrane normal. According to the Helmholtz equation, an electric potential difference exists across a polarized surface. In view of Equation 1, the curvature-dependent part of this potential difference, the direct flexoelectric effect, is given by
| (2) |
where Ps is the polarization per unit area, and ε0 is the absolute dielectric permittivity of free space. This is the expression of the direct flexoelectric effect. We can determine the flexoelectric coefficient by measuring the curvature and the curvature-induced potential difference.
Figure 2.
The parameters of flexoelectricity (taken from the logo of the 1st and 2nd Flexoelectric Congresses, SUNY-Buffalo, 2001 and Rice-Houston, 2003). On the right, flexoelectric (curvature-induced) polarization of the membrane Ps and sign convention about the flexocoefficient f. For the case shown, f is positive. R1 and R2 are the principal radii of membrane curvature.
Like piezoelectricity, flexoelectricity also displays the converse effect of electric field–induced curvature:17
| (3) |
where E is the transmembrane electric field, and K is the curvature elastic modulus. Equation 3 is valid for a tension-free membrane. The total flexocoefficient typically contains the three lowest order electric multipoles of the membrane molecules (charge, dipole, and quadrupole).17
Flexoelectricity and Membrane Lipids
Summing the surface potential for lipids that are both charged and dipolar allows us to express the dual contribution to the flexocoefficient (Figure 3). For Debye lengths shorter than half the membrane thickness, we derived a simple expression:18
| (4) |
where f M is the monopole component, f D is the dipole component, d is the membrane thickness, Ao is the area per lipid molecule on the outside, and Ai is the area on the inside. In Equation 4, the charge and dipole components of the double layer surface potential of the outer (o) and inner (i) membrane surface are lumped into one: = (Figure 3). The surface potential ΔV is an experimentally measurable quantity.
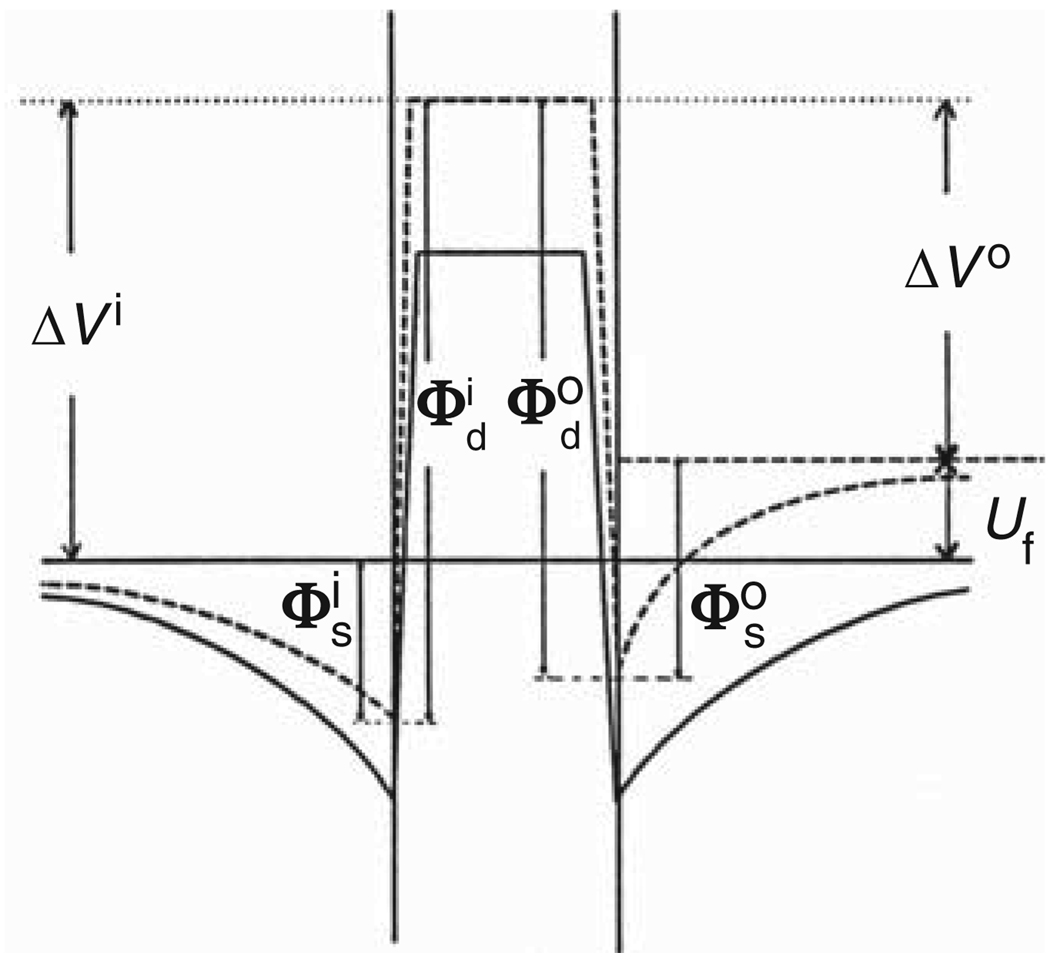
Figure 3.
