Abstract
Objective
Less than half of youths achieve remission (minimal to no symptoms) after acute antidepressant treatment. Early identification of who will or will not respond to treatment and achieve remission may help clinicians formulate treatment decisions and shorten the time spent on ineffective treatments. In a prospective open-label fluoxetine study, we investigate indicators of acute treatment response and remission.
Method
One hundred sixty-eight children and adolescents, ages 7 to 18 years, with primary diagnoses of major depressive disorder received 12 weeks of fluoxetine treatment. The youths were evaluated using the Kiddie Schedule for Affective Disorders and Schizophrenia. The outcome measure included the Children’s Depression Rating Scale-Revised.
Results
Positive first-degree family history of depression was the only baseline demographic and clinical characteristic that predicted a favorable treatment response (p = .01). The rate of symptom improvement, however, is a good indicator of acute treatment response. A significant symptom reduction (approximately 50%) by week 4 is needed to achieve remission at the end of acute treatment.
Conclusions
This study demonstrated that the rate of symptom improvement during early weeks of acute fluoxetine treatment is a good indicator of remission. Treatment approach may be reevaluated and modified as early as week 4 during acute treatment.
Keywords: acute treatment, pediatric depression, fluoxetine
For the past 10 years, research for the treatment of pediatric major depressive disorder (MDD), particularly the acute phase of treatment, has increased remarkably. Although most clinical trials use treatment response as the primary outcome, remission (i.e., minimal or no depressive symptoms) is the goal of acute treatment.1 Previous double-blind placebo-controlled acute pediatric antidepressant trials reveal response rates of 40% to 70%, with remission rates of 30% to 40%.2,3 Clearly, most depressed youths do not reach remission in 8 to 12 weeks of acute treatment in randomized controlled trials.
Clinical guidelines and algorithms suggest changing medication treatment if remission is not achieved by 3 months of treatment using an adequate dose.4,5 Most recommendations also allow for earlier changes when patients are showing minimal to no improvement (i.e., after 4–8 weeks).4,6 However, these recommendations are based on clinical experience. No studies to date have carefully examined how early we can identify who will achieve remission at the end of acute treatment.
What are the early indicators of treatment response or remission? Baseline demographic and clinical features may be potential predictors of treatment response. Some studies have found that greater baseline depression severity or more depressive episodes may predict poorer treatment response.7–9 However, findings in neither pediatric7,9 nor adult10,11 depression studies have been consistent. The few studies to date have yet to yield clinically useful predictors of response or remission.
Recently, adult MDD studies have found that post-baseline patterns of symptom change, especially during the early weeks of acute treatment, may predict ultimate response at the end of acute treatment.12 Investigating the speed and extent of response during the early weeks of acute treatment could be fruitful in identifying early indicators of remission, which in turn would help clinicians formulate treatment decisions. Earlier identification of patients who will not respond or remit with treatment could shorten the time spent on ultimately ineffective treatments, improve outcome, and potentially increase adherence.13
Response has been previously defined as a significant symptom reduction (i.e., 30%–50%) from baseline depression score, which has been measured by a rating scale such as the Children’s Depression Rating Scale-Revised (CDRS-R)14 and/or a score on the Clinical Global Impression-Improvement Scale (CGI-I)15 of 1 (very much improved) or 2 (much improved). A CDRS-R score of 28 or lower, however, is often used as a criterion for remission in pediatric trials.2 Both pediatric and adult depression trials have used an arbitrary percent reduction score as an indicator of positive response to a treatment. There are, however, few empirical data to support the use of a particular percent reduction score as a criterion for positive treatment effect. It is even less clear whether early positive “response” to treatment will ultimately lead to remission. One strategy is to identify the degree of symptom reduction that predicts ultimate response or remission by identifying cutoff scores that yield the best combined sensitivity and specificity. This cutoff score (i.e., percent reduction) would help clinicians inform their patients about the likelihood of remission and help patients, in turn, evaluate their options of either continuing their current treatment or making changes. To date, however, no study has examined this possibility in pediatric depression.
This article addresses several important questions using a well-defined and characterized pediatric sample with MDD. The aims of this article are to investigate whether there are baseline demographic or clinical predictors of remission status at the end of acute treatment, how early remitters and nonremitters can be distinguished based on the pattern of response during acute treatment, and the amount of symptom improvement during the early weeks of acute treatment that provides the best combined sensitivity and specificity to identify eventual remission for depressed children and adolescents.
METHOD
The data presented here are from the acute phase treatment of a single-site continuation study, Childhood Depression: Remission and Relapse (R01 MH39188; principal investigator: G.E.), the primary results of which have been published previously.16 The study was approved by the University of Texas Southwestern Medical Center at Dallas institutional review board. All participants and their parents provided written informed consent and assent before entering the study.
Participants
A total of 331 children and adolescents were consented for the study and received a diagnostic evaluation. Of those, 169 were eligible for acute phase treatment. Of the 162 screen drops, 88 did not meet MDD criteria, 52 withdrew consent, 17 met one or more exclusion criteria, and 5 improved during the evaluation period. Fluoxetine was given to 169 participants, of whom one did not have a postbaseline visit. Data from the sample (n = 168) who entered the acute phase of treatment and had at least one postbaseline visit were used in the baseline analyses.
