Abstract
Although specific pesticides have been associated with wheeze in farmers, little is known about pesticides and asthma. We used data from 19,704 male farmers in the Agricultural Health Study to evaluate lifetime use of 48 pesticides and prevalent adult-onset asthma, defined as doctor-diagnosed asthma after age 20. We categorized asthma cases as allergic (N=127) and non-allergic (N=314) based on their history of eczema or hayfever. We used polytomous logistic regression controlling for age, state, smoking, and body mass to assess pesticide associations.
High pesticide exposure events were associated with a doubling of both allergic and non-allergic asthma. For ever use, 12 individual pesticides were associated with allergic asthma and four with non-allergic asthma. For allergic asthma, coumaphos (odds ratio (OR) =2.34, 95% Confidence Interval (CI) =1.49,3.70), heptachlor (OR=2.01, 95%CI=1.30,3.11), parathion (OR=2.05, 95%CI=1.21,3.46), 80/20 mix (carbon tetrachloride/carbon disulfide) (OR=2.15, 95%CI=1.23,3.76) and ethylene dibromide (OR=2.07, 95%CI=1.02,4.20), all had odds ratios greater than 2.0 and significant exposure-response trends. For non-allergic asthma, DDT had the strongest association (OR=1.41, 95%CI=1.09,1.84) but with little evidence of increasing asthma with increasing use. Current animal handling and farm activities did not confound these results. We saw little evidence that allergy alone was driving these associations.
Pesticides may be an overlooked contributor to asthma risk among farmers.
Keywords: allergy, farming, occupational exposure, pesticides, respiratory disease
Introduction
Pesticides may contribute to asthma among farmers (1), farm women (2), and insecticide applicators (3), but data for individual pesticides are limited to a few studies. Pesticide use, particularly use of organophosphate insecticides, has been associated with wheeze among US farmers (4), US commercial pesticide applicators (5), Kenyan farmworkers (6), and with atopic asthma in farm women (2). In guinea pigs, organophosphate insecticides induce airway hyperreactivity at doses below those causing acetylcholinesterase inhibition (7, 8). These effects are stronger in allergen-sensitized animals (9).
Farmers are more likely to be diagnosed with non-atopic asthma than atopic asthma when compared to other occupational groups (10). This observation along with evidence of differential response to pesticides in allergen-sensitized animals (9) indicates the importance of evaluating risk factors asthma separately based on atopic status. In a cross-sectional analysis of enrollment data from the Agricultural Health Study (AHS), we evaluated the association of pesticide use with allergic and non-allergic asthma in farmers. We used self-reported allergy as a surrogate for atopy because we lacked clinical measurement of atopy.
Materials and Methods
The AHS is a large, prospective study of Iowa and North Carolina pesticide applicators and their spouses (11). The study enrolled over 52,000 licensed private pesticide applicators, mostly farmers, from 1993 to 1997. After completing the enrollment questionnaire, 22,916 (44%) applicators returned a second mailed questionnaire; applicators who did or did not return this second questionnaire were similar regarding demographics, farming practices, and medical history (12). Our analysis was limited to private applicators who returned both questionnaires because information on respiratory disease was obtained in the second questionnaire. Questionnaires are available at www.aghealth.org/questionnaires.html.
Participants provided information on medical history and potential confounders, including smoking, body mass, respiratory and allergic symptoms, and other respiratory diseases, as well as detailed information on their farming practices. Applicators provided detailed information on lifetime pesticide use for 50 pesticides (ever use, frequency of use, number of years used, and decade of first use). Individuals also provided information on their pesticide use practices, including application method, mixing, equipment repair, and use of personal protective equipment (PPE). We used this information to create two metrics of lifetime pesticide use: lifetime days of use and intensity-adjusted lifetime days of use. The intensity-adjusted metric accounted for potential differential exposure to pesticides based on application methods, mixing habits, repairs, and use of PPE (13). For general farming exposures, we collected information on current crops and animals raised as well as current farm tasks and maintenance activities.
In addition to pesticide application information, we also had two measures indicative of ever having elevated pesticide exposure: pesticide poisoning and high pesticide exposure events (HPEE). Pesticide poisoning was based on a self-report of doctor diagnosis of pesticide poisoning. HPEE was based on the response to the question “Have you ever had an incident or experience while using any type of pesticide which caused you unusually high personal exposure?”
All male private applicators age 20 and older with complete information on smoking, asthma history, age, body mass index (BMI), and HPEE were included in this analysis; female applicators were excluded because there were too few cases to evaluate (N=487 female applicators, 19 asthma cases). We included as cases those subjects who reported a doctor-diagnosis of asthma after age 19 and then further subdivided the cases by allergic status based on a history of doctor-diagnosed eczema or hayfever. Given our interest in adult asthma and occupational exposures, we excluded individuals diagnosed with asthma before age 20 (N=545).
We used polytomous logistic regression to evaluate associations between the farming exposures and prevalent adult-onset asthma. This allowed us to assess separate associations for allergic and non-allergic asthma simultaneously and formally test differences between odds ratios. Using goodness-of-fit tests, we developed a base model that consisted of age (4 categories: 20-39, 40-49, 50-59, 60+), state (IA, NC), smoking status (current, past, never), and BMI (<25, 25-29, 30+). We assessed each exposure individually adjusting for base-model covariates. We restricted our analysis to those exposures with at least five exposed cases. We also evaluated exposure response by creating a three-level variable for each pesticide. For each pesticide, we split the distribution of intensity-adjusted days among those who had ever used the pesticide at the median to create two exposed groups (≤median, >median); never users were the referent group. We used these ordinal categories for chi-square tests for trend.
To evaluate potential confounding by related exposures, we first assessed pairwise Spearman correlations between those exposure variables that were statistically significant individually. Second, for pairs of moderately correlated variables (r>0.3), we added both exposure variables simultaneously to the base model to assess changes in the odds ratio estimates compared to modeling each exposure separately.