Distribution of electric potential across a flat (solid line) and a curved (broken line) bilayer lipid membrane. The membrane is composed of lipids carrying surface charge and permanent dipoles. The potential distribution of a curved membrane corresponds to the open circuit condition. Δ V = Фd + Фs is the total surface potential of a monolayer (measurable by a vibrating electrode on the same lipid monolayer spread over the air-water interface of a classical Langmuir trough); Фd is the dipolar potential of the monolayer; Фs is the surface charge potential of the monolayer; the superscripts i and o stand for the inner and outer lipid monolayer, respectively. Uf is the curvature-generated (flexoelectric) potential difference. Reprinted with permission from Reference 13.
Flexoelectricity and Membrane Proteins
Integral membrane proteins can have a profound effect on the curvature-induced polarization,17 with the quadrupole contribution possibly larger than the dipolar contribution. The quadrupole contribution is expected in membranes with high protein concentration in ordered arrays,19 such as the inner mitochondrial membrane and the purple membranes of the bacterium Halobacterium halobium.
Electromotility of Native Membranes
Investigations of the electromotility of biological membranes have benefitted from the sensitivity of the AFM9 coupled to an amplifier that controls membrane potential. In the prototype of this experiment, an AFM cantilever tip is pressed against a cell, and an ac carrier (±10 mVp-p [peak-to-peak], ~100 Hz) is applied to the cell’s potential. The membrane moves with the voltage at a sensitivity of about 0.15 ± 0.05 nm/mVp-p, with an outward tip displacement as the inside is made positive. From the parameters in Reference 9, the flexoelectric coefficient f is estimated to be ~10−19 C.
Electromotility with Pulsed Electric Excitation
To improve measurements of the kinetics, step stimulations in voltage helped resolve the kinetics.10 Voltage pulses produced membrane displacements proportional to the voltage amplitude (~1 nm/100 mV, Figure 4). It is important to note that membrane movement and ionic currents were uncorrelated so that the motor is driven by the electric field, not the current (cf. Figure 4). The response was offset by the surface potential of the membrane so that at a low ionic strength of the bath solution (10 mM), the response became symmetric because the inner and outer interfaces had equal surface potential.
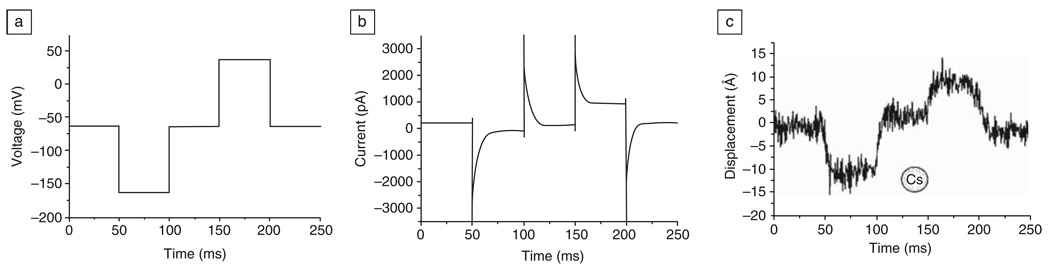
Figure 4.