Participants in the present study were children 7 to 18 years of age with MDD. All participants had at least 4 weeks of history of nonpsychotic MDD based on the DSM-IV17 criteria with a Clinical Global Impression-Severity Scale (CGI-S)15 score of 4 or higher for depression and CDRS-R total score of 40 or higher. Whereas other concurrent disorders were allowed, MDD was the primary disorder. Participants were excluded for lifetime history of psychotic depression or bipolar I and II disorder. In addition, participants were excluded for alcohol or substance abuse or dependence within the past 6 months. Treatment with other psychotropic medications was not allowed, with the exception of stimulant treatment for ADHD, which could be initiated before or during acute treatment. Twenty-three participants (13.7%) were taking a stimulant before study entry. An additional 13.7% began stimulants during acute treatment. For those participants requiring initiation stimulants during the study, the stimulant was started sequentially after the antidepressant.
Procedures and Measures
Study procedures have been detailed previously.16 Generally, after obtaining informed consent and assent, the children were evaluated for a 2-week period (three visits) using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL).18 At the initial visit, the K-SADS-PL and CDRS-R were administered by a trained research evaluator. Depressive disorder and other potential comorbid diagnoses, as well as depression severity, were confirmed 1 week later by a psychologist or psychiatrist using the K-SADS-PL and CDRS-R. Eligible participants were scheduled for a third evaluation visit (baseline visit) to confirm the diagnosis using the Affective portion of the K-SADS-PL and to measure continued depression severity (CDRS-R). At this visit, the children who continued to meet criteria for MDD and had a CDRS-R score of 40 or higher were started on fluoxetine treatment. At all evaluations, the children and parents were interviewed separately, and ratings were based on a synthesis of all available information.
Beginning at week 0, all of the children received 10 mg/day fluoxetine, with dose increased to 20 mg at week 1 if the child was able to tolerate the medication. The dose could be increased to 30 or 40 mg after week 6 if the patient was not adequately improving. Although supportive clinical management was provided during each visit, no disorder-specific psychotherapy (e.g., cognitive behavioral therapy, interpersonal therapy) was allowed. The children were followed up weekly for the first 4 weeks of treatment, then biweekly for the last 8 weeks of treatment by a child and adolescent psychiatrist. Medication compliance was monitored by pill count and medication diary at each visit. Participants with a pill count compliance rate below 80% at two consecutive visits were withdrawn from the study. The average compliance rate by pill count across all visits was more than 80% for all of the participants.
Depression Severity and Remission Status
Participants’ depression severity was measured by the physician-rated CDRS-R and CGI-S at each visit. The CDRS-R is a 17-item clinician-rated pediatric depression scale that is widely used in pediatric depression trials.2 The 17 items are rated on a Likert-type scale of 1 to 7, with higher scores indicating greater depression severity (range 17–113). Remission was operationally defined a priori as a CDRS-R total score of 28 or lower either at week 12 or at acute exit for patients who discontinued earlier. The patients with a CDRS-R total score greater than 28 were considered nonremitters. Thus, for the present study, remission status was a binomial outcome variable operationally defined as “remission” or “nonremission” of depression severity.
Improvement in Depression Severity and Cut Points
Improvement in depression severity was evaluated by the percent reduction of the CDRS-R total score from the baseline to each of the postbaseline assessment periods up to week 12 (e.g., weeks 1, 2, 3, 4, 6, 8, 10, 12). This percent reduction of CDRS-R total score was calculated by correcting for the nonzero base score (i.e., subtracting the 17-point minimum from the denominator). The following formula was used:
Each improvement in depression severity cut point was a binomial variable operationally defined as the proportion of patients who had higher than the cut point percent symptom improvement and the proportion of patients who had equal or lower than the cut point percent symptom improvement.
Participant Characteristics
Demographic information and baseline clinical features were collected, including age, sex, ethnicity, MDD episodes, number of concurrent psychiatric comorbidities, length of illness, current episode length, baseline depression severity, global functioning of the child and family, suicidal behaviors, and family history of depression among first-degree relatives (mother, father, and siblings). Family history was gathered during the diagnostic interview using the Family History Research Diagnostic Criteria.19 A positive family history of depression indicates that at least one of the first-degree relatives had received a depression diagnosis from a health care provider (and confirmed through symptom review using the Family History Research Diagnostic Criteria) and/or treatment for depression. Global functioning of the child was assessed by the Children’s Global Assessment Scale,20 and the overall functioning of the family was assessed by the Family Global Assessment Scale (D. Mrazek, unpublished, 1992). Both the Children’s Global Assessment Scale and Family Global Assessment Scale are measured on a Likert-type scale (range 0–100), with lower scores indicating more impairment in functioning.
Data Analysis
All of the analyses were conducted post hoc. Logistic regression (with ORs) was used to predict remission status at the end of acute treatment from the baseline demographic/clinical characteristics. Each baseline predictor variable was evaluated in a separate logistic regression model as well as in a multiple (stepwise) logistic regression model. The likelihood ratio χ2 statistic was used to test for a significant association between each predictor and remission status. Analyses for baseline characteristics were based on participants who received fluoxetine treatment at baseline and had at least one post-baseline visit (n = 168).
A 2 remission group (remitters, nonremitters) × 8 time point (weeks 1, 2, 3, 4, 6, 8, 10, and 12) mixed linear model analysis of repeated measures was used to assess the pattern of symptom improvement (percent reduction in CDRS-R total score) among remitters versus nonremitters and how early (which week) we can distinguish remitters from nonremitters based on their percent improvement score. Restricted maximum likelihood estimation and type 3 tests of fixed effects were used, with the Kenward-Roger correction21 applied to the antedependence covariance model. Age group (children and adolescents) and family history of depression (yes and no) were included as covariates in the mixed model analysis. The main effects of age group and remission group, as well as the remission group × time point (weeks) interaction effect were examined. Simple remission group effects at each time point were also assessed. The level of significance was set at p ≤ .05.