We conducted two sensitivity analyses to evaluate the impact of our choice of disease definition. First, because our allergic-asthma group contained two different conditions (asthma and allergy), we evaluated whether the results for pesticides might reflect an association with allergy alone. We removed the controls with allergy from the comparison group and constructed a polytomous model with four groups: controls without allergy, allergy alone, asthma with allergy, and asthma alone. Second, because asthma can co-occur with other respiratory diseases, we repeated our analyses after excluding individuals with other respiratory diseases (i.e., chronic bronchitis, farmers lung)
Analyses were done using SAS v9.1 (SAS Institute Inc., Cary, NC) and AHS dataset release P1REL0310.
Results
Of the 19,704 male farmers included in this analysis, 441 individuals (2.2%) reported adult-onset asthma, with 127 classified as allergic and 314 classified as non-allergic. Men with asthma tended to be older and heavier and slightly more likely to have smoked compared to those without asthma (Table 1). Wheeze was reported by 17% of controls and 74% of those with asthma. Allergic symptoms were most common in those with allergic asthma. While use of any PPE for pesticide application was similar for those with and without asthma, individuals with asthma were much more likely to report the use of respiratory protection than those without asthma.
Table 1.
Demographic, Medical and Farming Characteristics by adult-onset asthma status for 19,704 male farmers from the Agricultural Health Study
| Control (N=19263) |
Allergic Asthma (N=127) |
Non-allergic Asthma (N=314) |
||||
|---|---|---|---|---|---|---|
| Characteristic | n | % | n | % | n | % |
| Age (years) | ||||||
| 20-39 | 5143 | 27 | 19 | 15 | 31 | 10 |
| 40-49 | 5003 | 26 | 28 | 22 | 68 | 22 |
| 50-59 | 4520 | 24 | 33 | 26 | 83 | 26 |
| 60+ | 4597 | 24 | 47 | 37 | 132 | 42 |
| State | ||||||
| Iowa | 13046 | 68 | 90 | 71 | 226 | 72 |
| North Carolina | 6217 | 32 | 37 | 29 | 88 | 28 |
| Body Mass Index (kg/m2) | ||||||
| <25 | 5023 | 26 | 25 | 20 | 68 | 22 |
| 25-29 | 9874 | 51 | 68 | 54 | 157 | 50 |
| 30+ | 4366 | 23 | 34 | 27 | 89 | 28 |
| Smoking History | ||||||
| Never | 10408 | 54 | 66 | 52 | 145 | 46 |
| Past | 6359 | 33 | 54 | 43 | 143 | 46 |
| Current | 2496 | 13 | 7 | 6 | 26 | 8 |
| Race | ||||||
| White | 18907 | 98 | 125 | 98 | 310 | 99 |
| Other Race | 301 | 2 | 2 | 2 | 2 | 1 |
| Education | ||||||
| High School Graduate or less | 10811 | 57 | 64 | 53 | 198 | 64 |
| Greater than High School | 8055 | 43 | 58 | 48 | 111 | 36 |
| Grew up on a farm | 17728 | 92 | 118 | 93 | 287 | 92 |
| Respiratory and Allergic Conditions | ||||||
| Farmer's Lung | 386 | 2 | 22 | 17 | 32 | 10 |
| Chronic Bronchitis | 603 | 3 | 33 | 26 | 86 | 28 |
| Allergy* | 1856 | 10 | 127 | 100 | 0 | |
| Eczema | 406 | 2 | 36 | 28 | 0 | |
| Hayfever | 1519 | 8 | 105 | 83 | 0 | |
| Wheeze | 3152 | 17 | 105 | 84 | 216 | 70 |
| Runny Nose in the Past Year | 12413 | 66 | 110 | 88 | 224 | 74 |
| Watery Itchy Eyes in the Past | 6343 | 34 | 85 | 69 | 119 | 40 |
| PPE Use, 10 years before enrollment** | ||||||
| Never used PPE | 4071 | 21 | 24 | 19 | 62 | 20 |
| Cartridge respirator | 920 | 5 | 11 | 9 | 20 | 6 |
| Dust mask | 2117 | 11 | 20 | 16 | 56 | 18 |
| Chemical gloves | 6971 | 36 | 49 | 39 | 119 | 38 |
| PPE Use at enrollment | ||||||
| Never use PPE | 1768 | 9 | 15 | 12 | 31 | 10 |
| Cartridge respirator | 1827 | 9 | 14 | 11 | 25 | 8 |
| Dust mask | 3937 | 20 | 36 | 28 | 95 | 30 |
| Chemical gloves | 13361 | 69 | 92 | 72 | 208 | 66 |
History of doctor-diagnosed ezcema or hayfever
PPE= Personal Protective Equipment; PPE use is not mutually exclusive. Individuals may report more than one method.
While overall pesticide use history (e.g., total years applied pesticides) was not related to either type of asthma, a history of HPEE was associated with an almost doubling of asthma risk for both types of asthma (ORallergic=1.98, 95% CI=1.30, 2.99; ORnon-allergic=1.96, 95% CI=1.49, 2.56) (Table 2). After adjusting for HPEE and other base model covariates, a history of doctor-diagnosed pesticide poisoning was non-significantly associated with an almost two-fold increased prevalence of allergic asthma (OR=1.95, 95%CI=0.86, 4.39. Due to the strong association of HPEE with asthma, all subsequent analyses controlled for HPEE.
Table 2.
