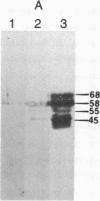
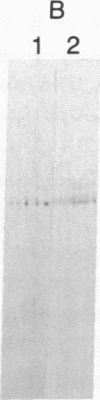
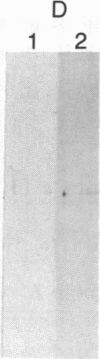
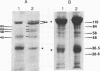
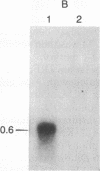
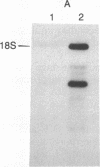
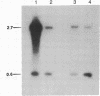
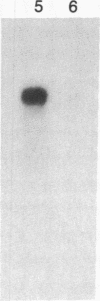
Abstract
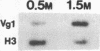
The localized maternal RNA Vg1 resides in the cortical region of the vegetal pole of fully grown Xenopus oocytes and is inherited by only a subset of blastomeres in the early embryo [Weeks, D. L. & Melton, D. A. (1987) Cell 51, 861-867]. Because RNA-cytoskeletal interactions may play a role in RNA localization, we have examined the association of Vg1 RNA with components of the oocyte's cytoskeleton. Gel and immunoblot analysis of a detergent-insoluble fraction revealed a greatly simplified protein pattern composed largely of cytokeratins and vimentin. In sharp contrast to the nonlocalized histone H3 mRNA, Vg1 RNA was concentrated some 35- to 50-fold in this insoluble fraction. Extractions at higher salt concentrations yielded preparations further enriched in cytokeratins and in the Vg1 RNA. Upon ovulation, VG1 RNA is released into the soluble fraction. This change in Vg1 RNA distribution coincides with the observed breakdown of cortical cytokeratin filaments [Klymkowsky, M. W., Maynell, L. A. & Polson, A. G. (1987) Development 100, 543-557] and the loss of Vg1 RNA from the cortical region. Our findings are consistent with the hypothesis that RNA-cytoskeletal interactions are involved in the localization and segregation of information during development.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. V., Nüsslein-Volhard C. Information for the dorsal--ventral pattern of the Drosophila embryo is stored as maternal mRNA. Nature. 1984 Sep 20;311(5983):223–227. doi: 10.1038/311223a0. [DOI] [PubMed] [Google Scholar]
- Anderton B. H. Intermediate filaments: a family of homologous structures. J Muscle Res Cell Motil. 1981 Jun;2(2):141–166. doi: 10.1007/BF00711866. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bray D., Heath J., Moss D. The membrane-associated 'cortex' of animal cells: its structure and mechanical properties. J Cell Sci Suppl. 1986;4:71–88. doi: 10.1242/jcs.1986.supplement_4.5. [DOI] [PubMed] [Google Scholar]
- Brown P. M., Kalthoff K. Inhibition by ultraviolet light of pole cell formation in Smittia sp (Chironomidae, Diptera): action spectrum and photoreversibility. Dev Biol. 1983 May;97(1):113–122. doi: 10.1016/0012-1606(83)90069-6. [DOI] [PubMed] [Google Scholar]
- Brown S., Levinson W., Spudich J. A. Cytoskeletal elements of chick embryo fibroblasts revealed by detergent extraction. J Supramol Struct. 1976;5(2):119–130. doi: 10.1002/jss.400050203. [DOI] [PubMed] [Google Scholar]
- Campanella C., Andreuccetti P. Ultrastructural observations on cortical endoplasmic reticulum and on residual cortical granules in the egg of Xenopus laevis. Dev Biol. 1977 Mar;56(1):1–10. doi: 10.1016/0012-1606(77)90150-6. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolecki G. J., Smith L. D. Poly(A)+ RNA metabolism during oogenesis in Xenopus laevis. Dev Biol. 1979 Mar;69(1):217–236. doi: 10.1016/0012-1606(79)90287-2. [DOI] [PubMed] [Google Scholar]
- Drummond D. R., McCrae M. A., Colman A. Stability and movement of mRNAs and their encoded proteins in Xenopus oocytes. J Cell Biol. 1985 Apr;100(4):1148–1156. doi: 10.1083/jcb.100.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin M. B., Kay B. K., Hershey J. W., Dawid I. B. Mitochondrial RNAs are abundant in the poly(A)+RNA population of early frog embryos. Dev Biol. 1981 Sep;86(2):502–504. doi: 10.1016/0012-1606(81)90208-6. [DOI] [PubMed] [Google Scholar]
- Franz J. K., Gall L., Williams M. A., Picheral B., Franke W. W. Intermediate-size filaments in a germ cell: Expression of cytokeratins in oocytes and eggs of the frog Xenopus. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6254–6258. doi: 10.1073/pnas.80.20.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall L., Karsenti E. Soluble cytokeratins in Xenopus laevis oocytes and eggs. Biol Cell. 1987;61(1-2):33–38. doi: 10.1111/j.1768-322x.1987.tb00566.x. [DOI] [PubMed] [Google Scholar]
- Gerton G. L., Hedrick J. L. The vitelline envelope to fertilization envelope conversion in eggs of Xenopus laevis. Dev Biol. 1986 Jul;116(1):1–7. doi: 10.1016/0012-1606(86)90036-9. [DOI] [PubMed] [Google Scholar]
- Godsave S. F., Anderton B. H., Heasman J., Wylie C. C. Oocytes and early embryos of Xenopus laevis contain intermediate filaments which react with anti-mammalian vimentin antibodies. J Embryol Exp Morphol. 1984 Oct;83:169–187. [PubMed] [Google Scholar]
- Godsave S. F., Wylie C. C., Lane E. B., Anderton B. H. Intermediate filaments in the Xenopus oocyte: the appearance and distribution of cytokeratin-containing filaments. J Embryol Exp Morphol. 1984 Oct;83:157–167. [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. 10 nm filaments in normal and transformed cells. Cell. 1978 Jan;13(1):151–163. doi: 10.1016/0092-8674(78)90146-0. [DOI] [PubMed] [Google Scholar]