Movement of a human embryonic kidney cell as the voltage is stepped. (a) Stimulus voltage; (b) cell current; (c) membrane movement at a set point of 0.7 nN. The inset is a scaled cesium atom (5.2 Å diameter). Adapted with permission from Reference 42.
Zhang et al.10 explained the effect of ionic strength in terms of the Lippmann equation for membrane tension in the presence of an electric field. The Lippmann equation predicts that symmetrically charged interfaces will move with a quadratic dependence on the transmembrane voltage, but membranes with asymmetric surface charges may appear monotonically dependent on the voltage. The membrane can be treated as having a Lippmann tension at each interface. The Lippmann tension arises from lateral repulsion of charges in the double layer and is numerically equal to the electric energy stored in the interface capacitance. The Lippmann tension at each interface sum provides a leading term that is linear with respect to the voltage, thus resembling flexoelectricity. Flexoelectric torque is produced by the difference of the two interfacial tensions rather than by their sum.
In a recent paper, Beyder and Sachs20 examined the electromotility of cell membranes that contained genetically introduced voltage-sensitive potassium channels. These ion channels are membrane proteins that are non-conducting when the membrane potential is significantly negative, and when the potential becomes more positive, they open an internal pore and freely conduct potassium ions. They behave similar to an ionic diode. Contradicting the Lippmann model that predicts a smooth movement with potential, Beyder and Sachs found that when the channel opened, the electromotility transiently saturated. To explain this unique data, they suggested that the conformational change associated with channel opening (a fanning out of the inner half of the channel) caused a buckling of the lipid membrane so that voltage could not produce a significant change in tension at the interfaces.
Membranes in Hearing
Hair Cells of the Cochlea
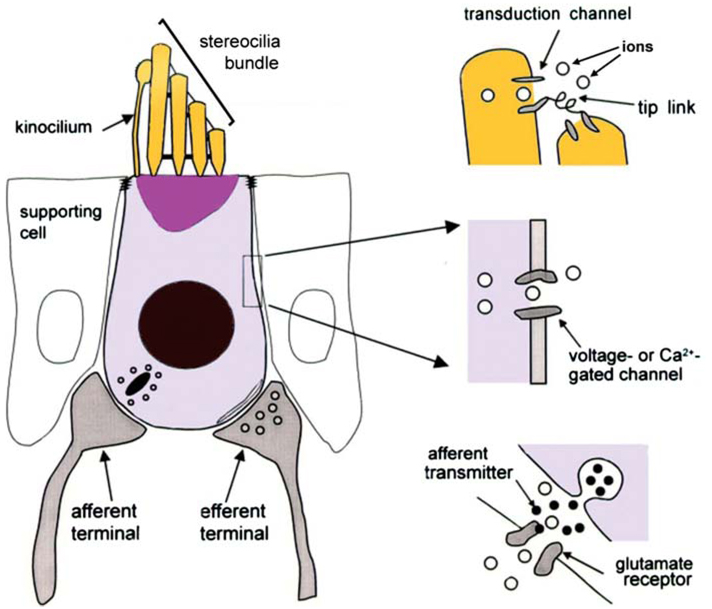
Hair cells are sensory receptor cells that convert the mechanical vibrations of sound into changes in membrane potential. They get their name from the stereociliary bundle on their surface (Figure 5). Stereocilia are giant microvilli (hair-like projections) arranged in three rows that increase in length toward the side of the cell located away from the central axis of the cochlear spiral (Figure 6a). The stereociliary bundle has an axis of symmetry perpendicular to the rows (see upper right insert of Figure 6b). The 0.1–0.2-µm diameter stereocilia have a dense inner core of tightly packed actin filaments anchored into a matrix of cytoskeletal proteins called the cuticular plate, which is located at the apical surface of the cell. Mecha-nosensitive ion channels are located in the wall of the stereocilia near the top and are hypothesized to be tethered to adjacent stereocilia by thin fibers called “tip links” (Figure 5). The bending of the stereociliary bundle toward the tallest row causes the tip links to pull on channels in the shorter neighbor causing them to open. This leads to an influx of K+ and Ca+2, making the cell interior more positive. Bending the bundle in the opposite direction closes the channels, and the cell becomes more negative. The electric field across the hair cell membrane changes within microseconds of bending the bundle. The field affects electromechanical force generation in the OHCs, tunes the resonance, and modulates neurotransmitter release by the inner hair cells (IHCs). The neurotransmitter controls the rate of firing in the nerve fibers that carry information about the acoustic environment to the brain. The events that convert the acoustic vibrations of sound into neural firing are coordinated by the organ of Corti.
