Abstract
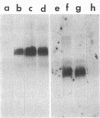
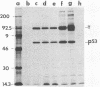
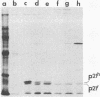
Cellular and viral oncogenes are usually defined on the basis of their ability to elicit neoplastic transformation. However, oncogene activity has also been implicated in the control of differentiation. We have tested whether transfection of primary cultured granulosa cells with various oncogenes can yield cell lines that maintain their differentiated properties. Primary granulosa cells were prepared from diethylstilbestrol-treated immature female rats and transfected with simian virus 40 (SV40) DNA or with SV40 plus activated human Ha-RAS oncogene. Transfection with SV40 plus Ha-RAS yielded cell lines that lost response to gonadotropins but, after 48 hr of stimulation with isoproterenol, cholera toxin, forskolin, or 8-bromoadenosine 3',5'-cyclic monophosphate (8-Br-cAMP), produced progesterone at levels comparable to those of differentiated primary cells. In contrast, cells transformed only by SV40 lost their ability to produce progesterone. Whereas in primary cell cultures progesterone production was already evident after a 3-hr incubation with 1 mM 8-Br-cAMP, in cotransfected cells progesterone production became evident only after 12 hr. All cotransformed cell lines produced SV40 large tumor antigen as well as human RAS p21 protein. The expression of the expected oncogenes in the various cell lines was confirmed by mRNA analysis. These results suggest that the expression of an activated RAS oncogene in granulosa cells can play a role in preserving inducible steroidogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alemà S., Casalbore P., Agostini E., Tatò F. Differentiation of PC12 phaeochromocytoma cells induced by v-src oncogene. Nature. 1985 Aug 8;316(6028):557–559. doi: 10.1038/316557a0. [DOI] [PubMed] [Google Scholar]
- Amsterdam A., Berkowitz A., Nimrod A., Kohen F. Aggregation of luteinizing hormone receptors in granulosa cells: a possible mechanism of desensitization to the hormone. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3440–3444. doi: 10.1073/pnas.77.6.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A., Knecht M., Catt K. J. Hormonal regulation of cytodifferentiation and intercellular communication in cultured granulosa cells. Proc Natl Acad Sci U S A. 1981 May;78(5):3000–3004. doi: 10.1073/pnas.78.5.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A., Nimrod A., Lamprecht S. A., Burstein Y., Lindner H. R. Internalization and degradation of receptor-bound hCG in granulosa cell cultures. Am J Physiol. 1979 Feb;236(2):E129–E138. doi: 10.1152/ajpendo.1979.236.2.E129. [DOI] [PubMed] [Google Scholar]
- Amsterdam A., Ohad I., Schramm M. Dynamic changes in the ultrastructure of the acinar cell of the rat parotid gland during the secretory cycle. J Cell Biol. 1969 Jun;41(3):753–773. doi: 10.1083/jcb.41.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A., Rotmensch S. Structure-function relationships during granulosa cell differentiation. Endocr Rev. 1987 Aug;8(3):309–337. doi: 10.1210/edrv-8-3-309. [DOI] [PubMed] [Google Scholar]
- Anderson C. M., Mendelson C. R. Regulation of steroidogenesis in rat Leydig cells in culture: effect of human chorionic gonadotropin and dibutyryl cyclic AMP on the synthesis of cholesterol side chain cleavage cytochrome P-450 and adrenodoxin. Arch Biochem Biophys. 1985 May 1;238(2):378–387. doi: 10.1016/0003-9861(85)90178-x. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985 Oct;42(3):841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Amsterdam A. In vitro regulation of granulosa cell differentiation. Involvement of cytoskeletal protein expression. J Biol Chem. 1987 Apr 15;262(11):5366–5376. [PubMed] [Google Scholar]
- Ben-Ze'ev A., Amsterdam A. Regulation of cytoskeletal proteins involved in cell contact formation during differentiation of granulosa cells on extracellular matrix. Proc Natl Acad Sci U S A. 1986 May;83(9):2894–2898. doi: 10.1073/pnas.83.9.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Kohen F., Amsterdam A. Gonadotropin-induced differentiation of granulosa cells is associated with the co-ordinated regulation of cytoskeletal proteins involved in cell-contact formation. Differentiation. 1987;34(3):222–235. doi: 10.1111/j.1432-0436.1987.tb00070.x. [DOI] [PubMed] [Google Scholar]
