Abstract
The long-term health benefits of caloric restriction (CR) are well known but the associated molecular mechanisms are poorly understood despite increasing knowledge of transcriptional and related metabolic changes. We report new metabolic insights into long-term CR in nonhuman primates revealed by the holistic inspection of plasma 1H-NMR spectroscopic metabolic and lipoprotein profiles. The results revealed attenuation of aging-dependant alterations of lipoprotein and energy metabolism by CR, noted by relative increase in HDL and reduction in VLDL levels. Metabonomic analysis also revealed animals exhibiting distinct metabolic trajectories from aging that correlated with higher insulin sensitivity. The plasma profiles of insulin-sensitive animals were marked by higher levels of gluconate and acetate suggesting a CR-modulated increase in metabolic flux through the pentose phosphate pathway. The metabonomic findings, particularly those that parallel improved insulin sensitivity, are consistent with diminished adiposity in CR monkeys despite aging. The metabolic profile and the associated pathways are compatible with our previous findings that CR-induced gene transcriptional changes in tissue suggest the critical regulation of peroxisome proliferator-activated receptors as a key mechanism. The metabolic phenotyping provided in this study can be used to define a reference molecular profile of CR-associated health benefits and longevity in symbiotic superorganisms and man.
Keywords: Ageing, Amino acids, Biomarkers, Caloric restriction, Chemometrics, Insulin sensitivity, Lipoproteins, Metabonomics/metabolomics, Nuclear magnetic resonance spectroscopy
Introduction
Caloric restriction (CR) has long been known to extend maximum lifespan and oppose the development of a broad array of age-associated biological and pathological changes in a diverse range of organisms (Weindruch and Walford, 1988). Accordingly, CR is widely viewed as the most potent dietary means of slowing the aging process. Although the precise molecular mechanisms for this action remain controversial, it is axiomatic that at some level major shifts in energy metabolism are of central importance (Anderson et al., 2008).
Since 1989 we have been testing the ability of adult-onset (8-14 years of age at initiation) CR to retard the aging process in a nonhuman primate model, the rhesus monkey (Ramsey et al., 2000a; Ramsey et al., 2000b). Rhesus macaques at the Wisconsin National Primate Research Center have an average lifespan of ∼27 years and a maximum lifespan of ∼40 years. In the present study we have sought to capture a global view of the metabolic effects of long-term CR in primates using well-validated plasma NMR spectroscopy-based metabolic screening techniques (Nicholson et al., 1995).
Metabonomics provides a powerful approach to study regulatory physiological processes through the quantitative analysis of metabolites in biofluids and tissues of living organisms (Nicholson et al., 1999). This approach efficiently characterizes metabolic phenotypes of mammals via data mining of complex metabolic profiles that encapsulate the expression of both host genome and gut microbiome (Martin et al., 2007; Nicholson et al., 2004). The approach was also successfully applied to the diagnosis of pathophysiological states (Brindle et al., 2002) and the pharmacometabonomic prediction of drug metabolism and toxicity from pre-dose metabolic models (Clayton et al., 2006). Recent applications also revealed metabonomics to be particularly well-suited for assessing the effects of nutritional interventions (Rezzi et al., 2007a). As a result of this, we have recently developed the “nutrimetabonomics” concept which opens up new possibilities for characterizing imprinted metabolic signatures associated with dietary patterns and lifestyle (Rezzi et al., 2007b).
Metabonomics has recently been used to study CR-induced metabolic changes in mouse (Selman et al., 2006) and dog models (Richards et al., 2008; Wang et al., 2007). The results indicate that mice responded to acute CR by rapidly switching from lipid biosynthesis to fatty acid catabolism, β-oxidation, and gluconeogenesis, as evidenced by liver and muscle transcripts analyses (Selman et al., 2006). The CR-induced switch in energy metabolism towards energy conservation and gluconeogenesis was sustained by the observed increased plasma levels of lactate, 3-D-hydroxybutyrate, creatine and the glucogenic amino acids, methionine, glutamine, alanine, and valine, as revealed by metabonomic analysis (Selman et al., 2006). In addition, the alteration of the plasma lipoprotein profile by CR was reported as a major metabonomic outcome in both mouse and dog models (Richards et al., 2008; Selman et al., 2006). In addition, metabonomics associated long-term CR with modulations of basal energy metabolism via decreased urinary excretion of creatine, 1-methylnicotinamide, lactate, acetate and succinate as well as changes of gut microbial activity with significantly higher levels of hippurate, phenylacetylglycine, 4-hydroxyphenylacetate, and dimethylamine (Wang et al., 2007).
For the first time, we report a metabonomic investigation of phenotypic changes associated with long-term CR in nonhuman primates. NMR-based metabolic profiling coupled with multivariate statistics were applied to plasma taken from monkeys subjected to CR for 15 years. Metabolic fluctuations differentiating normally aging subjects from CR animals are identified and discussed.
Materials and methods
Experimental design
This trial was conducted at the Wisconsin National Primate Research Center (Madison, WI, USA) and was reviewed and approved by the University of Wisconsin, Graduate School Animal Care and Use Committee. This study of adult (8-14 years of age at study onset) male rhesus monkeys included 9 control-fed animals and 11 animals subjected to a 30% reduction in dietary intake (CR). Prior to study initiation, animals were monitored for baseline food intake and body weight (Table 1). Individuals were then equally randomized to either control or CR group based upon age, body weight and baseline food intake levels. CR animals' food allotments were then reduced by 10% per month over a 3-month period to achieve the goal of 30% reduction from individual baseline food intake levels (Colman et al., 1998; Ramsey et al., 2000a). As voluntary food intake levels change with aging, in recent years we have occasionally altered CR animals' food allotments in order to maintain health. At years 2, 9 and 15 of study, fasted morning blood samples were drawn from each animal using potassium oxalate and sodium fluoride as preservatives.
Table 1. Baseline animal characteristics.
| Baseline | ||
|---|---|---|
| Control | CR | |
| Age (years) | 9.0 ± 0.4 | 9.0 ± 0.5 |
| Weight (kg) | 11.3 ± 0.5 | 11.3 ± 0.4 |
| Food intake (kcal/day) | 730 ± 52 | 701 ± 43 |
Values are given as means ± SE; CR = caloric restriction.