A receiver operating characteristic (ROC) analysis was conducted to determine the optimal “depression severity improvement” cut point (percent reduction scores) that best identified patients who experienced remission status at the end of acute treatment. Each individual area under the curve (AUC) for weeks 1 to 8 was tested against a nominal area of 0.50 using the z statistic. The area under the ROC curve is a parameter used to quantify the ability or accuracy of the test (which, in our case, was the depression severity improvement cut points for a given treatment week) to correctly classify or discriminate remitters from nonremitters. Mathematically, an AUC can range from 0.50 to 1. Thus, an AUC of 1 represents a perfect test (cut point)—perfect discrimination of remitters from nonremitters. An AUC of 0.50, however, represents a useless test (cut point)—the inability to discriminate between remitters and nonremitters. In practice, an AUC generally falls somewhere between 0.50 and 1.
Because the goal was to evaluate how well weeks 4, 6, and 8 CDRS-R percent reduction scores identify (or discriminate) eventual remitters from nonremitters, a subsample of 145 participants who completed at least 8 weeks of treatment was used for the ROC analysis. The ROC analysis was then based on week 4, and the cut point that produced the best combined sensitivity and specificity at week 4 was calculated. In addition, positive predictive value (PPV) and negative predictive value (NPV) are reported. An ROC analysis was also performed separately for children (11 years or younger) and adolescents (older than 11 years). Odds ratios were estimated (using logistic regression) from the corresponding two independent proportions (optimal cut point for improvement in depression severity versus remission status). The 95% likelihood ratio confidence intervals (CIs) were calculated, and the likelihood ratio χ2 statistic was used to test for a significant association between each cut point and remission status.
All of the analyses were intent-to-treat with minor modifications: participants who did not have at least one postbaseline visit were excluded from the analyses. The level of significance for all tests was set at p ≤ .05, and to address multiple testing, p values were adjusted using the false discovery rate.22
RESULTS
Participant Characteristics
More than 80% of the 168 participants completed the entire 12 weeks of acute phase of treatment. Reasons for discontinuation during the acute treatment were lack of efficacy (n = 6/168; 3.6%), moved or lost to follow-up (n = 5/168; 3.0%), withdrew consent (n = 11/168; 6.5%), noncompliance (n = 2/168; 1.2%), and adverse events (n = 8/168; 4.8%). Adverse events leading to discontinuation included rash (n = 2/168; 1.2%), multiple physical adverse events (n = 1; 0.6%), and suicide-related behaviors (n = 5/168; 3.0%), which included suicidal ideation (n = 3), suicide attempt (n = 1), and selfinjurious behavior (n = 1).
The mean age of participants was 11.8 ± 2.8 years, with children (ages 7–11 years) comprising 48% of the sample. Forty-two percent of the participants were girls; the sample was predominantly white (75%). The majority (70%) of the youths were in their first MDD episode. Concurrent comorbid disorders were common; 74% had at least one comorbid psychiatric diagnosis, with ADHD being the most common comorbid illness. Approximately 71% of the sample had a positive first-degree family history of depression. The mean baseline CDRS-R total score was 57.6 (SD 7.3), with no significant differences between boys (mean 56.8, SD 6.0) and girls (mean 58.7, SD 8.8; t = −1.7, p = .94) or between children (mean 56.4, SD 6.6) and adolescents (mean 58.6, SD 7.8; t = −2.0, p = .52). Baseline characteristics for participants who entered the acute phase of treatment are shown in Table 1. Patient features were similar between remitters (n = 110) and nonremitters (n = 58; Table 1).
TABLE 1.