Associations of Overall Pesticide Exposure variables with Allergic and Non-allergic Asthma among 19,704 male farmers from the Agricultural Health Study
| Exposure | Controls (N=19,263) |
Allergic asthma (N=127) | Non-allergic asthma (N=314) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | Odds Ratio* | 95% Confidence Interval | n | % | Odds Ratio* | 95% Confidence Interval | |||
| Days per year apply pesticides | ||||||||||||
| <5 | 3464 | 19 | 27 | 21 | 1.00 | 52 | 18 | 1.00 | ||||
| 5-9 | 4649 | 25 | 29 | 23 | 0.78 | 0.46 | 1.32 | 69 | 23 | 0.95 | 0.66 | 1.37 |
| 10-19 | 5683 | 31 | 41 | 32 | 0.94 | 0.57 | 1.54 | 97 | 33 | 1.18 | 0.84 | 1.67 |
| 20-39 | 3303 | 18 | 22 | 17 | 0.92 | 0.52 | 1.65 | 55 | 19 | 1.29 | 0.87 | 1.90 |
| 40+ | 1366 | 7 | 8 | 6 | 0.84 | 0.38 | 1.88 | 23 | 8 | 1.40 | 0.84 | 2.32 |
| Total Years applied pesticides | ||||||||||||
| <5 | 2206 | 12 | 11 | 9 | 1.00 | 21 | 7 | 1.00 | ||||
| 6-10 | 2640 | 14 | 7 | 6 | 0.51 | 0.20 | 1.33 | 34 | 11 | 1.29 | 0.75 | 2.24 |
| 11-20 | 6102 | 33 | 36 | 28 | 1.07 | 0.54 | 2.12 | 85 | 29 | 1.23 | 0.75 | 1.99 |
| 21-30 | 4710 | 25 | 48 | 38 | 1.53 | 0.77 | 3.06 | 83 | 28 | 1.11 | 0.68 | 1.82 |
| 31+ | 2908 | 16 | 25 | 20 | 0.99 | 0.46 | 2.12 | 74 | 25 | 1.18 | 0.71 | 1.98 |
| HPEE** | 2744 | 14 | 30 | 24 | 1.98 | 1.30 | 2.99 | 73 | 23 | 1.96 | 1.49 | 2.56 |
| HPEE w/o respiratory exp | 1745 | 9 | 16 | 13 | 1.70 | 0.99 | 2.91 | 39 | 12 | 1.72 | 1.22 | 2.44 |
| HPEE w/respiratory exposure | 955 | 5 | 14 | 11 | 2.53 | 1.44 | 4.46 | 34 | 11 | 2.41 | 1.67 | 3.49 |
| Decade of first HPEE | ||||||||||||
| 1940s-1960s | 363 | 2 | 6 | 5 | 2.11 | 0.91 | 4.89 | 16 | 5 | 2.09 | 1.24 | 3.52 |
| 1970s | 700 | 4 | 9 | 7 | 2.26 | 1.13 | 4.53 | 16 | 5 | 1.58 | 0.94 | 2.65 |
| 1980s-1990s | 1419 | 7 | 14 | 11 | 2.10 | 1.18 | 3.73 | 30 | 10 | 1.95 | 1.32 | 2.88 |
| Pesticide Poisoning | 381 | 2 | 7 | 6 | 1.95 | 0.86 | 4.39 | 11 | 4 | 1.10 | 0.58 | 2.07 |
Odds ratios adjusted for age, state, smoking, HPEE, and BMI
HPEE=High Pesticide Exposure Event
We evaluated 48 pesticides for asthma risk among farmers (ziram and trichlorfon had too few exposed cases to be assessed). While a minority of the cases were allergic asthma, more individual pesticides were associated with allergic asthma than non-allergic asthma based on ever use (12 vs. 4, Table 3). For allergic asthma, three herbicides (2,4,5-TP, EPTC, and paraquat), six insecticides (organochlorines: chlordane, heptachlor, and lindane; and organophosphates: diazinon, parathion, and coumaphos), one fungicide (captan), and two fumigants (ethylene dibromide and 80/20 mix – carbon tetrachloride and carbon disulfide) were positively associated. For non-allergic asthma, one herbicide (petroleum oil) and three insecticides (organochlorine: DDT and organophosphates; phorate and malathion) were associated. No chemical was significantly associated with both asthma subgroups, though the odds ratios were almost identical in both groups for DDT and phorate. Four pesticides had odds ratios that were significantly different (coumaphos, paraquat, captan, and lindane) between allergic and non-allergic asthma.
Table 3.
Associations of ever-use of individual pesticides with allergic and non-allergic asthma among 19,704 male farmers in the Agricultural Health Study
| Exposure | Controls (N=19,263) | Allergic asthma (N=127) | Non-allergic asthma (N=314) | p-for difference ** | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | Odds Ratio* | 95% Confidence Interval | n | % | Odds Ratio* | 95% Confidence Interval | |||||
| Herbicides | ||||||||||||||
| 2,4,5-T | 3564 | 19 | 38 | 31 | 1.44 | 0.96 | 2.14 | 88 | 29 | 1.20 | 0.93 | 1.56 | ||
| 2,4,5-TP | 925 | 5 | 13 | 11 | 1.91 | 1.06 | 3.44 | 23 | 8 | 1.24 | 0.80 | 1.91 | ||
| 2,4-D | 15054 | 79 | 110 | 87 | 1.56 | 0.91 | 2.69 | 264 | 85 | 1.19 | 0.86 | 1.64 | ||
| Alachlor | 10219 | 56 | 69 | 57 | 0.93 | 0.64 | 1.34 | 180 | 62 | 1.15 | 0.90 | 1.47 | ||
| Atrazine | 14034 | 73 | 94 | 75 | 0.95 | 0.63 | 1.45 | 241 | 78 | 1.12 | 0.85 | 1.49 | ||
| Butylate | 5058 | 27 | 42 | 33 | 1.23 | 0.84 | 1.81 | 96 | 31 | 1.10 | 0.85 | 1.42 | ||
| Chlorimuron-ethyl | 6124 | 32 | 45 | 36 | 1.21 | 0.83 | 1.75 | 99 | 32 | 1.05 | 0.82 | 1.35 | ||