Figure 5.
Sensory hair cell anatomy. Stereocilia are arranged in rows of different length and collectively make up the stereocilia bundle. Reprinted with permission from Reference 43.
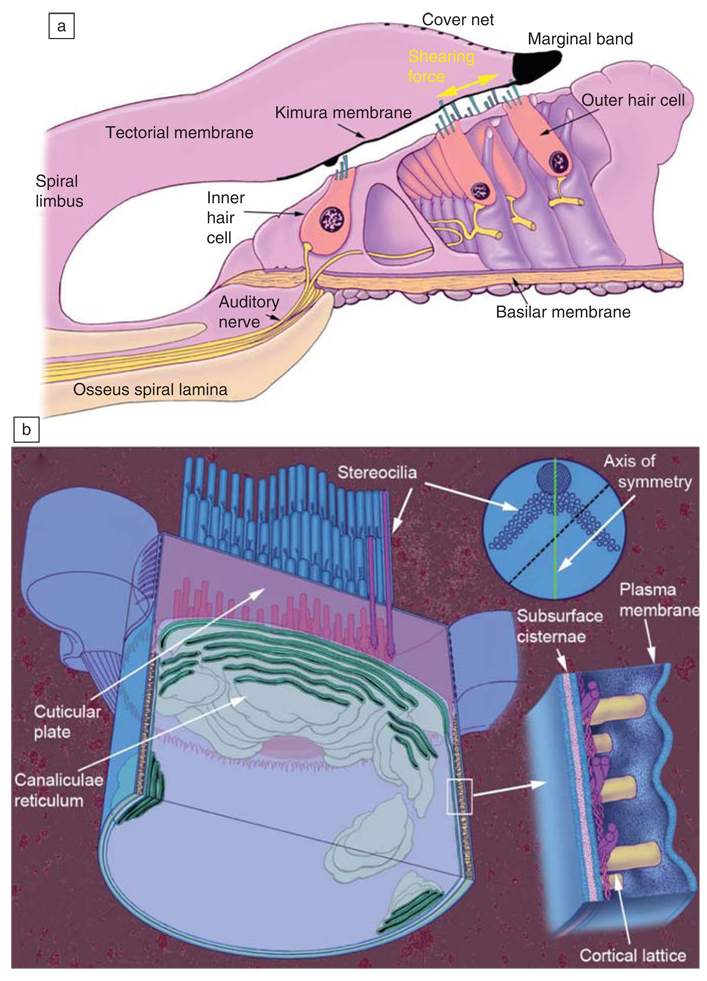
Figure 6.
The organ of Corti and outer hair cell. (a) The central axis of the spiraling cochlea is to the left of the drawing. Adapted from Reference 44. (b) Adapted from Reference 45.
Organ of Corti
The sensory cells of the cochlea are located in an elegant matrix of supporting cells, and the entire structure is called the organ of Corti (Figure 6a). There are three rows of OHCs and a single row of IHCs. The IHCs are located nearer the central axis of the cochlear spiral, while the OHCs are farther away. Both OHCs and IHCs are mechanoreceptors, but only IHCs trigger action potentials in afferent nerve fibers that convey information to the brain.
The inner ear is fluid filled, imposing damping forces on acoustically driven vibrations, and this effect increases with frequency. Auditory sensitivity and frequency discrimination require a mechanism to counteract this damping. When mammals appeared over 220 million years ago and the relevant sounds were propagated in air rather than water, they modified some of the sensory cells (OHCs) to become electric motors that served as active filters to tune the resonance. This cochlear amplifier gain is a function of the magnitude of the vibration, operating with the greatest gain with low intensity sound.