Demographic and Baseline Clinical Features
| Remission Status | ||||
|---|---|---|---|---|
| Whole Sample (n = 168) | Remitter (n = 110) | Nonremitter (n = 58) | Test Statistic and pa | |
| Sex | χ2 = 0.66, p = .41 | |||
| Male | 58% (97) | 60% (66) | 53.4% (31) | |
| Female | 42% (71) | 40% (44) | 46.6% (27) | |
| Age, y | 11.8 ± 2.8 | 11.7 ± 2.8 | 12.1 ± 2.9 | t = 0.88, p = .41 |
| Age group | χ2 = 2.26, p = .26 | |||
| Children (11 y and younger) | 48% (80) | 51.8% (57) | 39.7% (23) | |
| Adolescents (12 y and older) | 52% (88) | 48.2% (53) | 60.3% (35) | |
| Ethnicity | χ2 = 6.28, p = .26 | |||
| African American | 11% (18) | 10.9% (12) | 10.3% (6) | |
| White | 75% (126) | 70.0% (77) | 84.5% (49) | |
| Hispanic | 11% (18) | 14.6% (16) | 3.5% (2) | |
| Other | 4% (6) | 4.5% (5) | 1.7% (1) | |
| MDD episode | χ2 = 0.05, p = .99 | |||
| Single | 70% (117) | 69.1% (76) | 70.7% (41) | |
| Recurrent | 30% (51) | 30.9% (34) | 29.3% (17) | |
| No. of concurrent comorbidities | 1.2 ± 1.0 | 1.1 ± 0.98 | 1.2 ± 1.1 | t = 0.10, p = .99 |
| Dysthymia | 32% (53) | 30.0% (33) | 34.5% (20) | χ2 = 0.35, p = .89 |
| Behavioral disorders | 43% (72) | 45.5% (50) | 37.9% (22) | χ2 = 0.88, p = .87 |
| Anxiety disorders | 26% (44) | 25.4% (28) | 27.6% (16) | χ2 = 0.08, p = .99 |
| Length of illness, mo | 14.6 ± 17.6 | 14.5 ± 17.6 | 14.8 ± 17.8 | t = 0.11, p = .99 |
| Current episode length, wk | 25.3 ± 21.1 | 24.4 ± 22.2 | 26.9 ± 18.8 | t = 0.72, p = .87 |
| Baseline CDRS-R total score | 57.6 ± 7.3 | 57.2 ± 6.5 | 58.3 ± 8.6 | t = 0.90, p = .87 |
| Suicidal behaviors | χ2 = 9.26, p = .32 | |||
| None | 22.6% (38) | 24.6% (27) | 18.9% (11) | |
| Morbid thoughts/death wishes | 38.1% (64) | 33.6% (37) | 46.6% (27) | |
| Suicidal thoughts | 31.6% (53) | 32.7% (36) | 29.3% (17) | |
| Suicidal plans | 6.5% (11) | 9.1% (10) | 1.7% (1) | |
| Suicidal attempts | 1.2% (2) | 0.0% (0) | 3.5% (2) | |
| CGI-S | 4.8 ± 0.6 | 4.8 ± 0.64 | 4.6 ± 0.62 | t = 0.01, p = .99 |
| CGAS | 51.7 ± 6.2 | 52.1 ± 5.8 | 50.7 ± 6.7 | t = 1.35, p = .73 |
| FGAS | 61.9 ± 10.6 | 62.3 ± 11.0 | 61.1 ± 9.7 | t = 0.73, p = .87 |
| Positive family history of depression | 71% (119)b | 79.8% (87)b | 55.2% (32) | χ2 = 10.92, p = .01 |
Note: CGI-S = Clinical Global Impression-Severity Scale; CGAS = Clinical Global Assessment Scale; FGAS = Family Global Assessment Scale; CDRS-R = Children’s Depression Rating Scale-Revised; MDD = major depressive disorder. Data are presented as mean ± SD (continuous variables) or % (n; categorical variables).
Tested for differences between remitters and nonremitters on each demographic/clinical characteristic in a separate model, and the p values are adjusted using the false discovery rate described by Benjamini and Hochberg.22
Data unavailable for one adopted patient (total n = 167; remitted n = 109).
Predicting Remission Status From Baseline Characteristics
Based on previous studies,7,8 age, sex, ethnicity, MDD episodes, number of concurrent psychiatric comorbidities, length of illness, length of current episode, baseline depression severity, global functioning of the child and family, suicidal behaviors, and first-degree family history of depression were assessed in the regression model to test their predictability of remission status at the end of acute treatment. Of these variables, only positive family history of depression was a significant predictor of remission status at the end of acute treatment (p values adjusted using the false discovery rate). The logistic regression revealed that, among the 168 participants, those with a positive first-degree family history of depression were 3.21 (95% CI 1.60–6.51) times more likely to remit than those without a family history of depression (χ2 = 10.92, p = .01).
Patterns of Response: Remitters Versus Nonremitters
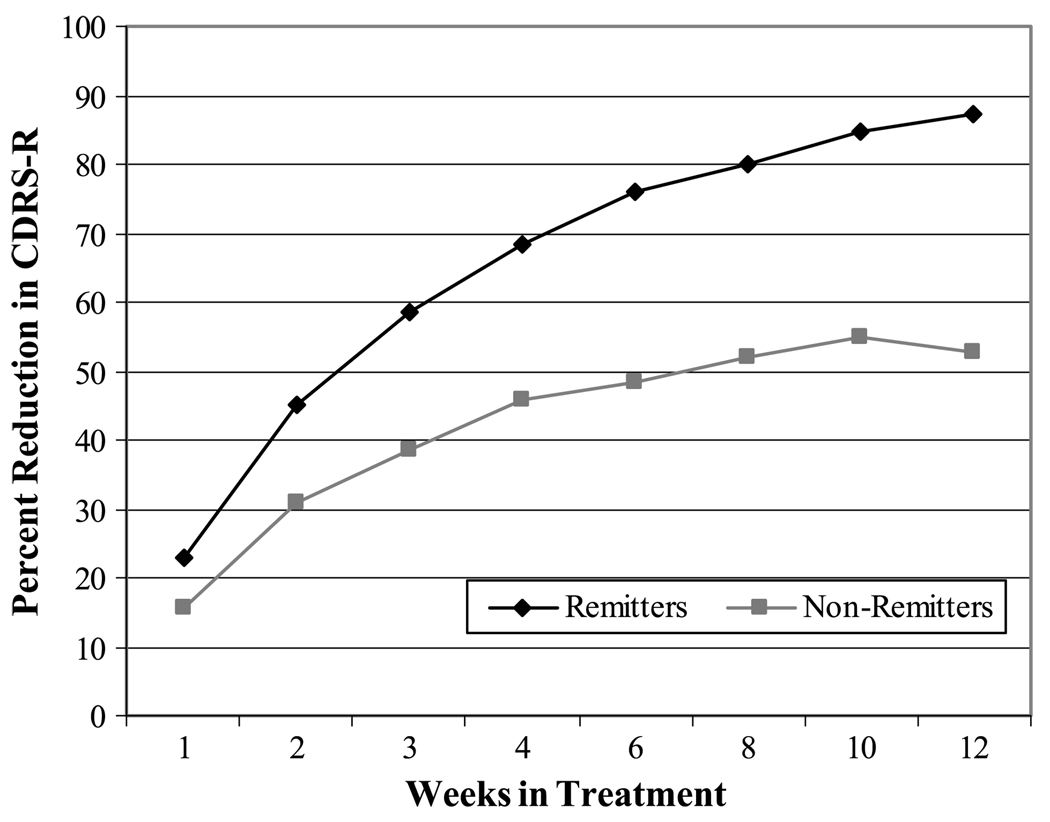
The mixed linear model analysis of repeated measures revealed that the patterns of symptom improvement (percent reduction in CDRS-R total score) were significantly different for remitters and nonremitters (F = 116.34, p = .0001) over the 12 weeks of acute treatment (Fig. 1). In addition, a significant remission group × time interaction effect was found (F = 9.36, p = .0001).