| Cyanazine | 7842 | 43 | 58 | 49 | 1.14 | 0.77 | 1.70 | 149 | 51 | 1.24 | 0.96 | 1.60 | ||
| Dicamba | 9607 | 53 | 71 | 59 | 1.19 | 0.78 | 1.81 | 173 | 61 | 1.28 | 0.97 | 1.69 | ||
| EPTC | 3611 | 20 | 35 | 29 | 1.61 | 1.06 | 2.43 | 71 | 25 | 1.25 | 0.94 | 1.66 | ||
| Glyphosate | 14788 | 77 | 104 | 82 | 1.37 | 0.86 | 2.17 | 247 | 79 | 1.15 | 0.87 | 1.51 | ||
| Imazethapyr | 8042 | 45 | 52 | 44 | 0.97 | 0.64 | 1.48 | 123 | 43 | 0.88 | 0.67 | 1.14 | ||
| Metolachlor | 8624 | 47 | 58 | 48 | 0.99 | 0.69 | 1.44 | 148 | 51 | 1.12 | 0.88 | 1.43 | ||
| Metribuzin | 7179 | 38 | 55 | 44 | 1.16 | 0.79 | 1.70 | 127 | 42 | 1.01 | 0.79 | 1.29 | ||
| Paraquat | 3068 | 16 | 28 | 22 | 1.67 | 1.05 | 2.65 | 40 | 13 | 0.82 | 0.58 | 1.18 | 0.02 | |
| Pendimethalin | 7104 | 38 | 44 | 35 | 0.94 | 0.64 | 1.36 | 120 | 39 | 1.14 | 0.90 | 1.44 | ||
| Petroleum Oil | 3933 | 21 | 33 | 27 | 1.28 | 0.85 | 1.92 | 84 | 28 | 1.35 | 1.04 | 1.74 | ||
| Trifluralin | 9964 | 55 | 62 | 53 | 0.79 | 0.54 | 1.16 | 164 | 55 | 0.89 | 0.70 | 1.14 | ||
| Insecticides | ||||||||||||||
| Carbamates | ||||||||||||||
| Aldicarb | 1508 | 8 | 7 | 6 | 0.79 | 0.35 | 1.79 | 14 | 5 | 0.66 | 0.37 | 1.17 | ||
| Carbaryl | 8089 | 43 | 63 | 50 | 1.26 | 0.85 | 1.85 | 140 | 45 | 0.98 | 0.77 | 1.26 | ||
| Carbofuran | 5266 | 29 | 42 | 35 | 1.10 | 0.75 | 1.61 | 106 | 37 | 1.17 | 0.92 | 1.49 | ||
| Organochlorines | ||||||||||||||
| Aldrin | 3247 | 17 | 33 | 27 | 1.19 | 0.77 | 1.86 | 79 | 26 | 0.95 | 0.72 | 1.26 | ||
| Chlordane | 3592 | 19 | 42 | 34 | 1.77 | 1.19 | 2.63 | 86 | 28 | 1.22 | 0.94 | 1.59 | ||
| DDT | 4344 | 23 | 47 | 37 | 1.42 | 0.93 | 2.17 | 124 | 40 | 1.41 | 1.09 | 1.84 | ||
| Dieldrin | 704 | 4 | 10 | 8 | 1.47 | 0.75 | 2.90 | 13 | 4 | 0.61 | 0.35 | 1.09 | 0.05 | |
| Heptachlor | 2289 | 12 | 34 | 27 | 2.01 | 1.30 | 3.11 | 64 | 21 | 1.20 | 0.89 | 1.61 | 0.05 | |
| Lindane | 2528 | 14 | 28 | 23 | 1.57 | 1.01 | 2.41 | 44 | 14 | 0.85 | 0.61 | 1.17 | 0.03 | |
| Toxaphene | 2156 | 11 | 18 | 15 | 1.06 | 0.63 | 1.78 | 43 | 14 | 0.96 | 0.69 | 1.35 | ||
| Organophosphates | ||||||||||||||
| Chlorpyrifos | 8037 | 42 | 60 | 48 | 1.26 | 0.89 | 1.80 | 135 | 43 | 1.08 | 0.86 | 1.36 | ||
| Coumaphos | 1646 | 9 | 24 | 21 | 2.34 | 1.49 | 3.70 | 26 | 9 | 0.88 | 0.58 | 1.32 | 0.002 | |
| Diazinon | 3891 | 21 | 38 | 30 | 1.57 | 1.05 | 2.35 | 70 | 23 | 1.03 | 0.78 | 1.36 | 0.09 | |
| Dichlorvos | 2092 | 12 | 21 | 18 | 1.47 | 0.90 | 2.39 | 39 | 14 | 1.05 | 0.74 | 1.49 | ||
| Fonofos | 4079 | 23 | 37 | 31 | 1.43 | 0.95 | 2.16 | 84 | 29 | 1.22 | 0.93 | 1.60 | ||
| Malathion | 12150 | 64 | 87 | 69 | 1.08 | 0.74 | 1.59 | 229 | 74 | 1.35 | 1.04 | 1.75 | ||
| Parathion | 1501 | 8 | 19 | 16 | 2.05 | 1.21 | 3.46 | 30 | 10 | 1.11 | 0.75 | 1.66 | 0.07 | |
| Phorate | 5776 | 31 | 48 | 39 | 1.23 | 0.84 | 1.81 | 126 | 41 | 1.29 | 1.01 | 1.65 | ||
| Terbufos | 7224 | 40 | 51 | 43 | 1.05 | 0.72 | 1.53 | 132 | 45 | 1.16 | 0.91 | 1.48 | ||
| Pyrethroids | ||||||||||||||
| Permethrin (animals) | 2365 | 13 | 21 | 18 | 1.51 | 0.92 | 2.45 | 33 | 11 | 0.91 | 0.63 | 1.32 | ||
| Permethrin (crops) | 2328 | 13 | 20 | 18 | 1.52 | 0.93 | 2.48 | 42 | 15 | 1.28 | 0.91 | 1.79 | ||
| Fungicides | ||||||||||||||
| Benomyl | 1519 | 8 | 10 | 8 | 0.97 | 0.49 | 1.94 | 22 | 7 | 0.87 | 0.54 | 1.38 | ||
| Captan | 1951 | 11 | 22 | 19 | 1.83 | 1.15 | 2.94 | 29 | 10 | 0.88 | 0.60 | 1.30 | 0.02 | |
| Chlorothalonil | 1427 | 7 | 6 | 5 | 0.64 | 0.27 | 1.51 | 22 | 7 | 1.10 | 0.69 | 1.76 | ||
| Maneb/Mancozeb | 1530 | 8 | 13 | 11 | 1.40 | 0.74 | 2.67 | 20 | 7 | 0.80 | 0.49 | 1.31 | ||
| Metalaxyl | 3695 | 20 | 26 | 21 | 1.26 | 0.77 | 2.06 | 50 | 16 | 0.89 | 0.63 | 1.25 | ||
| Fumigants | ||||||||||||||
| 80/20 mix*** | 818 | 4 | 15 | 12 | 2.15 | 1.23 | 3.76 | 24 | 8 | 1.25 | 0.81 | 1.93 | ||
| Aluminum phosphide | 641 | 3 | 6 | 5 | 1.34 | 0.58 | 3.06 | 12 | 4 | 1.14 | 0.63 | 2.04 | ||
| Ethylene dibromide | 818 | 4 | 10 | 8 | 2.07 | 1.02 | 4.20 | 16 | 5 | 1.25 | 0.73 | 2.15 | ||
| Methyl bromide | 2838 | 15 | 16 | 13 | 0.86 | 0.46 | 1.60 | 41 | 13 | 0.98 | 0.66 | 1.45 | ||
Odds ratios adjusted for age, state, smoking, HPEE, and BMI
P for difference shown if p-value <0.1
80/20 mix is carbon tetrachloride/carbon disulfide.