Outer Hair Cells
OHCs are cylindrical, ~9 µm in diameter (Figure 6a, 6b, and Figure 7), and vary in length from ~12 µm at the basal or high-frequency end of the cochlea to >90 µm at the low-frequency end. They have a bundle of stereocilia at one end, a synapse at the other (Figure 5), and a motor in the middle. Each of these three regions (the apical stereociliary bundle, middle cylinder, and hemispheric base) has a specific function. The stereocilia at the top are responsible for converting mechanical energy into electrical energy. The nerve connections (afferent—to the brain and efferent—from the brain) are found at the base. The efferent synapses modulate the gain of the cochlear amplifier. The cylindrical middle portion is where electrical energy is converted into mechanical energy, and no other cell is known to significantly change its length at acoustic frequencies in response to electrical stimulation. This electromotility can be greater than 1% of the cell’s length. The force alters the resonance of the organ of Corti, enhancing the perception and discrimination of high-frequency sounds. There are only about 12,000 hair cells (3000 IHCs and 9000 OHCs) in a normal human cochlea, a small number compared to the hundreds of millions of sensory cells in the retina, nose, or skin. Most of these sensory cells in the ear do not talk directly to the brain. To detect thermal level vibrations, the auditory system must be able to detect very small forces, and this requires a low mass.
Figure 7.
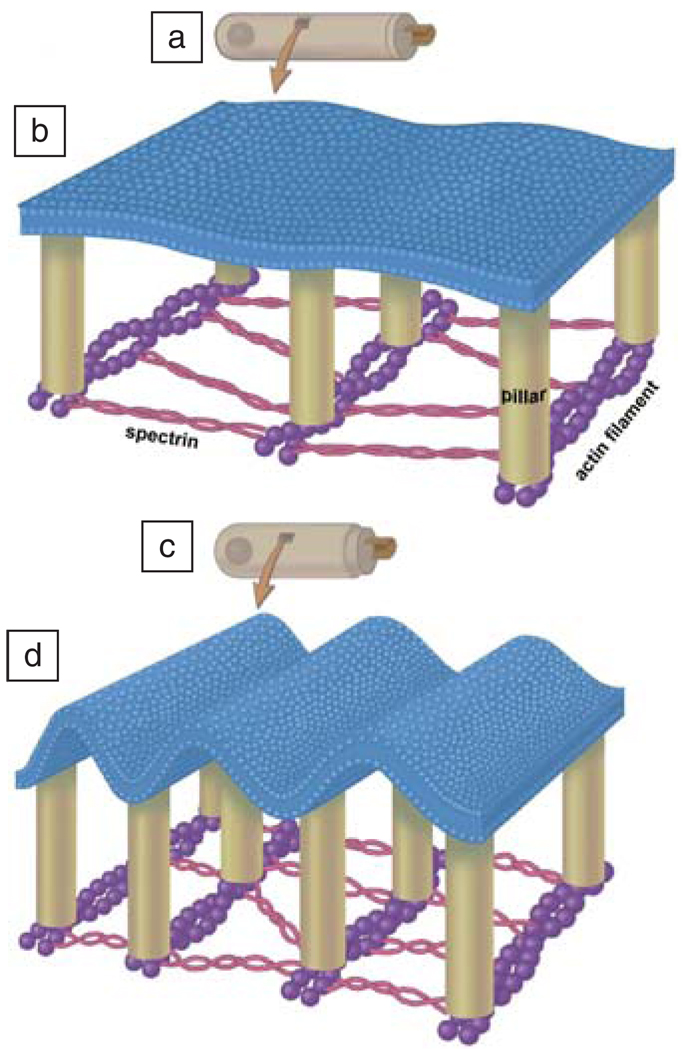
Postulated nanoscale rippling of the outer hair cell (OHC) lateral wall plasma membrane. OHC is shown at low magnification when hyperpolarized (a) and depolarized (c). A portion of the lateral wall is shown at higher magnification in (b) and (d). These cartoons portray flexoelectric alterations in membrane curvature associated with electromotile length changes. The plasma membrane is attached to cortical lattice pillars (tan), which, in turn, are attached to actin filaments (purple). These are cross linked with the elastic spectrin (thin red) filaments. Reprinted with permission from Reference 46.