- Jamrich M., Mahon K. A., Gavis E. R., Gall J. G. Histone RNA in amphibian oocytes visualized by in situ hybridization to methacrylate-embedded tissue sections. EMBO J. 1984 Sep;3(9):1939–1943. doi: 10.1002/j.1460-2075.1984.tb02073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery W. R., Meier S. A yellow crescent cytoskeletal domain in ascidian eggs and its role in early development. Dev Biol. 1983 Mar;96(1):125–143. doi: 10.1016/0012-1606(83)90317-2. [DOI] [PubMed] [Google Scholar]
- Jeffery W. R. Spatial distribution of messenger RNA in the cytoskeletal framework of ascidian eggs. Dev Biol. 1984 Jun;103(2):482–492. doi: 10.1016/0012-1606(84)90335-x. [DOI] [PubMed] [Google Scholar]
- Kasai M. Thermodynamical aspect of G-F transformations of actin. Biochim Biophys Acta. 1969 Jun 24;180(2):399–409. doi: 10.1016/0005-2728(69)90124-8. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987 Dec 4;51(5):869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W., Maynell L. A., Polson A. G. Polar asymmetry in the organization of the cortical cytokeratin system of Xenopus laevis oocytes and embryos. Development. 1987 Jul;100(3):543–557. doi: 10.1242/dev.100.3.543. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A. Translocation of a localized maternal mRNA to the vegetal pole of Xenopus oocytes. Nature. 1987 Jul 2;328(6125):80–82. doi: 10.1038/328080a0. [DOI] [PubMed] [Google Scholar]
- Neff A. W., Malacinski G. M., Wakahara M., Jurand A. Pattern formation in amphibian embryos prevented from undergoing the classical "rotation response" to egg activation. Dev Biol. 1983 May;97(1):103–112. doi: 10.1016/0012-1606(83)90068-4. [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Traub P. Intermediate (10 nm) filament proteins and the Ca2+-activated proteinase specific for vimentin and desmin in the cells from fish to man: an example of evolutionary conservation. J Cell Sci. 1982 Oct;57:25–49. doi: 10.1242/jcs.57.1.25. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Frohnhöfer H. G., Lehmann R. Determination of anteroposterior polarity in Drosophila. Science. 1987 Dec 18;238(4834):1675–1681. doi: 10.1126/science.3686007. [DOI] [PubMed] [Google Scholar]
- Rastl E., Dawid I. B. Expression of the mitochondrial genome in Xenopus laevis: a map of transcripts. Cell. 1979 Oct;18(2):501–510. doi: 10.1016/0092-8674(79)90067-9. [DOI] [PubMed] [Google Scholar]
- Rebagliati M. R., Weeks D. L., Harvey R. P., Melton D. A. Identification and cloning of localized maternal RNAs from Xenopus eggs. Cell. 1985 Oct;42(3):769–777. doi: 10.1016/0092-8674(85)90273-9. [DOI] [PubMed] [Google Scholar]
- Rosa F., Roberts A. B., Danielpour D., Dart L. L., Sporn M. B., Dawid I. B. Mesoderm induction in amphibians: the role of TGF-beta 2-like factors. Science. 1988 Feb 12;239(4841 Pt 1):783–785. doi: 10.1126/science.3422517. [DOI] [PubMed] [Google Scholar]
- Rosbash M. Polyadenylic acid-containing RNA in Xenopus laevis oocytes. J Mol Biol. 1974 May 5;85(1):87–101. doi: 10.1016/0022-2836(74)90131-4. [DOI] [PubMed] [Google Scholar]
- Ruderman J. V., Pardue M. L. A portion of all major classes of histone messenger RNA in amphibian oocytes is polyadenylated. J Biol Chem. 1978 Mar 25;253(6):2018–2025. [PubMed] [Google Scholar]
- Sagata N., Shiokawa K., Yamana K. A study on the steady-state population of poly(A)+RNA during early development of Xenopus laevis. Dev Biol. 1980 Jun 15;77(2):431–448. doi: 10.1016/0012-1606(80)90486-8. [DOI] [PubMed] [Google Scholar]
- Scharf S. R., Gerhart J. C. Axis determination in eggs of Xenopus laevis: a critical period before first cleavage, identified by the common effects of cold, pressure and ultraviolet irradiation. Dev Biol. 1983 Sep;99(1):75–87. doi: 10.1016/0012-1606(83)90255-5. [DOI] [PubMed] [Google Scholar]
- Schliwa M., van Blerkom J., Porter K. R. Stabilization and the cytoplasmic ground substance in detergent-opened cells and a structural and biochemical analysis of its composition. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4329–4333. doi: 10.1073/pnas.78.7.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P., Zackroff R., Aynardi-Whitman M., Goldman R. D. Isolation and characterization of intermediate filaments. Methods Cell Biol. 1982;24:399–419. doi: 10.1016/s0091-679x(08)60667-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdonk N. H. The effect of removing the polar lobe in centrifuged eggs of Dentalium. J Embryol Exp Morphol. 1968 Feb;19(1):33–42. [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Weeks D. L., Melton D. A. A maternal mRNA localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGF-beta. Cell. 1987 Dec 4;51(5):861–867. doi: 10.1016/0092-8674(87)90109-7. [DOI] [PubMed] [Google Scholar]
- Wolosewick J. J., Porter K. R. Microtrabecular lattice of the cytoplasmic ground substance. Artifact or reality. J Cell Biol. 1979 Jul;82(1):114–139. doi: 10.1083/jcb.82.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]