Fig. 1.
Percent reduction in CDRS-R scores at each week for remitters versus nonremitters. CDRS-R = Children’s Depression Rating Scale-Revised.
Remitters had an overall higher percent symptom reduction (adjusted least squares mean 65.3%) than nonremitters (42.3%; p = .0001). In addition, percent symptom reduction scores were significantly different at each of the assessment weeks (p < .01) for remitters and nonremitters. As early as 1 week, remitters experienced greater symptom reduction (least squares mean 22.7%) than nonremitters (least squares mean 15.6%), p = .01, after adjustments for age group and family history of depression.
The mixed model analysis also revealed a significant omnibus main effect of age group (F = 13.17, p = .0004) while controlling for (or independent of) family history of depression and remission status. Adjusted least squares means for percent symptom reduction were higher for children than adolescents (56.85 versus 50.78). In other words, children had a higher mean percent reduction over the 12 weeks of treatment than adolescents regardless of family history of depression or remission status.
How Early Can We Identify Patients Who Will Achieve Remission?
The ROC analysis was used to assess how well the rate of symptom reduction during the early weeks of treatment identified remission status at the end of acute treatment. A subsample of 145 participants who had completed at least 8 weeks of acute treatment were included in the ROC analysis. Table 2 presents the AUC and CIs for weeks 1, 2, 3, 4, 6, and 8. All AUCs were significant (z’s > 3.08, p’s < .0021), indicating that the rate of symptom reduction at each early week identified remission status (or discriminated remitters from nonremitters) at the end of acute treatment (when compared with the nominal AUC of 0.50). The changes in the AUCs from weeks 4 to 8 were rather small, and there was overlap in the CIs of the AUCs among weeks 4, 6, and 8. Pairwise comparisons of the AUCs, with p values adjusted via the false discovery rate, indicated that week 4 percent reduction was as good at discriminating remission status when compared with weeks 6 (p = .12) and 8 (p = .32), respectively. In other words, an additional 4 weeks of continuing on the same treatment (from week 4 to week 8) did not significantly increase the ability to discriminate remitters from nonremitters. The area under the ROC curve at week 4 was 0.788, which would be considered “good” at discriminating remitters from nonremitters.
TABLE 2.
Receiver Operating Characteristic Analysis: Area Under the Curve for Percent Reduction in CDRS-R Score at Each Week Relative to Remission Status at the End of Acute Treatment
| Week | AUC | SE | 95% Confidence Interval for AUC |
|---|---|---|---|
| 1 | 0.653 | 0.0496 | 0.569–0.730 |
| 2 | 0.719 | 0.045 | 0.638–0.790 |
| 3 | 0.741 | 0.0432 | 0.661–0.810 |
| 4 | 0.788 | 0.0388 | 0.713–0.852 |
| 6 | 0.834 | 0.0337 | 0.764–0.891 |
| 8 | 0.826 | 0.0347 | 0.754–0.884 |
Note: AUC = area under the curve; CDRS-R = Children’s Depression Rating Scale<Revised; SE = standard error. All AUCs were significant (z ’s > 3.08, p’s < .0021) when compared with the nominal AUC of 0.50.
What Percent of Symptom Improvement at Week 4 Provides the Best Combined Sensitivity and Specificity in Identifying Eventual Remission Status?