The OHC Lateral Wall
The cylindrical lateral wall (Figure 6b, Figure 7b, and 7d) of the OHC is about 100 nm thick and contains a plasma membrane reinforced by a structural array of proteins (Figure 7), and further inside the cell is the membrane bound structure called the subsurface cisternae (Figure 6b). Electron microscopy reveals the presence of hexagonally packed particles in the lateral wall plasma membrane. Structural actin filaments are oriented circumferentially around the cell and are cross-linked by spectrin molecules (Figure 7). Actin and spectrin are long chain protein polymers that provide a structural framework that maintains the shape of most cells. Actin is less compliant than spectrin, so the OHC changes its length more than its diameter in response to electromotile forces generated by the membrane.
The OHC Membrane Motor Involves Prestin and Intracellular Chloride
OHC electromotility uses a membrane-based motor in the lateral wall. The motor mechanism is piezoelectric-like in that mechanical deformation of the membrane changes the transmembrane potential (direct piezoelectric effect), while electromotility is comparable to converse piezoelectricity. 21–24 The mechanical force generated by the membrane is communicated to the ends of the cell hydraulically and mechanically through the cortical lattice.
The membrane protein prestin and intracellular chloride ions are required for membrane motor function. Prestin was discovered by using molecular biology techniques that identified proteins that expressed in the OHC but not the IHC (which are nonmotile).11 Prestin is a member of the SLC26A family of anion transporters with up to 12 transmembrane helices.25,26 Prestin facilitates the generation of large displacement currents by allowing intracellular anions such as chloride and bicarbonate to enter the membrane. Electromotility and the prestin-associated charge movement are fragile, inhibited by single point mutations25 in which one amino acid out of the ~800 that make up the protein is replaced by another. It should be of interest to the modelers that prestin increases charge movement by at least three orders of magnitude but appears to only double the electromotile force.8,27 The role of prestin in the conversion of electrical to mechanical energy has been modeled as a molecule that changes its area in response to a change in the transmembrane electric field,28 or it acts by flexoelectricity, causing changes in curvature and surface charge.29 The debate boils down to whether prestin is the motor or part of the motor (is it a piston or a distributor?).
The dependence of electromotility and the prestin-associated charge movement on the material properties of the membrane have long been known. Changes in membrane tension shift the voltage dependence of both electromotility and charge movement. The membranous nature of the motor mechanism is highlighted by altering the lipid composition. The voltage-charge movement function can be shifted by 100 mV by adding or depleting cholesterol to the membrane, while the amount of charge moved is unaffected.30,31 The positive feedback of mechanical energy by OHCs in normal ears results in the production of sound (otoacoustic emissions—OAEs) that can be measured with microphones in the ear canal. Reducing the cholesterol content in the living cochlea eliminates nonlinear distortion products otoacoustic emissions (DPOAEs). Cholesterol enrichment produces a small increase in sensitivity (~3 dB), followed by elimination of DPOAEs. Cholesterol is thought to act by altering the mechanical properties of the membrane.30 Unsaturated omega-3 fatty acids also shift the voltage-charge movement of prestin31 but are thought to exert their effect by changing the surface charge.32
Electromotility in Membrane Tethers
It is possible to measure the electro-motility of membranes by measuring the force on cylindrical lipid tethers pulled from a membrane while varying the transmembrane potential.27,33 The tethers typically have a radius of ~100 nm, display large flexoelectric effects, and can move at acoustic frequencies. One end of the tether is held in a laser trap, and the other end is held by a glass pipette that is also used to apply voltage. Changes in voltage change the curvature and thus change the force on the bead in the laser trap. Since many biological structures have a similar radius of curvature,34 these forces could be generated in situ.