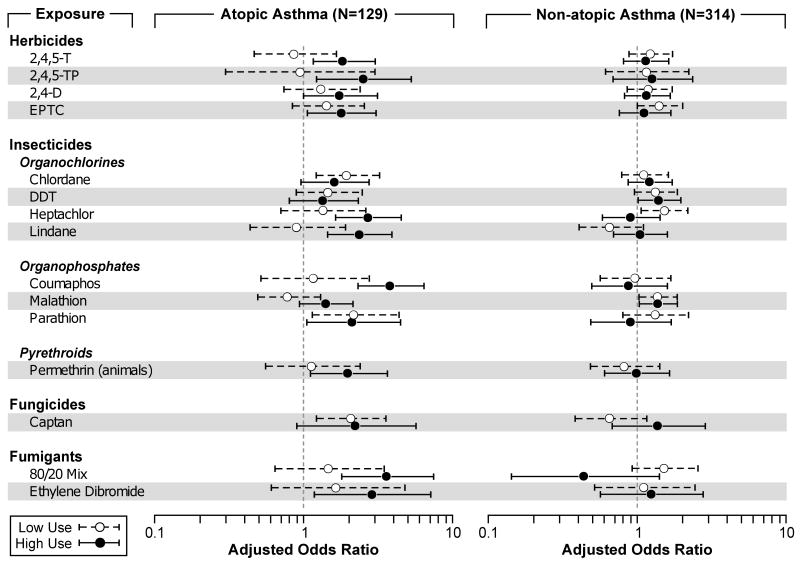
We evaluated exposure-response for pesticides and adult asthma using three cumulative pesticide exposure metrics: total years of use, lifetime days of use, and intensity-adjusted lifetime days of use. The results for all metrics were similar and because the intensity-adjusted metric accounts for potential differences in exposure as a result of application practices, we present those results in Figure 1 for those pesticides with significant trend tests for either allergic or non-allergic asthma (the online supplement Table E1 includes the exposure-response models for all 48 pesticides evaluated). Ten of the 12 pesticides associated with allergic-asthma also had significant exposure-response trends, and, with the exception of chlordane and parathion, all had higher odds ratios at the higher exposure levels. The herbicides 2,4-D and 2,4,5-T had significant exposure-response trends for allergic asthma, though the models for ever use were not statistically significant. For non-allergic asthma, DDT and malathion had significant dose-response trends.
Figure 1. Selected Pesticide Exposure-Response Models for Adult Asthma.
Pesticides selected for presentation had a significant p-value for trend for either allergic or non-allergic asthma. For allergic asthma, 2,4,5-T; 2,4,5-TP; 2,4-D; EPTC; chlordane; heptachlor; lindane; coumaphos; parathion; permethrin on animals; captan; 80/20 mix; and ethylene dibromide had significant trend tests (p<0.05). For non-allergic asthma, DDT and malathion had significant trend tests (P<0.05).
Cutpoints for pesticide use were based on the users of that specific pesticide and were then split at the median number of intensity-adjusted lifetime days for that pesticide.
We were also able to assess the association for two fungicides (captan and metalaxyl) related to use of treated seed. Captan-treated seed was associated with allergic asthma only (OR=2.49, 95% CI=1.42, 4.36). Similarly, users of metalaxyl-treated seed were over 5 times more likely to report allergic asthma (OR=5.18, 95% CI =2.48, 10.8).
We saw some evidence of correlations among specific pesticides and attenuation of some associations, but no strong evidence of confounding for any specific pesticide. The highest observed correlation among the controls was 0.37 for 2,4,5-T and 2,4,5-TP. Below, we present the results for the odds ratios that changed in the multiple chemical models. For allergic asthma, parathion attenuated the association of paraquat (OR from 1.67 to 1.36, 95% CI=0.82, 2.25). For the organochlorine pesticides, there was evidence of confounding for the associations with allergic asthma. Chlordane attenuated the associations with DDT and allergic asthma (OR from 1.42 to 1.17, 95% CI=0.75,1.83) and diazinon (OR from 1.57 to 1.39, 95% CI=0.91,2.12) while heptachlor attenuated the association of chlordane (OR from 1.77 to 1.53, 95%CI=1.02,2.31). In a model containing DDT, heptachlor, and chlordane, the association for DDT was reduced to 1.05 (95%CI=0.67, 1.67), while the ORs for heptachlor and chlordane remained elevated (1.93 and 1.52, respectively). When heptachlor and chlordane were included in a model with diazinon, the ORallergic asthma for diazinon was reduced to 1.25 (95% CI=0.82,1.93) with little change in the estimates for heptachlor and chlordane (1.93 and 1.46, respectively). We saw similar attenuation of the exposure-response models for DDT when chlordane and heptachlor were included in the model. None of the significant associations for non-allergic asthma could be explained by correlations with other pesticides.
We saw little evidence of association between prevalent asthma and current farming activities (data not shown). Allergic asthma was associated with using gasoline as a solvent to clean (OR= 1.48, 95%CI=1.04, 2.13) and with performing veterinary services (OR=1.51, 95%CI=1.03, 2.21). Veterinary services (OR=0.77, 95%CI =0.60,0.97) and driving combines (OR=0.72, 95%CI=0.53,0.97) were inversely associated with non-allergic asthma. There were no positive associations between non-allergic asthma and current farming activities. Current farm activities did not appear to confound the pesticide results.
To determine whether the differential results for allergic and non-allergic asthma were due to allergy alone, we removed allergic individuals from our control group and reran the analysis (Online supplement Table E2). While some pesticides were associated with allergy alone, the odds ratios were stronger for allergic asthma than for allergy alone for all pesticides associated with allergic asthma in earlier models.