- Beug H., Blundell P. A., Graf T. Reversibility of differentiation and proliferative capacity in avian myelomonocytic cells transformed by tsE26 leukemia virus. Genes Dev. 1987 May;1(3):277–286. doi: 10.1101/gad.1.3.277. [DOI] [PubMed] [Google Scholar]
- Beug H., Leutz A., Kahn P., Graf T. Ts mutants of E26 leukemia virus allow transformed myeloblasts, but not erythroblasts or fibroblasts, to differentiate at the nonpermissive temperature. Cell. 1984 Dec;39(3 Pt 2):579–588. doi: 10.1016/0092-8674(84)90465-3. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Campbell K. L. Ovarian granulosa cells isolated with EGTA and hypertonic sucrose: cellular integrity and function. Biol Reprod. 1979 Nov;21(4):773–786. doi: 10.1095/biolreprod21.4.773. [DOI] [PubMed] [Google Scholar]
- Carnegie J. A., Dardick I., Tsang B. K. Microtubules and the gonadotropic regulation of granulosa cell steroidogenesis. Endocrinology. 1987 Feb;120(2):819–828. doi: 10.1210/endo-120-2-819. [DOI] [PubMed] [Google Scholar]
- Cherington V., Morgan B., Spiegelman B. M., Roberts T. M. Recombinant retroviruses that transduce individual polyoma tumor antigens: effects on growth and differentiation. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4307–4311. doi: 10.1073/pnas.83.12.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola J. A., Cole M. D. Constitutive c-myc oncogene expression blocks mouse erythroleukaemia cell differentiation but not commitment. Nature. 1986 Apr 24;320(6064):760–763. doi: 10.1038/320760a0. [DOI] [PubMed] [Google Scholar]
- Davis J. S., Weakland L. L., Farese R. V., West L. A. Luteinizing hormone increases inositol trisphosphate and cytosolic free Ca2+ in isolated bovine luteal cells. J Biol Chem. 1987 Jun 25;262(18):8515–8521. [PubMed] [Google Scholar]
- Dmitrovsky E., Kuehl W. M., Hollis G. F., Kirsch I. R., Bender T. P., Segal S. Expression of a transfected human c-myc oncogene inhibits differentiation of a mouse erythroleukaemia cell line. Nature. 1986 Aug 21;322(6081):748–750. doi: 10.1038/322748a0. [DOI] [PubMed] [Google Scholar]
- Epstein-Almog R., Orly J. Inhibition of hormone-induced steroidogenesis during cell proliferation in serum-free cultures of rat granulosa cells. Endocrinology. 1985 May;116(5):2103–2112. doi: 10.1210/endo-116-5-2103. [DOI] [PubMed] [Google Scholar]
- Ezra E., Salomon Y. Mechanism of desensitization of adenylate cyclase by lutropin. Impaired introduction of GTP into the regulatory site. J Biol Chem. 1981 Jun 10;256(11):5377–5382. [PubMed] [Google Scholar]
- Funkenstein B., Waterman M. R., Simpson E. R. Induction of synthesis of cholesterol side chain cleavage cytochrome P-450 and adrenodoxin by follicle-stimulating hormone, 8-bromo-cyclic AMP, and low density lipoprotein in cultured bovine granulosa cells. J Biol Chem. 1984 Jul 10;259(13):8572–8577. [PubMed] [Google Scholar]
- Furman A., Rotmensch S., Kohen F., Mashiach S., Amsterdam A. Regulation of rat granulosa cell differentiation by extracellular matrix produced by bovine corneal endothelial cells. Endocrinology. 1986 May;118(5):1878–1885. doi: 10.1210/endo-118-5-1878. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia I., Sordat B., Rauccio-Farinon E., Dunand M., Kraehenbuhl J. P., Diggelmann H. Establishment of two rabbit mammary epithelial cell lines with distinct oncogenic potential and differentiated phenotype after microinjection of transforming genes. Mol Cell Biol. 1986 Jun;6(6):1974–1982. doi: 10.1128/mcb.6.6.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring N. B., Durica J. M., Lifka J., Hedin L., Ratoosh S. L., Miller W. L., Orly J., Richards J. S. Cholesterol side-chain cleavage P450 messenger ribonucleic acid: evidence for hormonal regulation in rat ovarian follicles and constitutive expression in corpora lutea. Endocrinology. 1987 May;120(5):1942–1950. doi: 10.1210/endo-120-5-1942. [DOI] [PubMed] [Google Scholar]
- Goldring N. B., Orly J. Concerted metabolism of steroid hormones produced by cocultured ovarian cell types. J Biol Chem. 1985 Jan 25;260(2):913–921. [PubMed] [Google Scholar]
- Hanley M. R., Jackson T. The ras gene. Transformer and transducer. Nature. 1987 Aug 20;328(6132):668–669. doi: 10.1038/328668a0. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh A. J., Adashi E. Y., Jones P. B., Welsh T. H., Jr Hormonal regulation of the differentiation of cultured ovarian granulosa cells. Endocr Rev. 1984 Winter;5(1):76–127. doi: 10.1210/edrv-5-1-76. [DOI] [PubMed] [Google Scholar]