Metabonomic analysis of plasma
Plasma samples (550 μL) were introduced into a 5 mm NMR tube with 50 μL of deuterium oxide (D2O) used as locking substance and measured on a Bruker Avance 600 MHz spectrometer equipped with an inverse probe and an automatic sample changer (Bruker Biospin, Rheinstetten, Germany) as previously reported (Rezzi et al., 2007b); see supplementary information (SI). NMR data were prepared and analyzed using unsupervised and supervised pattern recognition methods as previously reported (Rezzi et al., 2007b); see SI. Briefly, after conversion into 22 K data points over the range of δ 0.2-10.0 and removal of residual water resonance (δ 4.5-5.19), the spectra were normalized to a constant total sum of all intensities within the specified range. Multivariate pattern recognition techniques used in this study were based on principal component analysis (PCA)(Wold, 1987) and projection to latent structure (PLS) (Wold et al., 1987) using the software package SIMCA-P+ (version 11.5, Umetrics AB, Umeå, Sweden) and in-house developed MATLAB (The MathWorks Inc., Natick, MA, USA) routines. PCA was first applied to NMR variables (subjected to Pareto scaling, by dividing each variable by the square root of its standard deviation) to detect the presence of inherent similarities between metabolic profiles. Variations between the different plasma metabolic phenotypes were analyzed using scores and loadings plots. Biochemical components (NMR spectral variables) responsible for the differences between individual plasma samples detected in the scores plot can be extracted from the corresponding loadings plot. Additional detailed classification studies were performed using PLS and O-PLS-DA to exclusively focus on the effects of CR on aging (Trygg and Wold, 2002).
Clinical quantitative measurements of plasma lipids
Triglycerides (TG) were measured using a Wako enzymatic method on a XPAND™ system (Dade Behring, Switzerland). HDL and LDL were determined using the AHDL and ALDL Cholesterol assay systems (Dade Behring, Switzerland). Statistical analysis of the clinical parameters was performed using a two-tailed Mann-Whitney test.
Insulin sensitivity
Insulin sensitivity was determined by intravenous glucose tolerance testing and analyzed according to the Modified Minimal Model protocol as adapted for rhesus monkeys (Bergman, 1989; Gresl et al., 2003); see SI. Plasma insulin was measured in duplicate by double antibody radioimmunoassay (Linco Research, St. Charles, MO). Total glucose was measured in duplicate with an automated analyzer by use of the glucose oxidase method (Yellow Springs Instruments, Yellow Springs, OH).
Body composition
Dual-energy x-ray absorptiometry (DXA, Model DPX-L, GE/Lunar Corp., Madison, WI) was used to assess total body fat and lean tissue mass as previously described (Colman et al., 1998; Colman et al., 1999); see SI.
Results
Changes in food intake, weight, lean and fat masses for the CR subjects are reported in Table 2. A standard 1H-NMR spectrum of rhesus monkeys blood plasma exhibits a set of resonances arising from lipoprotein lipids and many sharper peaks from major low molecular weight molecules (Nicholson et al., 1995) as shown in Figure 1 A. Principal component analysis (PCA) and projection to latent structure discriminant analysis (PLS-DA) were performed on standard NMR spectra of plasma. Two subjects in the control group developed type 2 diabetes and were removed from statistical models to avoid any confounding effects due to this metabolic disorder.
Table 2. Characteristics of experimental and control groups.
| Group 2 years | Group 9 years | Group 15 years | ||||
|---|---|---|---|---|---|---|
| Control | CR | Control | CR | Control | CR | |
| Age (years) | 11.1 ± 0.3 | 11.0 ± 0.5 | 18.1 ± 0.4 | 18.1 ± 0.5 | 24.1 ± 0.4 | 24.1 ± 0.5 |
| Weight (kg) | 12.8 ± 0.6 | 9.9 ± 0.5* | 14.0 ± 0.6 | 9.6 ± 0.4* | 13.3 ± 0.6 | 9.6 ± 0.3* |
| Lean mass (kg) | 8.4 ± 0.3 | 7.7 ± 0.3* | 10.3 ± 0.4† | 8.8 ± 0.3 *,† | 9.0 ± 0.3 | 8.1 ± 0.2* |
| Fat mass (kg) | 4.1 ± 0.3 | 2.1 ± 0.2* | 4.0 ± 0.4 | 1.1 ± 0.2*,† | 4.3 ± 0.5 | 1.6 ± 0.2* |
| Fat mass (%) | 31.3 ± 1.6 | 19.7 ± 1.6* | 26.5 ± 2.1† | 10.2 ± 1.3*,† | 30.8 ± 2.5 | 15.9 ± 1.7*,‡ |
| Food intake (kcal / day) | 688.8 ± 38.9 | 514.2 ± 18.0* | 674.1 ± 38.1 | 487.0 ± 18.3* | 581.4 ± 39.5 | 480.0 ± 21.5* |
NB: Values are given as means ± SE; CR, caloric restriction. The values for CR monkeys were compared to controls;
designates significant difference at 95% confidence level;
designates significant differences at 95% confidence level of the corresponding variable between 2 and 9 years groups.
designates significant differences at 95% confidence level of the corresponding variable between 9 and 15 years groups.
Fig. 1.
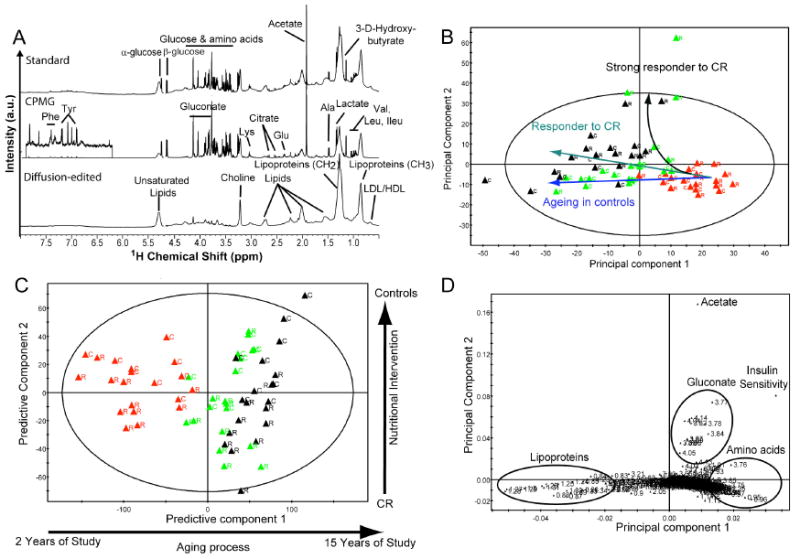
Multivariate data analysis of 1H-NMR plasma metabolic profiles. (A) Typical 600 MHz plasma 1H-NMR spectra (standard, CPMG, diffusion-edited). (B) 2D PCA scores plot obtained from 1H-NMR CPMG spectral data for subjects under CR (black) and normal (grey) aging animals at 2 years (triangle), 9 years (dot), and 15 years (square). Data were pareto scaled, PC1 and PC2 explain 37.6 and 25.1% of the total variance, respectively. (C) 2D PLS cross-validated scores plot obtained from 1H-NMR CPMG spectra highlighting discrepancies in the aging-related metabolic trajectories between CR (black) and normal (grey) aging animals. Data were pareto scaled, R2X = 0.36.5 and Q2Y = 0.39, 7 fold cross validation. (D) 2D PCA loadings plot obtained from combined 1H-NMR CPMG spectral and insulin sensitivity (IS) data for all subjects highlighting a positive correlation between (IS) and plasma metabolic profile for several CR “strong responders”. Data were pareto scaled, PC1 and PC2 explain 37.3 and 25.1% of the total variance, respectively.