The voltage-sensitive ion channels, such as those responsible for the nerve action potential, change conformational shape upon opening and thus cause membrane deformation.20 The voltage sensing in these channels is the result of translation of a specific group of charges in the protein (known as the S4 segment), and the force is the product of the electric field times the charge
| (5) |
In the case of potassium channels,35,36 there are four charges per sensor and four monomers per channel, so the force could be on the order of 0.3 pN. The fluctuation dissipation theorem predicts that the force will alter the conformation equilibrium of coupled proteins and lipids37 and, as expected from the presence of a substantial conformational change, are modulated by the membrane tension.38–40
Summary
Cells move with the electric fields that appear across their membranes, and this electromotility occurs on a scale of nanometers to microns. The motility results from two effects: the general properties of polarizable interfaces and protein rearrangement in response to the electric field. These effects allow animals to hear sounds at thermal noise levels at frequencies above 100 kHz. Electromotility provides cells with mechanical responses in response to changes in voltage that are much faster than those that occur in muscles. Nature’s ability to utilize soft materials under salt water to produce high-frequency low-noise acoustic amplifiers should serve as design guides for industry.
Acknowledgments
Work supported by NIH research grants to FS, and DC 00354 & DC 02775 to WEB, as well as a grant from the Bulgarian Fund “Scientific Studies” under the Project No. NT 1-03/2004 to AGP.
References
- 1.Hodgkin AL, Huxley AF. J. Physiol. (London) 1952;117:500. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catterall WA. Curr. Opin. Cell Biol. 1994;6:607. doi: 10.1016/0955-0674(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. Nature. 2003;423:33. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- 4.Helfrich W, Naturforsch Z. C: Biosci. 1973;28:693. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- 5.Cohen LB, Keynes RD, Hille B. Nature. 1968;218:438. doi: 10.1038/218438a0. [DOI] [PubMed] [Google Scholar]
- 6.Iwasa K, Tasaki I. Biochem. Biophys. Res. Commun. 1980;95:1328. doi: 10.1016/0006-291x(80)91619-8. [DOI] [PubMed] [Google Scholar]
- 7.Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Science. 1985;227:194. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- 8.Ludwig J, Oliver D, Frank G, Klocker N, Gummer AW, Fakler B. Proc. Nat. Acad. Sci. U.S.A. 2001;98:4178. doi: 10.1073/pnas.071613498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosbacher J, Langer M, Horber JK, Sachs F. J. Gen. Physiol. 1998;111:65. doi: 10.1085/jgp.111.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang PC, Keleshian AM, Sachs F. Nature. 2001;413:428. doi: 10.1038/35096578. [DOI] [PubMed] [Google Scholar]
- 11.Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Nature. 2000;405:149. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
- 12.Petrov AG, Sokolov VS. Eur. Biophys. J. 1986;13:139. [Google Scholar]
- 13.Todorov AT, Petrov AG, Fendler JH. J. Phys. Chem. 1994;98:3076. [Google Scholar]
- 14.Harden J, Diorio N, Petrov AG, Jakli A. Phys. Rev. E: Stat. Nonlin. Soft Matter Phys. 2009;79:011701. doi: 10.1103/PhysRevE.79.011701. [DOI] [PubMed] [Google Scholar]
- 15.Meyer RB. Phys. Rev. Lett. 1969;22:918. [Google Scholar]
- 16.De Gennes PG. The Physics of Liquid Crystals. Oxford: Clarendon Press; 1974. [Google Scholar]
- 17.Petrov AG. The Lyotropic State of Matter: Molecular Physics and Living Matter Physics. The Netherlands: Gordon and Breach Science Publishers; 1999. [Google Scholar]
- 18.Petrov AG, Sachs F. Phys. Rev. E: Stat. Nonlin. Soft Matter Phys. 2002;65:021905. doi: 10.1103/PhysRevE.65.021905. [DOI] [PubMed] [Google Scholar]
- 19.Green DE, Ji S, Brucker RF. J. Bioenerg. 1973;4:253. doi: 10.1007/BF01516061. [DOI] [PubMed] [Google Scholar]