- Knecht M., Amsterdam A., Catt K. J. Inhibition of granulosa cell differentiation by gonadotropin-releasing hormone. Endocrinology. 1982 Mar;110(3):865–872. doi: 10.1210/endo-110-3-865. [DOI] [PubMed] [Google Scholar]
- Knecht M., Amsterdam A., Catt K. The regulatory role of cyclic AMP in hormone-induced of granulosa cell differentiation. J Biol Chem. 1981 Oct 25;256(20):10628–10633. [PubMed] [Google Scholar]
- Knecht M., Ranta T., Catt K. J. Granulosa cell differentiation in vitro: induction and maintenance of follicle-stimulating hormone receptors by adenosine 3',5'-monophosphate. Endocrinology. 1983 Sep;113(3):949–956. doi: 10.1210/endo-113-3-949. [DOI] [PubMed] [Google Scholar]
- Maltzman W., Oren M., Levine A. J. The structural relationships between 54,000-molecular-weight cellular tumor antigens detected in viral- and nonviral-transformed cells. Virology. 1981 Jul 15;112(1):145–156. doi: 10.1016/0042-6822(81)90620-6. [DOI] [PubMed] [Google Scholar]
- Michalovitz D., Eliyahu D., Oren M. Overproduction of protein p53 contributes to simian virus 40-mediated transformation. Mol Cell Biol. 1986 Oct;6(10):3531–3536. doi: 10.1128/mcb.6.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Wagner E. F. Differentiation of F9 teratocarcinoma stem cells after transfer of c-fos proto-oncogenes. Nature. 1984 Oct 4;311(5985):438–442. doi: 10.1038/311438a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Ko M., Ogura A., Liu D. G., Amano T., Takano T., Ikawa Y. Sarcoma viruses carrying ras oncogenes induce differentiation-associated properties in a neuronal cell line. Nature. 1985 Nov 7;318(6041):73–75. doi: 10.1038/318073a0. [DOI] [PubMed] [Google Scholar]
- Oren M., Bienz B., Givol D., Rechavi G., Zakut R. Analysis of recombinant DNA clones specific for the murine p53 cellular tumor antigen. EMBO J. 1983;2(10):1633–1639. doi: 10.1002/j.1460-2075.1983.tb01637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren M., Maltzman W., Levine A. J. Post-translational regulation of the 54K cellular tumor antigen in normal and transformed cells. Mol Cell Biol. 1981 Feb;1(2):101–110. doi: 10.1128/mcb.1.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J. S. Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol Rev. 1980 Jan;60(1):51–89. doi: 10.1152/physrev.1980.60.1.51. [DOI] [PubMed] [Google Scholar]
- Sanders M. M., Midgley A. R., Jr Rat granulosa cell differentiation: an in vitro model. Endocrinology. 1982 Aug;111(2):614–624. doi: 10.1210/endo-111-2-614. [DOI] [PubMed] [Google Scholar]
- Schmitt H. D., Wagner P., Pfaff E., Gallwitz D. The ras-related YPT1 gene product in yeast: a GTP-binding protein that might be involved in microtubule organization. Cell. 1986 Nov 7;47(3):401–412. doi: 10.1016/0092-8674(86)90597-0. [DOI] [PubMed] [Google Scholar]
- Schramm M., Selinger Z. Message transmission: receptor controlled adenylate cyclase system. Science. 1984 Sep 21;225(4668):1350–1356. doi: 10.1126/science.6147897. [DOI] [PubMed] [Google Scholar]
- Shih C., Weinberg R. A. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell. 1982 May;29(1):161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Trahey M., McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987 Oct 23;238(4826):542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- Trzeciak W. H., Waterman M. R., Simpson E. R. Synthesis of the cholesterol side-chain cleavage enzymes in cultured rat ovarian granulosa cells: induction by follicle-stimulating hormone and dibutyryl adenosine 3',5'-monophosphate. Endocrinology. 1986 Jul;119(1):323–330. doi: 10.1210/endo-119-1-323. [DOI] [PubMed] [Google Scholar]
- Voutilainen R., Tapanainen J., Chung B. C., Matteson K. J., Miller W. L. Hormonal regulation of P450scc (20,22-desmolase) and P450c17 (17 alpha-hydroxylase/17,20-lyase) in cultured human granulosa cells. J Clin Endocrinol Metab. 1986 Jul;63(1):202–207. doi: 10.1210/jcem-63-1-202. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The action of oncogenes in the cytoplasm and nucleus. Science. 1985 Nov 15;230(4727):770–776. doi: 10.1126/science.2997917. [DOI] [PubMed] [Google Scholar]
- van der Eb A. J., Graham F. L. Assay of transforming activity of tumor virus DNA. Methods Enzymol. 1980;65(1):826–839. doi: 10.1016/s0076-6879(80)65077-0. [DOI] [PubMed] [Google Scholar]