The PCA and PLS-DA scores plot showed a clear clustering of plasma profiles related to aging in the first two principal components (Fig. 1 B, C). The plots also indicated a segregation of metabolic profiles after 9 and 15 years of CR when compared to aging in the controls. Interestingly, three CR animals exhibited a deviation of the metabolic trajectory from the other animals along the major age-related axis and co-mapped together along the second principal component (PC2) (Fig. 1B). A positive correlation was also observed between insulin sensitivity and several NMR derived metabolic variables including acetate and gluconate in these animals (Fig. 1D).
To improve the distinction of metabolic biomarkers associated with CR, a cross-validated orthogonal corrected PLS-DA (O-PLS-DA) was applied to characterize aging-related metabolism in CR and control animals using pairwise comparisons, e.g., years 2 vs. 9, and 9 vs. 15. The identification of statistically influential metabolites in aging and CR is achieved by the analysis of the corresponding coefficients plots (SI Fig. 1).
Interpretation of the back-scaled O-PLS-DA loadings highlighted metabolites associated with aging (SI Fig. 1). The main metabolic changes are listed in Table 3. Overall, aging-dependant decreases in concentrations of circulating amino acids (valine, isoleucine, leucine, alanine, lysine, glutamate, glycine, serine, histidine, tyrosine and tryptophan) and modulation of lipoprotein levels were observed in both groups. The careful examination of the O-PLS-DA loadings and NMR spectra, e.g., methyl (δ 0.77-1.02) and methylene (δ 1.16-1.36) signals indicated differences in the lipoprotein profile between the two dietary groups. The 1H-NMR spectroscopic plasma profile encapsulates quantitative information on the distribution of lipoprotein species, namely VLDL, LDL and HDL (Brindle et al., 2002; Otvos et al., 1991). The lipoprotein changes were further investigated with conventional clinical analyses (Fig. 2). Clinical data suggested an upward trend in plasma HDL median for CR animals between years 2 and 9, unlike control animals who underwent a slight but constant decrease of HDL with aging. Plasma HDL concentration in CR animals was higher on average than in the controls, particularly at year 15, as confirmed by O-PLS-DA obtained from diffusion-edited NMR profiles (SI Fig. 2). Notably, the controls exhibited significantly lower levels of HDL and higher concentrations of TG, whereas these variables were not altered by aging in CR animals. Total cholesterol and LDL levels were not aging-dependant in either group. Other metabolic differences between the groups involved levels of methylamines, specifically trimethylamine (TMA) and dimethylamine (DMA), which were markedly altered in CR animals (SI Fig. 1).
Table 3. Significant age dependent directional metabolic changes of plasma of normal and CR animals.
| Metabolites | NMR (ppm) | Aging Controls | Aging CR | Changes in CR | |||
|---|---|---|---|---|---|---|---|
| 9 years | 15 years | 9 years | 15 years | 9 years | 15 years | ||
| Lipoproteins | 1.27 | ↑ | - | ↑ | - | ↓ | - |
| Unsaturated lipids | 5.31 | ↑ | - | ↑ | - | - | ↓ |
| HDL | 0.82 | - | - | - | - | - | ↑ |
| Choline | 3.20 | - | - | ↑ | ↑ | - | ↑ |
| GPC | 3.23 | - | ↓ | ↓ | ↓ | - | ↑ |
| Ala | 1.47 | ↓ | ↓ | ↓ | - | - | - |
| Glu | 2.35 | ↓ | ↓ | ↓ | - | - | ↑ |
| Gly | 3.57 | ↓ | ↓ | - | ↓ | - | - |
| His | 7.01 | ↓ | ↓ | ↓ | - | - | - |
| Ileu | 1.01 | ↓ | ↓ | ↓ | ↓ | - | - |
| Leu | 0.96 | ↓ | ↓ | ↓ | ↓ | - | - |
| Lys | 1.72 | ↓ | ↓ | ↓ | ↓ | - | - |
| Pro | 3.35 | ↓ | - | ↓ | - | - | - |
| Ser | 3.83 | ↓ | ↓ | ↓ | ↓ | - | ↑ |
| Trp | 7.75 | ↓ | ↓ | ↓ | - | - | - |
| Tyr | 6.91 | ↓ | ↓ | ↓ | ↓ | - | ↑ |
| Val | 1.03 | - | ↓ | ↓ | - | - | - |
| Citrate | 2.54 | ↓ | - | ↓ | - | - | - |
| Lactate | 4.11 | - | - | ↓ | - | - | - |
| Creatinine | 4.05 | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ |
| DMA | 2.74 | - | ↓ | ↓ | ↓ | - | ↓ |
| TMA | 2.92 | ↓ | ↓ | ↓ | - | - | ↑ |
Key: Significant metabolic changes at the level of p < 0.05 are reported; ↑: increased concentration; ↓: decreased concentration; -: no significant concentration change; Aging-related changes are reported at 9 years of treatment compared to 2 years and at 15 years compared to 9 years, CR-related changes are reported in CR animals compared to controls; NMR (ppm): NMR chemical shifts calibrated against the lactate signal at δ 1.33.
Fig. 2.
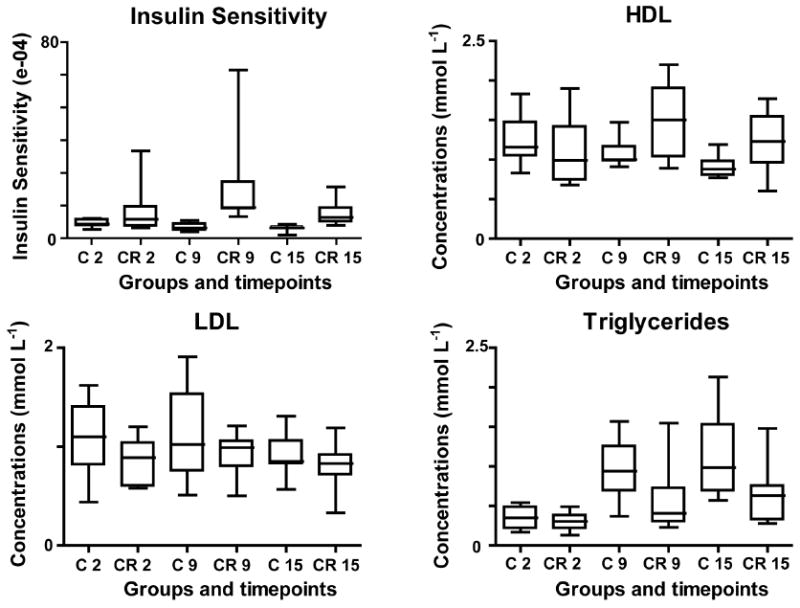
Box and whisker plots of clinical measurements on plasma lipid profile (HDL, LDL, TG) and insulin sensitivity for CR and normal aging subjects. Statistical analysis was performed using a Mann-Whitney test at a confidence level of 95%. The blood plasma levels of HDL were significantly reduced for controls between 2 and 15 years of study (n=18, P=0.011) and were significantly higher in CR at 15 years when compared to controls (n=19, P=0.018). Concentrations of TG were significantly increased with aging in controls (n=18, P=0.001 between 2 and 9 years, and P=0.0001 between 2 and 15 years). Controls also showed higher levels of TG when compared to CR animals at 9 (n=19, P=0.046) and 15 (n=19, P=0.039) year of study.