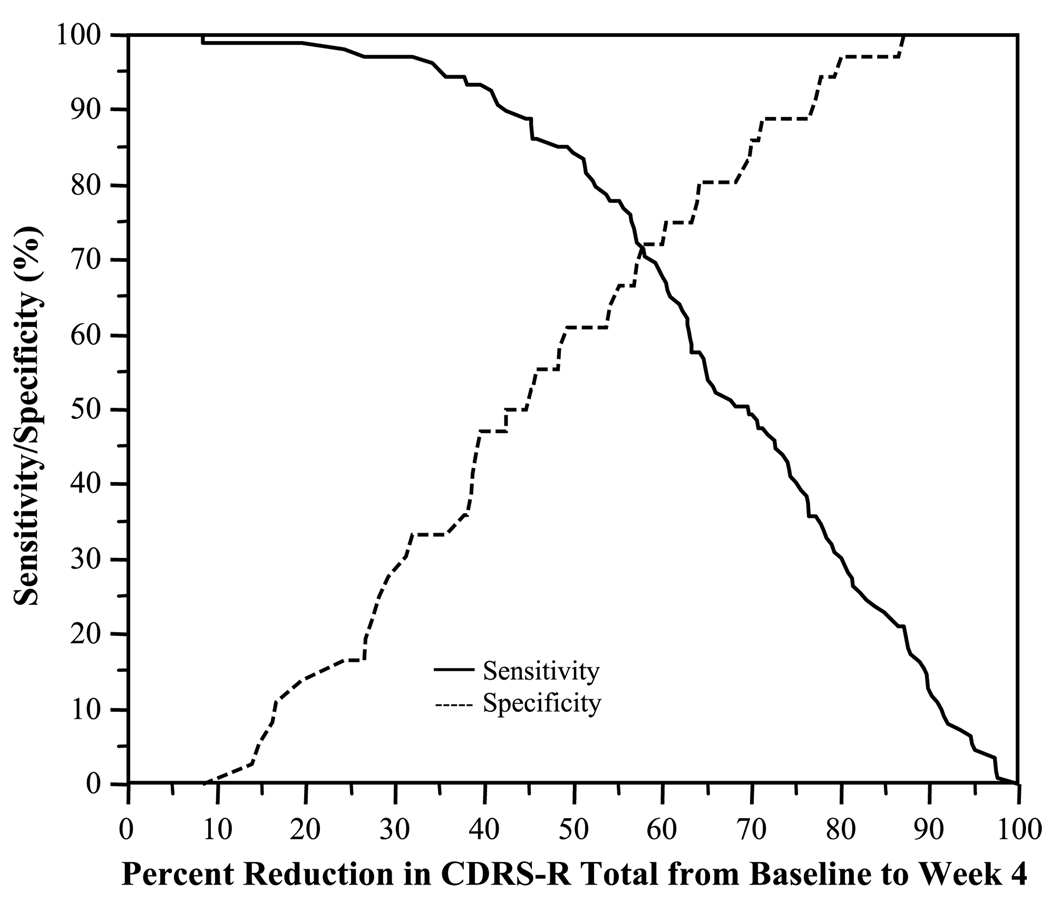
Figure 2 presents the combined sensitivity and specificity of CDRS-R percent reduction score at week 4 relative to remission status at week 12 or exit. The ROC analysis determined that, at week 4, a cutoff greater than 57.9% of reduction in the CDRS-R total score represented the best combined sensitivity (71.6%) and specificity (72.2%), and hence area under the ROC curve (0.72), with a PPV of 88.6% and NPV of 45.6% in identifying or discriminating patients who experienced remission status at week 12 or exit. That is, 71.6% of remitters had a greater than 57.9% of reduction in their CDRS-R score at week 4, whereas 72.2% of nonremitters had a 57.9% or lower of reduction. At week 4, the odds of achieving remission at the end of acute treatment was 6.54 (95% CI 2.90–15.76) times higher in patients with a CDRS-R percent reduction threshold of greater than 57.9% than those with a CDRS-R percent reduction threshold of 57.9% or lower (χ2 = 21.63, p < .0001). Using a lower percent reduction cutoff point (e.g., 30%), sensitivity is increased (95% for a 30% reduction); however, this leads to increased false positives.
Fig. 2.
Sensitivity and specificity associated with percent reduction in CDRS-R score at week 4 relative to remission status at week 12. CDRS-R = Children’s Depression Rating Scale-Revised.
To examine whether there were age-related differences on sensitivity and specificity, the ROC analysis was conducted separately for children (11 years or younger) and adolescents (older than 11 years). Although the cutoff scores for the best combined sensitivity and specificity were different for children and adolescents, week 4 percent reduction score was as good at discriminating remitters from nonremitters when compared with weeks 6 (p = .38) and 8 (p = .47) for children and with weeks 6 (p = .36) and 8 (p = .55) for adolescents. A slightly higher percent reduction score (>63.9%) for children than for adolescents (>55.2%) yielded the best combined sensitivity and specificity. For children (with a cutoff score higher than 63.9%), the sensitivity, specificity, PPV, and NPV were 70.2%, 72.7%, 93.0%, and 32.0%, respectively. For adolescents (with a cutoff score higher than 55.2%), the sensitivity, specificity, PPV, and NPV were 71.2%, 72.0%, 84.1%, and 54.5%, respectively. The odds of remitting at the end of acute treatment were 6.27 (95% CI 1.60–31.38) times higher in children with a CDRS-R percent reduction of greater than 63.9% at week 4 than in children with a CDRS-R percent reduction of 63.9% or lower (χ2 = 7.08, p = .007). For adolescents, the odds of achieving remission at the end of acute treatment was 5.24 (95% CI 1.92–15.40) times higher if their CDRS-R percent reduction score was higher than 55.2% at week 4 than if their CDRS-R percent reduction score was 55.2% or lower (χ2 = 10.71, p = .001).
DISCUSSION
Most demographic and baseline clinical features did not predict treatment outcome in this well-characterized pediatric sample with MDD. Although some earlier studies demonstrated a few clinical characteristics that predicted outcome (e.g., depression severity, previous episodes),7–9 this study found no demographic or illness characteristics at baseline that predicted remission. Previous studies of predictors of response have not yielded consistent results, so our lack of finding here is not entirely unexpected. This sample had a relatively high number of children and a high proportion with co-morbid diagnoses, which may have contributed to the findings, although baseline severity was comparable with previous studies.2 Positive first-degree family history of depression was predictive of remission at the end of 12 weeks of acute fluoxetine treatment. It is possible that a more “biological” depression (as would be expected in the youths with a positive family history) responds to biological treatment (fluoxetine) more favorably than depression without predisposed biological factors. In recent years, convergent evidence from neurophysiology, genetics, sleep research, brain imaging, and electroencephalogram have linked biological markers to antidepressant treatment response.23–26 Future studies focusing on biological indicators in youths will be helpful in identifying treatment response/remission predictors.