To evaluate whether our results were related to asthma, or to some co-morbid respiratory disease, we excluded all individuals with chronic bronchitis and farmers lung and reran our models (online supplement Table E3). A larger proportion of asthma cases, both allergic and non-allergic, reported some other respiratory illness compared to controls (35% vs. 5%). With these individuals excluded, the odds ratios for allergic asthma were statistically significant for five pesticides (2,4,5-T, parathion, coumaphos, captan, and 80/20 mix) and similar to the values reported in Table 3. For four other pesticides, the resulting odds ratios were attenuated: heptachlor (OR from 2.01 to 1.30), chlordane (OR from 1.77 to 1.27), paraquat (OR=1.67 to 1.36), and 2,4,5-TP (OR from 1.91 to 1.68). Of the four pesticides significantly associated with non-allergic asthma, only phorate remained statistically significant when the individuals with other lung diseases were removed. After removing the other respiratory diseases, the odds ratio for fonofos and non-allergic asthma increased from 1.22 to 1.39 (95% CI=1.00, 1.94) and the association between petroleum oil and non-allergic asthma went away (OR from 1.35 to 1.15).
Discussion
Grains, hays, and animals have been identified as important etiologic agents for respiratory disease among farmers for centuries (14); however, few studies have evaluated the association between specific pesticides and respiratory disease. Here we offer additional evidence that pesticides may also be a risk factor for asthma among farmers. Building on previous work in farmers (1), farmworkers (6, 15, 16), pesticide applicators (3), and farm women (2), we observed that specific pesticides were associated with allergic asthma and that self-reported high pesticide exposure events were associated with both allergic and non-allergic asthma. We also saw associations with lifetime days of use of individual pesticides suggesting an exposure-response relationship.
High pesticide exposure events are infrequent events on farms, however 14% of farmers report having at least one HPEE in their lifetime. These events often do not result in seeking care for pesticide poisoning (17). HPEE was an important risk factor for both allergic and non-allergic asthma. While we saw no evidence of an interaction between HPEE and individual pesticides, a history of HPEE was an important confounder of the pesticide findings. Before adjusting for HPEE, 14 pesticides were associated with allergic asthma and 10 with non-allergic asthma. HPEE has been associated with a twofold increased prevalence of the respiratory diseases chronic bronchitis (18) and farmers lung (19) as well as with a number of neurological outcomes including depression (20) and neurological symptoms (21).
Our findings are consistent with results from other respiratory analyses from the AHS and other studies. The herbicides, EPTC and paraquat, were associated with wheeze among farmers (4). Paraquat was also associated with allergic asthma among farm women (2) as well as respiratory symptoms and oxygen desaturation in studies of farmworkers in Costa Rica and South Africa (22, 23). Paraquat has also been associated with allergic symptoms in grape farmers in Crete (24). The organochlorine insecticides DDT and lindane were associated with allergic asthma in farm women; DDT was also associated with non-allergic asthma among farm women. The organophosphate insecticides coumaphos and parathion were associated with allergic asthma in farm women; coumaphos was associated with wheeze in the commercial pesticide applicators (5), while parathion was associated with wheeze among farmers (4). Other investigators have also found organophosphate insecticides to be associated with asthma or wheeze. Saskatchewan farmers who used organophosphate insecticides had increased asthma, though the association was stronger with users of carbamate insecticides (1); our findings suggest stronger evidence for organophosphates. Farmworkers with depressed acetyl cholinesterase (a measure of organophosphate exposure) have increased respiratory symptoms (6, 16).
For the pesticides associated with non-allergic asthma, all were previously associated with wheeze or asthma in the AHS. Petroleum oil was associated with non-allergic asthma in farm women (2) and with wheeze among farmers and commercial pesticide applicators (4, 5). Both phorate and malathion were associated with allergic, but not non-allergic, asthma in farm women (2) and were associated with wheeze. One pesticide that was not associated with asthma in this analysis was carbofuran; this pesticide was strongly associated with asthma among Saskatchewan farmers (1) and among AHS farm women (2).
Allergic asthma is far less common in adults than children (25) and far less common in farmers than in other occupational groups (10). Yet in this study the associations with pesticides were much stronger for allergic than non-allergic asthma; our analysis of farm women (2) showed a similar relationship. Pesticides may modulate inflammatory responses to farm bioaerosols such as endotoxin and allergens. Allergen-sensitized guinea pigs had a lower threshold for parathion-induced airway responsiveness (9). Carbaryl enhanced the allergenicity of dust mites in animals (26). Captan and metalaxyl, both commonly used seed treatments, were strongly associated with allergic asthma when pre-applied to seed, suggesting a possible interaction of allergen and pesticide. Given the variety of pesticides associated with allergic asthma, we cannot rule out the possibility that some common ingredient in the pesticidal products may explain these results.
We relied on self-reported exposure and outcome data. Farmers provide reliable information regarding their personal pesticide use (27, 28). By using an intensity-adjusted exposure metric, we were able to account for potential differences in exposure resulting from different application practices. The intensity metric is able to distinguish between high and low exposure intensity in field studies (29), thus we are better able to classify individuals with respect to total exposure. Even though individuals with allergic asthma appeared to have reduced their exposure through fewer pesticide application days annually and increased use of respiratory protection, we were still able to observe significant exposure-response trends using this intensity metric. We have no reason to believe that pesticide use reporting would differ by asthma or allergy status. Self-reported doctor-diagnosed asthma has been shown to be a reliable and valid endpoint (30, 31). Among farmers, self-reported asthmatic subjects have a higher symptom prevalence and lower lung function than non-asthmatics (1). In our sample, individuals with asthma were more likely to report wheeze than those without asthma and individuals classified as allergic had more allergic symptoms than those classified as non-allergic. Allergy was defined as a history of doctor-diagnosed eczema or hayfever. While we have no clinical measures to assess allergic status such as skin prick testing or IgE measurement, our measure is similar to ones used previously. Our two case groups are based on responses to three questions (asthma, eczema, and hayfever), similar the classification that Upton and colleagues used to define atopic and non-atopic asthma among adults in Scotland (25). We classified 71% of adult asthma among farmers as non-allergic, an estimate very similar to the 70% reported among Norwegian adults where atopy was defined by IgE (10). By limiting our definition of allergy to those with doctor-diagnosis of hayfever or eczema it is likely that these individuals received allergy testing at the time of diagnosis, however we have no data to evaluate that.