Finally, pairwise O-PLS-DA models between CR and normal aging animals were generated to characterize metabolic signatures of CR at years 9 and 15 (SI Fig. 3). The influential metabolites associated with CR are listed in Table 3. At year 9, the metabolic profiles of CR animals were marked by lower levels of lipoprotein (VLDL mainly), higher level of creatinine and an upward trend in gluconate and acetate. These CR-specific metabolic changes were maintained at year 15. In addition, higher plasma concentrations of glutamate, serine, tyrosine, choline, glycerophosphocholine (GPC), HDL, and TMA, lower concentrations of unsaturated lipids and DMA, and a downward trend in 3-D-hydroxybutyrate level were observed.
Discussion
Plasma metabotype analysis revealed characteristic age-related metabolic changes in both CR and control animals. The distinct differences in energy and lipoprotein metabolism suggest that CR preserves metabolic functions in aging animals, potentially delaying the onset of aging-associated diseases such as cardiovascular disease. The global metabonomic snapshot is highly consistent with our previous gene expression studies and further strengthen the notion that the regulation of PPARs may be central to the effects of CR (Corton and Brown-Borg, 2005; Masternak and Bartke, 2007; Weindruch et al., 2001).
An upward trend in gluconate, a key metabolite of PPP, and acetate, shown to increase flux through PPP (Flatt and Ball, 1966; Saggerson and Greenbaum, 1970) were observed under CR (Fig. 1 D). These changes were particularly significant in the three “strong responders” to CR that exhibited a distinct trajectory away from age-related metabolic shifts and were also strongly correlated with increased insulin sensitivity, a noted benefit of CR (Kemnitz et al., 1994; Roth et al., 2004). The modulation of energy metabolism by CR has already been reported in dogs with associated reductions in urinary excretion of creatine, 1-methylnicotinamide, lactate, acetate, and succinate (Wang et al., 2007). We previously showed in mice that aging is associated with a decline in the expression of PPP genes and that the changes are counteracted by CR (Lee et al., 1999; Lee et al., 2000). PPP is involved in the biosynthesis of NADPH, which is essential for various reductive biosynthetic processes (lipogenesis and cholesterol synthesis), and the synthesis of ribose-5-phosphate for nucleotide production. The importance of PPP in regulating hepatic glucose output, β-oxidation in muscle, and systemic insulin sensitivity is emerging (Wu et al., 2005). We previously reported that CR up-regulates the expression of PPARδ in skeletal muscle (Lee et al., 1999), the activation of which increases the insulin sensitivity of liver and peripheral tissue by increasing glucose flux through the PPP and enhancing fatty acid synthesis (Lee et al., 2006). The concomitant activation of related genes by PPARδ results in reduced hepatic glucose production, increased fatty acid oxidation in muscle, and improved peripheral insulin sensitivity (Tanaka et al., 2003).
Aging is associated with a decline in plasma levels of acetate (Skutches et al., 1979), a metabolite derived from both colonic fermentation of dietary fibers and the endogenous metabolism of glucose and fatty acids (Bergman, 1990). Studies suggest that acetate may positively influence insulin sensitivity (Ostman et al., 2005; Yamashita et al., 2007) and increase the flux of glucose-carbon through PPP in adipocytes (Flatt and Ball, 1966; Saggerson and Greenbaum, 1970). This is mediated via a G-protein-coupled receptor, GPR43, and is dependent on the up-regulation of PPARγ, a recognized insulin sensitizer (Hong et al., 2005). Acetate is a natural ligand for GPR41 and GPR43, which are highly expressed on adipocytes, immune cells and gastrointestinal tissue (Brown et al., 2005; Covington et al., 2006). These GPRs have been shown to play critical roles in nutrient sensing (including secretion of leptin), lipid and glucose metabolism, and regulation of inflammation. GPRs have recently become therapeutic targets for diabetes (Rayasam et al., 2007).
Aging is associated with decreased levels of free amino acids (FAAs) that can be attributed to declines in protein synthesis, lean body mass, renal tubular function and hormonal changes affecting the amino acid homeostasis (Lindeman and Goldman, 1986; Millward et al., 1997). The plasma levels of creatinine, a metabolite for which the urinary excretion was associated with lean mass variations (Davies et al., 2002), show a less marked decrease with age in CR animals as evidenced by body composition data. This aging-dependant creatinine change was also observed in the urinary 1H-NMR profiles of CR dogs compared to controls (Wang et al., 2007). The decrease in branched-chained amino acids (BCAA; leucine, isoleucine and valine) (Chan, 1999; Rudman et al., 1989), which are oxidized peripherally and serve as a fuel source to decrease protein degradation and to stimulate protein synthesis, indicate a reduced contribution of muscle to total body protein metabolism. Surprisingly, despite the attenuated loss of lean mass in CR animals (Colman et al., 2008), e.g., -5.5 % compared to - 13.8 % in control animals, very little differences in BCAAs levels were seen at year 15. However, higher levels of other FAAs, particularly serine, tyrosine and glutamate in aging CR animals (SI Fig. 1 and Table 3), suggest the maintenance of protein turnover rate despite aging (Tavernarakis and Driscoll, 2002). Furthermore, the maintenance of plasma serine, tyrosine and glutamate along with choline and glycerylphosphorylcholine (GPC), metabolites important for neurotransmitter biosynthesis and brain function, may have relevance to the preservation of neurological function often observed with CR (Ingram et al., 2007).