The rate of symptom reduction early in treatment identified remission status (or discriminated remitters from nonremitters) by the end of acute treatment. As early as 1 week, the patients who eventually achieved remission had significantly greater symptom improvement than the patients who did not. Improvement in depression severity by week 4 was as good at discriminating remitters from nonremitters as that of weeks 6 and 8. This finding is of substantial clinical value. Currently, most guidelines recommend waiting until after week 6 to increase dose and even later (after week 8) to change treatment approach (e.g., augment, switch).6 Based on the present study, both children and adolescents need to have sufficient (above 50%) improvement by week 4 to have a strong likelihood of achieving remission by 12 weeks. If there is no significant reduction in their depressive symptoms by week 4, the likelihood of achieving remission by week 12 decreases. Therefore, a change in treatment may be warranted earlier than previously recommended. Whether the change in treatment should be a dose increase or alteration to the treatment plan warrants further investigation. Clinically, if the child is showing at least some response by week 4, increasing the dose is probably justified, whereas those with no change in symptoms may benefit more from altering the treatment plan (e.g., change medication strategy, intensify psychosocial interventions).
One positive result from this study is the amount of improvement noted by week 4. Most clinicians do not use extensive rating scales such as the CDRS-R to guide treatment. However, a 50% improvement could be readily translated into clinical practice by evaluating whether patients’ symptoms are at least halfway improved. In fact, a 50% reduction on the CDRS-R correlates highly with a CGI Improvement score of 2 or lower (“much” or “very much” improved). More than 90% of the participants identified as responders by the CDRS-R (more than 50% decrease) were also responders based on CGI-I; similarly, more than 90% of nonresponders by CDRS-R were also nonresponders by CGI-I.
The differences between the children and adolescents were small. The children seemed to have slightly greater symptom reduction during the early weeks than the adolescents. It is unclear whether dose had an impact on early response, particularly for adolescents. In this study, dose was not increased until week 6 or later (after the week 4 response cut point), which may account for the slightly lower rate of symptom reduction in adolescents. Based on previous randomized controlled trials with fluoxetine, 20 mg may be adequate for children but low for adolescents.27
The study has several limitations. First, because this is an open-label study, clinicians were not blind to their patients’ treatment. The response and remission rates were high compared with double-blind placebo-controlled trials, which may be a reflection of clinicians’ positive bias toward estimating improvements. Future studies using independent evaluators may improve the objectivity of the assessment. However, the early response was sustained and continued to the end of acute treatment. Although more structured and intensive than clinical practice settings, open treatment resembles clinical practice settings. Consequently, results may be more generalizable to the community clinical setting. Second, the high remission rate may have inflated the cutoff percent reduction scores that yielded the best combined sensitivity and specificity.
Finally, the findings may be unique to fluoxetine treatment (i.e., they may not generalize to other medications or treatment modalities). Fluoxetine is the only antidepressant to have more than one positive randomized controlled trial in pediatric depression.28 The randomized continuation phase of the present study further demonstrated the efficacy of fluoxetine in pediatric depression treatment, because youths remaining on fluoxetine had lower relapse rates than those switched to placebo.16
In conclusion, this study raises an important question of the timing of treatment modifications that are currently recommended by treatment guidelines. It presents evidence for early modification of treatment strategies, which could shorten the time spent on ineffective or suboptimal treatments.
Acknowledgments
The study was supported by a grant entitled Childhood Depression: Remission and Relapse (R01 MH39188) from the National Institute of Mental Health (principal investigator: G.E.). In addition, support for Dr. Tao was provided in part by the Klingenstein Third Generation Foundation Fellowship in Child and Adolescent Psychiatry. Eli Lilly provided the medication for the study but had no role in the study design or implementation, analysis of data, or authorship of this article.
Footnotes
Clinical trials registration information—Determining Optimal Continuation Treatment Duration for Depressed Children and Adolescents. URL: http://www.clinicaltrials.gov. Unique identifier: NCT00332787.
Disclosure: Dr. Emslie has received research support from the National Institute of Mental Health, Shire, Somerset, Forest Laboratories, Eli Lilly, Organon, and Biobehavioral Diagnostics; has been a consultant to GlaxoSmithKline, Eli Lilly, Forest Laboratories, Wyeth-Ayerst, Shire, and Biobehavioral Diagnostics; and has been on the speakers’ bureau of McNeil Consumer and Specialty Pharmaceuticals. Dr. Hughes is a consultant to Biobehavioral Diagnostics. The other authors report no conflicts of interest.