Farms represent a complex occupational setting with opportunities for a number of concurrent exposures, including multiple pesticides. When we took into account correlated pesticides, the associations for DDT were attenuated, but the associations for other organochlorines remained elevated. While we saw no strong associations with current farm activities, we lack data on lifetime farming exposures to animals, hays, and grains, which may contribute to asthma. Hence, the findings for some specific pesticides may be confounded by previous exposures. For example, coumaphos is an insecticide used on animals, and thus, it may be a surrogate for historic animal exposures. However, a majority of the pesticides associated with asthma have not been used in animal production. Given the lack of commonality to the pesticides, both in their use patterns and toxicity, it seems unlikely that all the findings are due to uncontrolled confounding.
In this analysis of 19,704 male farmers with good information on lifetime pesticide use, specific pesticides were associated with allergic asthma. One of the pesticides, parathion, has also been associated with allergic asthma in farm women, wheeze in farmers, and with airway hyperresponsiveness in allergen-sensitized animals. Additionally individuals with a history of a high pesticide exposure event were twice as likely to report having adult-onset asthma. Because the analysis was cross-sectional we cannot evaluate the temporal order of exposure and disease, however, given the consistency of the results for epidemiologic and animal studies, more detailed studies are warranted to identify patterns of pesticide use which may contribute to asthma among farmers.
Supplementary Material
Table E1 Complete exposure-response models for all 48 pesticides evaluated.
Table E2 Sensitivity analysis for allergy alone
Table E3 Odds ratios for individual pesticide and allergic and non-allergic asthma after exclusion of other respiratory disease cases
Acknowledgments
The authors thank Stuart Long for data analysis. This work could not have happened without the hard work of the Iowa and North Carolina Field Stations (Ellen Heywood, Chuck Lynch, Margaret Hayslip, Charles Knott) and the AHS coordinating center (Ben Laimon, Marsha Dunn, Kate Torres, Stanley Legum). We thank the AHS cohort members for their contribution to this work. This work was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01-ES049030) and National Cancer Institute (Z01-CP010119).
Disclaimers: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health (NIOSH). Mention of any company or product does not constitute endorsement by NIOSH.
Abbreviations
- AHS
Agricultural Health Study
- BMI
body mass index
- CI
confidence interval
- HPEE
High Pesticide Exposure Event
- OR
odds ratio
- PPE
Personal Protective Equipment
References
- 1.Senthilselvan A, McDuffie HH, Dosman JA. Association of Asthma With Use of Pesticides - Results of a Cross-Sectional Survey of Farmers. Am Rev Respir Dis. 1992;146(4):884–7. doi: 10.1164/ajrccm/146.4.884. [DOI] [PubMed] [Google Scholar]
- 2.Hoppin JA, Umbach DM, London SJ, Henneberger PK, Kullman GJ, Alavanja MC, et al. Pesticides and atopic and nonatopic asthma among farm women in the Agricultural Health Study. Am J Respir Crit Care Med. 2008 Jan 1;177(1):11–8. doi: 10.1164/rccm.200706-821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beard J, Sladden T, Morgan G, Berry G, Brooks L, McMichael A. Health impacts of pesticide exposure in a cohort of outdoor workers. Environ Health Perspect. 2003 May;111(5):724–30. doi: 10.1289/ehp.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoppin JA, Umbach DM, London SJ, Alavanja MCR, Sandler DP. Chemical predictors of wheeze among farmer pesticide applicators in the agricultural health study. Am J Respir Crit Care Med. 2002 Mar 1;165(5):683–9. doi: 10.1164/ajrccm.165.5.2106074. [DOI] [PubMed] [Google Scholar]
- 5.Hoppin JA, Umbach DM, London SJ, Lynch CF, Alavanja MC, Sandler DP. Pesticides associated with wheeze among commercial pesticide applicators in the Agricultural Health Study. Am J Epidemiol. 2006 Jun 15;163(12):1129–37. doi: 10.1093/aje/kwj138. [DOI] [PubMed] [Google Scholar]
- 6.Ohayo-Mitoko GJA, Kromhout H, Simwa JM, Boleij JSM, Heederik D. Self reported symptoms and inhibition of acetylcholinesterase activity among Kenyan agricultural workers. Occup Environ Med. 2000;57(3):195–200. doi: 10.1136/oem.57.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fryer AD, Lein PJ, Howard AS, Yost BL, Beckles RA, Jett DA. Mechanisms of organophosphate insecticide-induced airway hyperreactivity. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2004 May 1;286(5):L963–L9. doi: 10.1152/ajplung.00343.2003. [DOI] [PubMed] [Google Scholar]
- 8.Lein PJ, Fryer AD. Organophosphorus insecticides induce airway hyperreactivity by decreasing neuronal M2 muscarinic receptor function independent of acetylcholinesterase inhibition. Toxicol Sci. 2005 Jan;83(1):166–76. doi: 10.1093/toxsci/kfi001. [DOI] [PubMed] [Google Scholar]
- 9.Proskocil BJ, Bruun DA, Lorton JK, Blensly KC, Jacoby DB, Lein PJ, et al. Antigen sensitization influences organophosphorus pesticide-induced airway hyperreactivity. Environ Health Perspect. 2008 Mar;116(3):381–8. doi: 10.1289/ehp.10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eduard W, Omenaas E, Bakke PS, Douwes J, Heederik D. Atopic and non-atopic asthma in a farming and a general population. Am J Ind Med. 2004 Oct;46(4):396–9. doi: 10.1002/ajim.20088. [DOI] [PubMed] [Google Scholar]