Alterations of the lipoprotein profile were remarkably different between the control and CR groups (SI Fig. 1 and Fig. 2). The lipoprotein profile changes with age between years 2 and 9 are dominated by significant increase in HDL in CR animals and VLDL in controls. These results were further supported by clinical quantitation of HDL and TG (Fig. 2). The trend in lipoprotein profile, the increasing TG with decreasing HDL levels, observed in the controls with age is recognized in humans as an atherogenic profile common to metabolic syndrome and/or diabetes. The lipoprotein profile affects 60% of high-risk humans and is especially associated with adverse cardiovascular outcomes (Szapary and Rader, 2004). Clinically, treatment with PPAR agonists such as fibrates often produces a profile that closely resembles that observed in the CR monkeys. Given the proposed role of PPARs in CR, the similarities in lipoprotein profile may suggest commonalities in mechanism. Clinically, PPAR agonists produce 30-50% reduction in TG and 10-20% increase in HDL, while having moderate, if any, effects on LDL or total cholesterol (Szapary and Rader, 2004). The effect is thought to precipitate as a consequence of increased lipoprotein lipolysis and hepatic fatty acid uptake, reduction of hepatic triglyceride production, and a change in lipoprotein metabolism (Staels et al., 1998). PPARδ and PPARγ agonists are also considered for this therapeutic approach (Barish et al., 2006; Robinson, 2008). In the present study, the CR animals at years 9 and 15, when compared to controls, showed lower TG and higher HDL with no noticeable differences in LDL. In rhesus monkeys, PPARδ agonist increases HDL, while lowering TG and fasting insulin (Robinson, 2008). The elevation of HDL in the CR monkeys was also seen in previous CR studies in our monkeys (Edwards et al., 2001) as well as those in another study (Verdery et al., 1997) and in humans on long-term CR (Fontana et al., 2004). Augmentation of plasma lipids and lipoproteins were also recently reported as a metabonomic outcome in a life-long CR study in dogs (Richards et al., 2008).
An association between HDL and extension of life expectancy was first observed over 40 years ago (Glueck et al., 1976), and later supported by data from the Framingham Heart Study (Schaefer et al., 1989) and a study in Ashkenazi Jews (Barzilai et al., 2003). The latter study further revealed that polymorphisms in the gene for cholesterol-ester transfer protein (CETP), which metabolizes HDL, were strongly associated with exceptional longevity. Interestingly, the increase in HDL by PPARα agonists is highly dependent on the concomitant decrease in the activity of CETP (Kersten, 2008).
Although the significance of HDL on aging and longevity in humans is still not fully understood, the relevance of increased HDL on cardiovascular health is well established. Serum HDL level has been consistently shown to be inversely related to the risk of cardiovascular disease (Toth, 2005). HDL has not only been recognized as a target for preventive measures but also as a target that may be able to successfully produce the regression of existing atherosclerosis (Dansky and Fisher, 1999). Furthermore, the combined effects of decreasing TG and increasing HDL levels in human populations without the traditional high-risk LDL levels produce about 22% relative reduction in the risk of major coronary events (Rubins et al., 1999). This illustrates the significance of the observation in the CR monkeys, especially in context of features of the metabolic syndrome.
In addition to the lipoprotein changes, we also observed lower levels of unsaturated lipids in old CR animals compared to old control animals. In a previous metabonomic study in humans, we observed that unsaturated lipids in plasma were higher in old compared to young, and in obese compared to lean males (Kochhar et al., 2006). Thus the observation in the current study may be explained in part by the lower fat mass of CR animals.
This work demonstrates the potential of data-driven metabolic approaches to generate global system information including gut microbial symbiotic interactions. The observed differences in plasma levels of mammalian gut microbial co-metabolites (i.e. acetate, choline, and methylamines) (Martin et al., 2008; Zeisel et al., 1983), highlight the importance of understanding the molecular basis of the host-microbiome interaction and its relation to nutritional stimuli. In particular, the changes of choline and methylamines (TMA, DMA) are a well documented example of metabolites derived from host-microbial interactions produced within the large intestine (Smith et al., 1994). The first reaction of the methylamine pathway involves conversion of dietary choline into TMA by gut microbiota (al-Waiz et al., 1992). Therefore, changes in plasma levels these compounds may reflect different bacterial production of methylamines (Allison and Macfarlane, 1989) in relation to age-dependent changes in gut microbial populations and activities. An implication of the gut microbiota activity in the metabolic response to CR was recently reported in a study of aging in dogs (Wang et al., 2007), with CR being associated with elevated urinary concentrations of aromatic metabolites (i.e. hippurate, phenylacetylglycine, 4-hydroxyphenylacetate and 3-hydroxyphenylpropionate) that provided additional evidence of age-dependent changes in diet processing by gut bacteria. Our findings provide a global view of aging- and CR-associated changes in energy metabolism in monkeys and consequential changes in lipoprotein metabolism that modulate immune responses (Chait et al., 2005; Murch et al., 2007) that potentially impact the onset of many aging associated diseases.
Supplementary Material
SI Fig. 1 O-PLS-DA scores and coefficients plots derived from 1H-NMR CPMG spectra of blood plasma describe metabolic changes associated with aging in control (C) and restricted (CR) animals from years 2 to 9 (B, D) and from years 9 to 15 (A, C). The cross-validated scores plot showed statistically significant separations between the plasma profiles with aging for controls and CR animals. The corresponding O-PLS-DA coefficients identified the specific metabolites contributing to the separation. The color code corresponds to the correlation coefficients of the NMR variables. 1 predictive and 1 orthogonal components were calculated, the respective (Q2Y, R2X) are: A (64.7%, 37.7%), B (70.8%, 40.7%), C (32.6%, 26.5%), D (70.0%, 35.5%). Aging-related metabolic changes were characterized by decreased levels of circulating plasma amino acids and increased concentrations of plasma lipoproteins.
SI Fig. 2 O-PLS-DA scores and coefficients plots derived from 1H-NMR diffusion-edited spectra of blood plasma describe metabolic changes, mainly lipoproteins, associated with aging in control (C) and restricted (CR) animals from year 2 to 9 (B, D) and from year 9 to 15 (A, C). The cross-validated scores plot showed statistically significant separations between the plasma profiles with aging for controls and CR animals. The corresponding O-PLS-DA coefficients identified the specific metabolites contributing to the separation. The color code corresponds to the correlation coefficients of the NMR variables. 1 predictive and 1 orthogonal components were calculated, the respective (QY2, RX2) are: A (64.0%, 65.0%), B (64.0%, 62.0%), C (59.0%, 52.0%), D (64.0%, 60.0%). Aging-related metabolic changes were characterized by decreased levels of circulating plasma amino acids and increased concentrations of plasma lipoproteins.
SI Fig. 3 O-PLS-DA scores and coefficients plots derived from 1H-NMR CPMG (left panel, A and B) and diffusion-edited (right panel, C and D) spectra of blood plasma describe the CR induced metabolic changes between control and CR animals at 9 and 15 years of study. The CPMG and diffusion-edited spectra favor the measurement of low molecular weight metabolites and large macromolecules (mainly lipoproteins), respectively. Signals that change in the negative and positive directions represent metabolites relatively increased and decreased in the CR group versus the control group. 1 predictive and 2 orthogonal components were calculated, the respective (Q2Y, R2X) are: (15.9%, 34.5%), (53.7%, 39.3%), (19.6%, 58.8%), (59.4%, 61.7%). CR subjects were characterized with relative lower concentrations of plasma VLDL associated with higher levels of HDL, creatinine, Glu, OAc and gluconate.