- 20.Beyder A, Sachs F. PNAS. 2009;106:6626. doi: 10.1073/pnas.0808045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong XX, Ospeck M, Iwasa KH. Biophys. J. 2002;82:1254. doi: 10.1016/S0006-3495(02)75481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabbitt RD, Ayliffe HE, Christensen D, Pamarthy K, Durney C, Clifford S, Brownell WE. Biophys. J. 2005;88:2257. doi: 10.1529/biophysj.104.050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spector AA, Brownell WE, Popel AS. J. Acoust. Soc. Am. 2003;113:453. doi: 10.1121/1.1526493. [DOI] [PubMed] [Google Scholar]
- 24.Weitzel EK, Tasker R, Brownell WE. J. Acoust. Soc. Am. 2003;114:1462. doi: 10.1121/1.1596172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajagopalan L, Greeson JN, Xia A, Liu H, Sturm A, Raphael RM, Davidson AL, Oghalai JS, Pereira FA, Brownell WE. J. Neurosci. 2006;26:12727. [Google Scholar]
- 26.Rajagopalan L, Pereira FA, Lichtarge O, Brownell WE. Methods Mol. Biol. 2009;493:287. doi: 10.1007/978-1-59745-523-7_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang R, Qian F, Rajagopalan L, Pereira FA, Brownell WE, Anvari B. Biophys. J. 2007;93:L07. doi: 10.1529/biophysj.107.107573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dallos P, Fakler B. Nat. Rev. Mol. Cell Biol. 2002;3:104. doi: 10.1038/nrm730. [DOI] [PubMed] [Google Scholar]
- 29.Marsh D. Biochim Biophys. Acta. 2008;1778:1545. doi: 10.1016/j.bbamem.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Rajagopalan L, Greeson JN, Xia A, Liu H, Sturm A, Raphael RM, Davidson AL, Oghalai JS, Pereira FA, Brownell WE. J. Biol. Chem. 2007;282:36659. doi: 10.1074/jbc.M705078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sfondouris J, Rajagopalan L, Pereira FA, Brownell WE. J. Biol. Chem. 2008;283:22473. doi: 10.1074/jbc.M803722200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borjesson SI, Hammarstrom S, Elinder F. Biophys. J. 2008;95:2242. doi: 10.1529/biophysj.108.130757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian F, Ermilov S, Murdock D, Brownell WE, Anvari B. Rev. Sci. Instrum. 2004;75:2937. doi: 10.1063/1.1781382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Science. 2004;303:1007. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 35.Chanda B, Asamoah OK, Blunck R, Roux B, Bezanilla F. Nature. 2005;436:852. doi: 10.1038/nature03888. [DOI] [PubMed] [Google Scholar]
- 36.Bezanilla F. Physiol. Rev. 2000;80:555. doi: 10.1152/physrev.2000.80.2.555. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt D, MacKinnon R. Proc. Nat. Acad. Sci. U.S.A. 2008;105:19276. doi: 10.1073/pnas.0810187105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calabrese B, Tabarean IV, Juranka P, Morris CE. Biophys. J. 2002;83:2560. doi: 10.1016/S0006-3495(02)75267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu CX, Juranka PF, Morris CE. Biophys. J. 2001;80:2678. doi: 10.1016/S0006-3495(01)76237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabarean IV, Morris CE. Biophys. J. 2002;82:2982. doi: 10.1016/S0006-3495(02)75639-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dowhan W, Bogdanov M, Mileykowskaya E. In: Biochemistry of Lipids, Lipoproteins and Membranes. Vance DE, Vance JE, editors. Cambridge: Elsevier; 2008. pp. 1–37. [Google Scholar]
- 42.Besch S, Snyder KV, Zhang RC, Sachs F. Cell Biochem. Biophys. 2003;39:195. doi: 10.1385/cbb:39:3:195. [DOI] [PubMed] [Google Scholar]
- 43.Brownell WE, Oghalai JS. In: Ballenger’s Otorhinolaryngology Head and Neck Surgery. Snow JB Jr., Wackym PA, editors. vol. 17. Philadelphia: BC Decker Inc; 2009. pp. 101–106. [Google Scholar]
- 44.Brownell WE. Volta Rev. 1999;99:9. [PMC free article] [PubMed] [Google Scholar]
- 45.Brownell WE. In: Hair Cell Micromechanics and Otoacoustic Emissions. Berlin CI, Hood LJ, Ricci A, editors. New Jersey: Delmar Learning; 2002. pp. 25–45. [Google Scholar]
- 46.Oghalai JS, Zhao HB, Kutz JW, Brownell WE. Science. 2000;287:658. doi: 10.1126/science.287.5453.658. [DOI] [PMC free article] [PubMed] [Google Scholar]