- 11.Alavanja MC, Sandler DP, McMaster SB, Zahm SH, McDonnell CJ, Lynch CF, et al. The Agricultural Health Study. Environ Health Perspect. 1996 Apr;104(4):362–9. doi: 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarone RE, Alavanja MC, Zahm SH, Lubin JH, Sandler DP, McMaster SB, et al. The Agricultural Health Study: factors affecting completion and return of self-administered questionnaires in a large prospective cohort study of pesticide applicators. Am J Ind Med. 1997;31(2):233–42. doi: 10.1002/(sici)1097-0274(199702)31:2<233::aid-ajim13>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Dosemeci M, Alavanja MC, Rowland AS, Mage D, Zahm SH, Rothman N, et al. A quantitative approach for estimating exposure to pesticides in the Agricultural Health Study. Ann Occup Hyg. 2002 Mar;46(2):245–60. doi: 10.1093/annhyg/mef011. [DOI] [PubMed] [Google Scholar]
- 14.Schenker MB, Christiani D, Cormier Y, Dimich-Ward H, Doekes G, Dosman J, et al. Respiratory health hazards in agriculture. Am J Respir Crit Care Med. 1998;158(5):S1–S76. doi: 10.1164/ajrccm.158.supplement_1.rccm1585s1. [DOI] [PubMed] [Google Scholar]
- 15.Castro-Gutierrez N, McConnell R, Andersson K, Pacheco-Anton F, Hogstedt C. Respiratory symptoms, spirometry and chronic occupational paraquat exposure. Scand J Work Environ Health. 1997 Dec;23(6):421–7. doi: 10.5271/sjweh.264. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez AF, Casado I, Pena G, Gil F, Villanueva E, Pla A. Low level of exposure to pesticides leads to lung dysfunction in occupationally exposed subjects. Inhal Toxicol. 2008 Jul;20(9):839–49. doi: 10.1080/08958370801905524. [DOI] [PubMed] [Google Scholar]
- 17.Bell EM, Sandler DP, Alavanja MC. High pesticide exposure events among farmers and spouses enrolled in the Agricultural Health Study. J Agric Saf Health. 2006 May;12(2):101–16. doi: 10.13031/2013.20385. [DOI] [PubMed] [Google Scholar]
- 18.Hoppin JA, Valcin M, Henneberger PK, Kullman GJ, Umbach DM, London SJ, et al. Pesticide use and chronic bronchitis among farmers in the Agricultural Health Study. Am J Ind Med. 2007 Dec;50(12):969–79. doi: 10.1002/ajim.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoppin JA, Umbach DM, Kullman GJ, Henneberger PK, London SJ, Alavanja MC, et al. Pesticides and other agricultural factors associated with self-reported farmer's lung among farm residents in the Agricultural Health Study. Occup Environ Med. 2007 May;64(5):334–41. doi: 10.1136/oem.2006.028480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beseler C, Stallones L, JA H, Alavanja M, Blair A, Keefe T, et al. Depression And Pesticide Exposures Among Private Pesticide Applicators Enrolled In The Agricultural Health Study. Environ Health Perspect. 2008 doi: 10.1289/ehp.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamel F, Engel LS, Gladen BC, Hoppin JA, Alavanja MC, Sandler DP. Neurologic symptoms in licensed pesticide applicators in the Agricultural Health Study. Hum Exp Toxicol. 2007 Mar;26(3):243–50. doi: 10.1177/0960327107070582. [DOI] [PubMed] [Google Scholar]
- 22.Dalvie MA, White N, Raine R, Myers JE, London L, Thompson M, et al. Long-term respiratory health effects of the herbicide, paraquat, among workers in the Western Cape. Occup Environ Med. 1999;56(6):391–6. doi: 10.1136/oem.56.6.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schenker MB, Stoecklin M, Lee K, Lupercio R, Zeballos RJ, Enright P, et al. Pulmonary function and exercise-associated changes with chronic low-level paraquat exposure. Am J Respir Crit Care Med. 2004 Oct 1;170(7):773–9. doi: 10.1164/rccm.200403-266OC. [DOI] [PubMed] [Google Scholar]
- 24.Chatzi L, Alegakis A, Tzanakis N, Siafakas N, Kogevinas M, Lionis C. Association of allergic rhinitis with pesticide use among grape farmers in Crete, Greece. Occup Environ Med. 2007 Jun;64(6):417–21. doi: 10.1136/oem.2006.029835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Upton MN, McConnachie A, McSharry C, Hart CL, Smith GD, Gillis CR, et al. Intergenerational 20 year trends in the prevalence of asthma and hay fever in adults: the Midspan family study surveys of parents and offspring. Bmj. 2000 Jul 8;321(7253):88–92. doi: 10.1136/bmj.321.7253.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong W, Gilmour MI, Lambert AL, Selgrade MK. Enhanced allergic responses to house dust mite by oral exposure to carbaryl in rats. Toxicol Sci. 1998 Jul;44(1):63–9. doi: 10.1006/toxs.1998.2475. [DOI] [PubMed] [Google Scholar]
- 27.Blair A, Zahm SH. Patterns of pesticide use among farmers: implications for epidemiologic research. Epidemiology. 1993;4(1):55–62. doi: 10.1097/00001648-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Hoppin JA, Yucel F, Dosemeci M, Sandler DP. Accuracy of self-reported pesticide use duration information from licensed pesticide applicators in the Agricultural Health Study. J Expo Anal Environ Epidemiol. 2002 Sep;12(5):313–8. doi: 10.1038/sj.jea.7500232. [DOI] [PubMed] [Google Scholar]
- 29.Coble J, Arbuckle T, Lee W, Alavanja M, Dosemeci M. The validation of a pesticide exposure algorithm using biological monitoring results. J Occup Environ Hyg. 2005 Mar;2(3):194–201. doi: 10.1080/15459620590923343. [DOI] [PubMed] [Google Scholar]
- 30.Burney PG, Laitinen LA, Perdrizet S, Huckauf H, Tattersfield AE, Chinn S, et al. Validity and repeatability of the IUATLD (1984) Bronchial Symptoms Questionnaire: an international comparison. European Respiratory Journal. 1989;2(10):940–5. [PubMed] [Google Scholar]
- 31.Jenkins MA, Clarke JR, Carlin JB, Robertson CF, Hopper JL, Dalton MF, et al. Validation of questionnaire and bronchial hyperresponsiveness against respiratory physician assessment in the diagnosis of asthma. International Journal of Epidemiology. 1996;25(3):609–16. doi: 10.1093/ije/25.3.609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table E1 Complete exposure-response models for all 48 pesticides evaluated.
Table E2 Sensitivity analysis for allergy alone
Table E3 Odds ratios for individual pesticide and allergic and non-allergic asthma after exclusion of other respiratory disease cases