Acknowledgments
The authors gratefully acknowledge the technical assistance provided by S. Baum, J. A. Adriansjach, C. E. Armstrong, and the Animal Care and Veterinary Staff of the Wisconsin National Primate Research Center. This work was supported by grants P01 AG-11915 and P51 RR000167. This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01.
Abbreviations
- NMR
Nuclear magnetic resonance
- PCA
principal component analysis
- PLS
projection to latent structure
- PLS-DA
projection to latent structure discriminant analysis
- O-PLS-DA
orthogonal-projection to latent structure discriminant analysis
- FAAs
free amino acids
- BCAAs
branched-chained amino acids
- PPP
pentose-phosphate pathway
- TMA
trimethylamine
- DMA
dimethylamine
- GPC
glycerophosphocholine
References
- al-Waiz M, Mikov M, Mitchell SC, Smith RL. The exogenous origin of trimethylamine in the mouse. Metabolism. 1992;41:135–136. doi: 10.1016/0026-0495(92)90140-6. [DOI] [PubMed] [Google Scholar]
- Allison C, Macfarlane GT. Influence of pH, nutrient availability, and growth rate on amine production by Bacteroides fragilis and Clostridium perfringens. Appl Environ Microbiol. 1989;55:2894–2898. doi: 10.1128/aem.55.11.2894-2898.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Barger JL, Edwards MG, Braun KH, O'Connor CE, Prolla TA, Weindruch R. Dynamic regulation of PGC-1alpha localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress response. Aging Cell. 2008;7:101–111. doi: 10.1111/j.1474-9726.2007.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish GD, Narkar VA, Evans RM. PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest. 2006;116:590–597. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Atzmon G, Schechter C, Schaefer EJ, Cupples AL, Lipton R, Cheng S, Shuldiner AR. Unique lipoprotein phenotype and genotype associated with exceptional longevity. JAMA. 2003;290:2030–2040. doi: 10.1001/jama.290.15.2030. [DOI] [PubMed] [Google Scholar]
- Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70:567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- Bergman RN. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes. 1989;38:1512–1527. doi: 10.2337/diab.38.12.1512. [DOI] [PubMed] [Google Scholar]
- Brindle JT, Antti H, Holmes E, Tranter G, Nicholson JK, Bethell HW, Clarke S, Schofield PM, McKilligin E, Mosedale DE, Grainger DJ. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat Med. 2002;8:1439–1444. doi: 10.1038/nm1202-802. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Jupe S, Briscoe CP. A family of fatty acid binding receptors. DNA Cell Biol. 2005;24:54–61. doi: 10.1089/dna.2005.24.54. [DOI] [PubMed] [Google Scholar]
- Chait A, Han CY, Oram JF, Heinecke JW. Thematic review series: The immune system and atherogenesis. Lipoprotein-associated inflammatory proteins: markers or mediators of cardiovascular disease? J Lipid Res. 2005;46:389–403. doi: 10.1194/jlr.R400017-JLR200. [DOI] [PubMed] [Google Scholar]
- Chan YCSMYS. A comparison of anthropometry, biochemical variables and plasma amino acids among centenarians, elderly and young subjects. J Am Coll Nutr. 1999;18:358–365. doi: 10.1080/07315724.1999.10718876. [DOI] [PubMed] [Google Scholar]
- Clayton TA, Lindon JC, Cloarec O, Antti H, Charuel C, Hanton G, Provost JP, Le Net JL, Baker D, Walley RJ, Everett JR, Nicholson JK. Pharmaco-metabonomic phenotyping and personalized drug treatment. Nature. 2006;440:1073–1077. doi: 10.1038/nature04648. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Allison DB, Weindruch R. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J Gerontol A Biol Sci Med Sci. 2008;63:556–559. doi: 10.1093/gerona/63.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Ramsey JJ, Roecker EB, Havighurst T, Hudson JC, Kemnitz JW. Body fat distribution with long-term dietary restriction in adult male rhesus macaques. J Gerontol A Biol Sci Med Sci. 1999;54:B283–B290. doi: 10.1093/gerona/54.7.b283. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Roecker EB, Ramsey JJ, Kemnitz JW. Aging. Vol. 10. Milano: 1998. The effect of dietary restriction on body composition in adult male and female rhesus macaques; pp. 83–92. [DOI] [PubMed] [Google Scholar]
- Corton JC, Brown-Borg HM. Peroxisome proliferator-activated receptor gamma coactivator 1 in caloric restriction and other models of longevity. J Gerontol A Biol Sci Med Sci. 2005;60:1494–1509. doi: 10.1093/gerona/60.12.1494. [DOI] [PubMed] [Google Scholar]
- Covington DK, Briscoe CA, Brown AJ, Jayawickreme CK. The G-protein-coupled receptor 40 family (GPR40-GPR43) and its role in nutrient sensing. Biochem Soc Trans. 2006;34:770–773. doi: 10.1042/BST0340770. [DOI] [PubMed] [Google Scholar]
- Dansky HM, Fisher EA. High-density lipoprotein and plaque regression: the good cholesterol gets even better. Circulation. 1999;100:1762–1763. doi: 10.1161/01.cir.100.17.1762. [DOI] [PubMed] [Google Scholar]
- Davies KM, Heaney RP, Rafferty K. Decline in muscle mass with age in women: a longitudinal study using an indirect measure. Metabolism. 2002;51:935–939. doi: 10.1053/meta.2002.33355. [DOI] [PubMed] [Google Scholar]
- Edwards IJ, Rudel LL, Terry JG, Kemnitz JW, Weindruch R, Zaccaro DJ, Cefalu WT. Caloric restriction lowers plasma lipoprotein (a) in male but not female rhesus monkeys. Exp Gerontol. 2001;36:1413–1418. doi: 10.1016/s0531-5565(01)00107-3. [DOI] [PubMed] [Google Scholar]
- Flatt JP, Ball EG. Studies on the metabolism of adipose tissue. XIX. An evaluation of the major pathways of glucose catabolism as influenced by acetate in the presence of insulin. J Biol Chem. 1966;241:2862–2869. [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glueck CJ, Gartside P, Fallat RW, Sielski J, Steiner PM. Longevity syndromes: familial hypobeta and familial hyperalpha lipoproteinemia. J Lab Clin Med. 1976;88:941–957. [PubMed] [Google Scholar]
- Gresl TA, Colman RJ, Havighurst TC, Byerley LO, Allison DB, Schoeller DA, Kemnitz JW. Insulin sensitivity and glucose effectiveness from three minimal models: effects of energy restriction and body fat in adult male rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1340–R1354. doi: 10.1152/ajpregu.00651.2002. [DOI] [PubMed] [Google Scholar]
- Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG, Katoh K, Roh SG, Sasaki S. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146:5092–5099. doi: 10.1210/en.2005-0545. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Young J, Mattison JA. Calorie restriction in nonhuman primates: assessing effects on brain and behavioral aging. Neuroscience. 2007;145:1359–1364. doi: 10.1016/j.neuroscience.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Kemnitz JW, Roecker EB, Weindruch R, Elson DF, Baum ST, Bergman RN. Dietary restriction increases insulin sensitivity and lowers blood glucose in rhesus monkeys. Am J Physiol. 1994;266:E540–E547. doi: 10.1152/ajpendo.1994.266.4.E540. [DOI] [PubMed] [Google Scholar]
- Kersten S. Peroxisome proliferator activated receptors and lipoprotein metabolism. PPAR Res. 2008;2008 doi: 10.1155/2008/132960. 132960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochhar S, Jacobs DM, Ramadan Z, Berruex F, Fuerholz A, Fay LB. Probing gender-specific metabolism differences in humans by nuclear magnetic resonance-based metabonomics. Anal Biochem. 2006;352:274–281. doi: 10.1016/j.ab.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Lee CH, Olson P, Hevener A, Mehl I, Chong LW, Olefsky JM, Gonzalez FJ, Ham J, Kang H, Peters JM, Evans RM. PPARdelta regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2006;103:3444–3449. doi: 10.1073/pnas.0511253103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- Lindeman RD, Goldman R. Anatomic and physiologic age changes in the kidney. Exp Gerontol. 1986;21:379–406. doi: 10.1016/0531-5565(86)90044-6. [DOI] [PubMed] [Google Scholar]
- Martin FP, Dumas ME, Wang Y, Legido-Quigley C, Yap IK, Tang H, Zirah S, Murphy GM, Cloarec O, Lindon JC, Sprenger N, Fay LB, Kochhar S, van Bladeren P, Holmes E, Nicholson JK. A top-down systems biology view of microbiome-mammalian metabolic interactions in a mouse model. Mol Syst Biol. 2007;3:112. doi: 10.1038/msb4100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FP, Wang Y, Sprenger N, Yap IK, Lundstedt T, Lek P, Rezzi S, Ramadan Z, van Bladeren P, Fay LB, Kochhar S, Lindon JC, Holmes E, Nicholson JK. Probiotic modulation of symbiotic gut microbial-host metabolic interactions in a humanized microbiome mouse model. Mol Syst Biol. 2008;4:157. doi: 10.1038/msb4100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak MM, Bartke A. PPARs in Calorie Restricted and Genetically Long-Lived Mice. PPAR Res. 2007;2007 doi: 10.1155/2007/28436. 28436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward DJ, Fereday A, Gibson N, Pacy PJ. Aging, protein requirements, and protein turnover. Am J Clin Nutr. 1997;66:774–786. doi: 10.1093/ajcn/66.4.774. [DOI] [PubMed] [Google Scholar]
- Murch O, Collin M, Hinds CJ, Thiemermann C. Lipoproteins in inflammation and sepsis. I. Basic science. Intensive Care Med. 2007;33:13–24. doi: 10.1007/s00134-006-0432-y. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Foxall PJ, Spraul M, Farrant RD, Lindon JC. 750 MHz 1H and 1H-13C NMR spectroscopy of human blood plasma. Anal Chem. 1995;67:793–811. doi: 10.1021/ac00101a004. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Holmes E, Lindon JC, Wilson ID. The challenges of modeling mammalian biocomplexity. Nat Biotechnol. 2004;22:1268–1274. doi: 10.1038/nbt1015. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Lindon JC, Holmes E. Metabonomics: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- Ostman E, Granfeldt Y, Persson L, Bjorck I. Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. Eur J Clin Nutr. 2005;59:983–988. doi: 10.1038/sj.ejcn.1602197. [DOI] [PubMed] [Google Scholar]
- Otvos JD, Jeyarajah EJ, Bennett DW. Quantification of plasma lipoproteins by proton nuclear magnetic resonance spectroscopy. Clin Chem. 1991;37:377–386. [PubMed] [Google Scholar]
- Ramsey JJ, Colman RJ, Binkley NC, Christensen JD, Gresl TA, Kemnitz JW, Weindruch R. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol. 2000a;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- Ramsey JJ, Harper ME, Weindruch R. Restriction of energy intake, energy expenditure, and aging. Free Radic Biol Med. 2000b;29:946–968. doi: 10.1016/s0891-5849(00)00417-2. [DOI] [PubMed] [Google Scholar]
- Rayasam GV, Tulasi VK, Davis JA, Bansal VS. Fatty acid receptors as new therapeutic targets for diabetes. Expert Opin Ther Targets. 2007;11:661–671. doi: 10.1517/14728222.11.5.661. [DOI] [PubMed] [Google Scholar]
- Rezzi S, Ramadan Z, Fay LB, Kochhar S. Nutritional metabonomics: applications and perspectives. J Proteome Res. 2007a;6:513–525. doi: 10.1021/pr060522z. [DOI] [PubMed] [Google Scholar]
- Rezzi S, Ramadan Z, Martin FP, Fay LB, van Bladeren P, Lindon JC, Nicholson JK, Kochhar S. Human metabolic phenotypes link directly to specific dietary preferences in healthy individuals. J Proteome Res. 2007b;6:4469–4477. doi: 10.1021/pr070431h. [DOI] [PubMed] [Google Scholar]
- Richards SE, Wang Y, Lawler D, Kochhar S, Holmes E, Lindon JC, Nicholson JK. Self-modeling curve resolution recovery of temporal metabolite signal modulation in NMR spectroscopic data sets: application to a lifelong caloric restriction study in dogs. Anal Chem. 2008;80:4876–4885. doi: 10.1021/ac702584g. [DOI] [PubMed] [Google Scholar]
- Robinson JG. Should We Use PPAR Agonists to Reduce Cardiovascular Risk. PPAR Res. 2008;2008 doi: 10.1155/2008/891425. 891425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. Aging in rhesus monkeys: relevance to human health interventions. Science. 2004;305:1423–1426. doi: 10.1126/science.1102541. [DOI] [PubMed] [Google Scholar]
- Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- Rudman D, Mattson DE, Feller AG, Cotter R, Johnson RC. Fasting plasma amino acids in elderly men. Am J Clin Nutr. 1989;49:559–566. doi: 10.1093/ajcn/49.3.559. [DOI] [PubMed] [Google Scholar]
- Saggerson ED, Greenbaum AL. The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue. Effects of altered dietary and hormonal conditions. Biochem J. 1970;119:221–242. doi: 10.1042/bj1190221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer EJ, Moussa PB, Wilson PW, McGee D, Dallal G, Castelli WP. Plasma lipoproteins in healthy octogenarians: lack of reduced high density lipoprotein cholesterol levels: results from the Framingham Heart Study. Metabolism. 1989;38:293–296. doi: 10.1016/0026-0495(89)90113-3. [DOI] [PubMed] [Google Scholar]