REFERENCES
- 1.Rush AJ, Kraemer HC, Sackeim HA, et al. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31:1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- 2.Cheung AH, Emslie GJ, Mayes TL. Review of the efficacy and safety of antidepressants in youth depression. J Child Psychol Psychiatry. 2005;46:735–754. doi: 10.1111/j.1469-7610.2005.01467.x. [DOI] [PubMed] [Google Scholar]
- 3.March J, Silva S, Petrycki S, et al. Treatment for Adolescents With Depression Study (TADS) Team: fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: treatment for Adolescents With Depression Study (TADS) randomized controlled Trial. JAMA. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 4.Hughes CW, Emslie GJ, Crismon ML, et al. The Texas Childhood Medication Algorithm Project: update from the Texas consensus conference panel on medication treatment for the treatment of childhood major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:667–686. doi: 10.1097/chi.0b013e31804a859b. [DOI] [PubMed] [Google Scholar]
- 5.Rush AJ, Trivedi M, Schriger D, Petty F. The development of clinical practice guidelines for the diagnosis and treatment of depression. Gen Hosp Psychiatry. 1992;14:230–236. doi: 10.1016/0163-8343(92)90093-p. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Child and Adolescent Psychiatry. Practice parameters for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:1503–1526. doi: 10.1097/chi.0b013e318145ae1c. [DOI] [PubMed] [Google Scholar]
- 7.Birmaher B, Brent DA, Kolko D, et al. Clinical outcome after short-term psychotherapy for adolescents with major depressive disorder. Arch Gen Psychiatry. 2000;57:29–36. doi: 10.1001/archpsyc.57.1.29. [DOI] [PubMed] [Google Scholar]
- 8.Curry J, Rohde P, Simons A, et al. Predictors and moderators of acute outcome in the Treatment for Adolescents with Depression Study (TADS) J Am Acad Child Adolesc Psychiatry. 2006;45:1427–1439. doi: 10.1097/01.chi.0000240838.78984.e2. [DOI] [PubMed] [Google Scholar]
- 9.Kowatch RA, Carmody TJ, Emslie GJ, Rintelmann JW, Hughes CW, Rush AJ. Prediction of response to fluoxetine and placebo in children and adolescents with major depression: a hypothesis generating study. J Affect Disord. 1999;54:269–276. doi: 10.1016/s0165-0327(98)00205-5. [DOI] [PubMed] [Google Scholar]
- 10.Saghafi R, Brown C, Butters MA, et al. Predicting 6-week treatment response to escitalopram pharmacotherapy in late-life major depressive disorder. Int J Geriatr Psychiatry. 2007;22:1141–1146. doi: 10.1002/gps.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moses T, Leuchter AF, Cook I, Abrams M. Does the clinical course of depression determine improvement in symptoms and quality of life? J Nerv Ment Dis. 2006;194:241–248. doi: 10.1097/01.nmd.0000207358.15230.80. [DOI] [PubMed] [Google Scholar]
- 12.Papakostas GI, Petersen T, Sklarsky KG, Nierenberg AA, Alpert JE, Fava M. Timing of clinical improvement and symptom resolution in the treatment of major depressive disorder. Psychiatry Res. 2007;149:195–200. doi: 10.1016/j.psychres.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Masand PS. Tolerability and adherence issues in antidepressant therapy. Clin Ther. 2003;25:2289–2304. doi: 10.1016/s0149-2918(03)80220-5. [DOI] [PubMed] [Google Scholar]
- 14.Poznanski E, Mokros H. Children’s Depression Rating Scale-Revised (CDRS-R) Los Angeles: WPS; 1996. [Google Scholar]
- 15.Guy W. ECDEU Assessment Manual for Psychopharmacology Revised. Rockville: National Institute of Mental Health; 1976. [Google Scholar]
- 16.Emslie GJ, Kennard BD, Mayes TL, et al. Fluoxetine vs. placebo to prevent relapse of MDD in children and Adolescents. Am J Psychiatry. 2008;165:459–467. doi: 10.1176/appi.ajp.2007.07091453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Psychiatric Association. Washington: American Psychiatric Association; Diagnostic and Statistical Manual of Mental Disorders. (4th ed.) 1994
- 18.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Andreasen NC, Rice J, Endicott J, Reich T, Coryell W. The family history approach to diagnosis. Arch Gen Psychiatry. 1986;43:421–429. doi: 10.1001/archpsyc.1986.01800050019002. [DOI] [PubMed] [Google Scholar]
- 20.Shaffer D, Gould MS, Brasic J, et al. A children’s global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 21.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 23.Brody AL, Saxena S, Silverman DH, et al. Brain metabolic changes in major depressive disorder from pre- to post-treatment with paroxetine. Psychiatry Res. 1999;91:127–139. doi: 10.1016/s0925-4927(99)00034-7. [DOI] [PubMed] [Google Scholar]
- 24.Bruder GE, Sedoruk JP, Stewart JW, McGrath PJ, Quitkin FM, Tenke CE. Electroencephalographic alpha measures predict therapeutic response to a selective serotonin reuptake inhibitor antidepressant: pre- and post-treatment findings. Biol Psychiatry. 2008;63:1171–1177. doi: 10.1016/j.biopsych.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayberg H. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry. 1997;9:474–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 26.Smeraldi E, Zanardi R, Benedetti F, Di Bella D, Perez J, Catalano M. Polymorphism within the promoter of the serotonin transporter gene and antidepressant efficacy of fluvoxamine. Mol Psychiatry. 1998;3:508–511. doi: 10.1038/sj.mp.4000425. [DOI] [PubMed] [Google Scholar]
- 27.Mayes TL, Tao R, Rintelmann JW, et al. Do children and adolescents have differential response rates in placebo-controlled trials of fluoxetine? CNS Spectr. 2007;12:147–154. doi: 10.1017/s1092852900020666. [DOI] [PubMed] [Google Scholar]
- 28.Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683–1696. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]