- Selman C, Kerrison ND, Cooray A, Piper MD, Lingard SJ, Barton RH, Schuster EF, Blanc E, Gems D, Nicholson JK, Thornton JM, Partridge L, Withers DJ. Coordinated multitissue transcriptional and plasma metabonomic profiles following acute caloric restriction in mice. Physiol Genomics. 2006;27:187–200. doi: 10.1152/physiolgenomics.00084.2006. [DOI] [PubMed] [Google Scholar]
- Skutches CL, Holroyde CP, Myers RN, Paul P, Reichard GA. Plasma acetate turnover and oxidation. J Clin Invest. 1979;64:708–713. doi: 10.1172/JCI109513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Wishnok JS, Deen WM. Metabolism and excretion of methylamines in rats. Toxicol Appl Pharmacol. 1994;125:296–308. doi: 10.1006/taap.1994.1076. [DOI] [PubMed] [Google Scholar]
- Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088–2093. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- Szapary PO, Rader DJ. The triglyceride-high-density lipoprotein axis: an important target of therapy? Am Heart J. 2004;148:211–221. doi: 10.1016/j.ahj.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yamamoto J, Iwasaki S, Asaba H, Hamura H, Ikeda Y, Watanabe M, Magoori K, Ioka RX, Tachibana K, Watanabe Y, Uchiyama Y, Sumi K, Iguchi H, Ito S, Doi T, Hamakubo T, Naito M, Auwerx J, Yanagisawa M, Kodama T, Sakai J. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci U S A. 2003;100:15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernarakis N, Driscoll M. Caloric restriction and lifespan: a role for protein turnover? Mechanisms of Ageing and Development. 2002;123:215–229. doi: 10.1016/s0047-6374(01)00341-4. [DOI] [PubMed] [Google Scholar]
- Toth PP. High-density lipoprotein as a therapeutic target: clinical evidence and treatment strategies. Am J Cardiol. 2005;96:50K–58K. doi: 10.1016/j.amjcard.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Trygg J, Wold S. Orthogonal projections to latent structures. O-PLS J Chemom. 2002;16:119–128. [Google Scholar]
- Verdery RB, Ingram DK, Roth GS, Lane MA. Caloric restriction increases HDL2 levels in rhesus monkeys (Macaca mulatta) Am J Physiol. 1997;273:E714–E719. doi: 10.1152/ajpendo.1997.273.4.E714. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lawler D, Larson B, Ramadan Z, Kochhar S, Holmes E, Nicholson JK. Metabonomic Investigations of Aging and Caloric Restriction in a LifeLong Dog Study. J Proteome Res. 2007;6:1846–1854. doi: 10.1021/pr060685n. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford R. The retardation of aging and disease by dietary restriction. Thomas, CC; Springfield, Illinois: 1988. [Google Scholar]
- Weindruch R, Kayo T, Lee CK, Prolla TA. Microarray profiling of gene expression in aging and its alteration by caloric restriction in mice. J Nutr. 2001;131:918S–923S. doi: 10.1093/jn/131.3.918S. [DOI] [PubMed] [Google Scholar]
- Wold S. Principal Component Analysis. Chemom Intell Lab Syst. 1987;2:37–52. [Google Scholar]
- Wold S, Hellberg S, Lundstedt T, Sjostrom M. PLS Modeling with Latent Variables in Two or More Dimensions. PLS Meeting; Frankfurt. 1987. [Google Scholar]
- Wu C, Kang JE, Peng LJ, Li H, Khan SA, Hillard CJ, Okar DA, Lange AJ. Enhancing hepatic glycolysis reduces obesity: differential effects on lipogenesis depend on site of glycolytic modulation. Cell Metab. 2005;2:131–140. doi: 10.1016/j.cmet.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Fujisawa K, Ito E, Idei S, Kawaguchi N, Kimoto M, Hiemori M, Tsuji H. Improvement of obesity and glucose tolerance by acetate in Type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci Biotechnol Biochem. 2007;71:1236–1243. doi: 10.1271/bbb.60668. [DOI] [PubMed] [Google Scholar]
- Zeisel SH, Wishnok JS, Blusztajn JK. Formation of methylamines from ingested choline and lecithin. J Pharmacol Exp Ther. 1983;225:320–324. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SI Fig. 1 O-PLS-DA scores and coefficients plots derived from 1H-NMR CPMG spectra of blood plasma describe metabolic changes associated with aging in control (C) and restricted (CR) animals from years 2 to 9 (B, D) and from years 9 to 15 (A, C). The cross-validated scores plot showed statistically significant separations between the plasma profiles with aging for controls and CR animals. The corresponding O-PLS-DA coefficients identified the specific metabolites contributing to the separation. The color code corresponds to the correlation coefficients of the NMR variables. 1 predictive and 1 orthogonal components were calculated, the respective (Q2Y, R2X) are: A (64.7%, 37.7%), B (70.8%, 40.7%), C (32.6%, 26.5%), D (70.0%, 35.5%). Aging-related metabolic changes were characterized by decreased levels of circulating plasma amino acids and increased concentrations of plasma lipoproteins.
SI Fig. 2 O-PLS-DA scores and coefficients plots derived from 1H-NMR diffusion-edited spectra of blood plasma describe metabolic changes, mainly lipoproteins, associated with aging in control (C) and restricted (CR) animals from year 2 to 9 (B, D) and from year 9 to 15 (A, C). The cross-validated scores plot showed statistically significant separations between the plasma profiles with aging for controls and CR animals. The corresponding O-PLS-DA coefficients identified the specific metabolites contributing to the separation. The color code corresponds to the correlation coefficients of the NMR variables. 1 predictive and 1 orthogonal components were calculated, the respective (QY2, RX2) are: A (64.0%, 65.0%), B (64.0%, 62.0%), C (59.0%, 52.0%), D (64.0%, 60.0%). Aging-related metabolic changes were characterized by decreased levels of circulating plasma amino acids and increased concentrations of plasma lipoproteins.
SI Fig. 3 O-PLS-DA scores and coefficients plots derived from 1H-NMR CPMG (left panel, A and B) and diffusion-edited (right panel, C and D) spectra of blood plasma describe the CR induced metabolic changes between control and CR animals at 9 and 15 years of study. The CPMG and diffusion-edited spectra favor the measurement of low molecular weight metabolites and large macromolecules (mainly lipoproteins), respectively. Signals that change in the negative and positive directions represent metabolites relatively increased and decreased in the CR group versus the control group. 1 predictive and 2 orthogonal components were calculated, the respective (Q2Y, R2X) are: (15.9%, 34.5%), (53.7%, 39.3%), (19.6%, 58.8%), (59.4%, 61.7%). CR subjects were characterized with relative lower concentrations of plasma VLDL associated with higher levels of HDL, creatinine, Glu, OAc and gluconate.


