Abstract
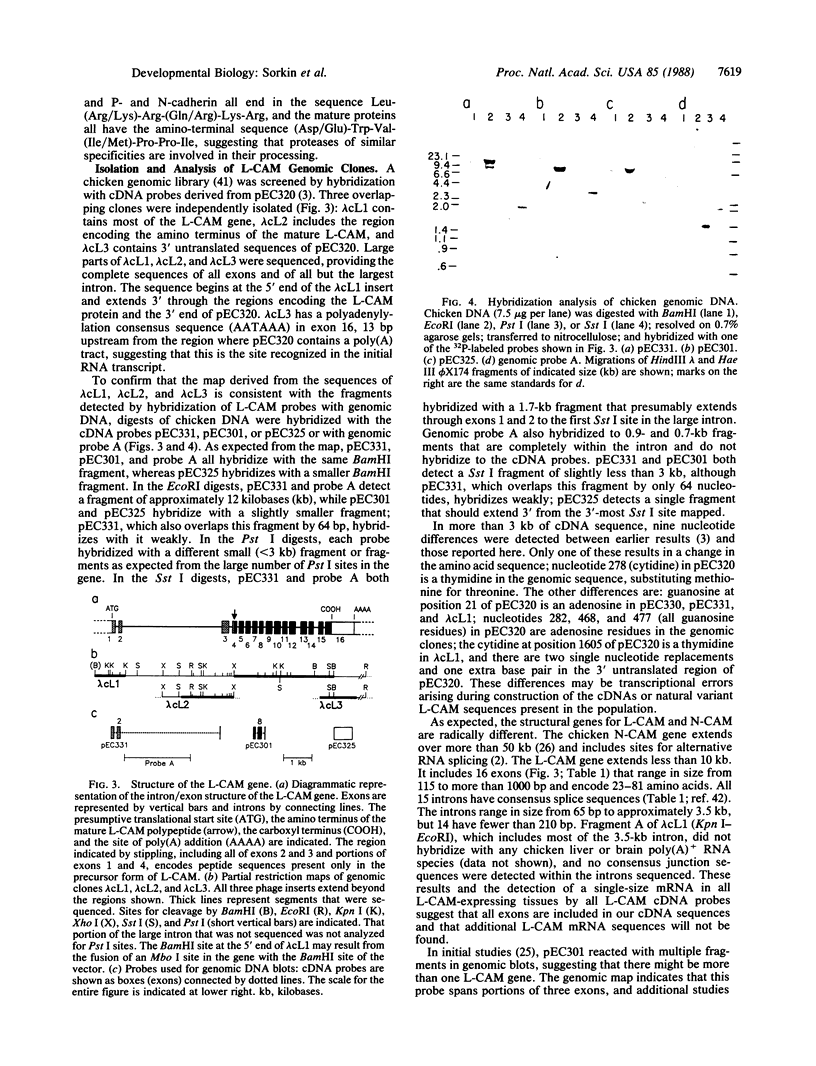
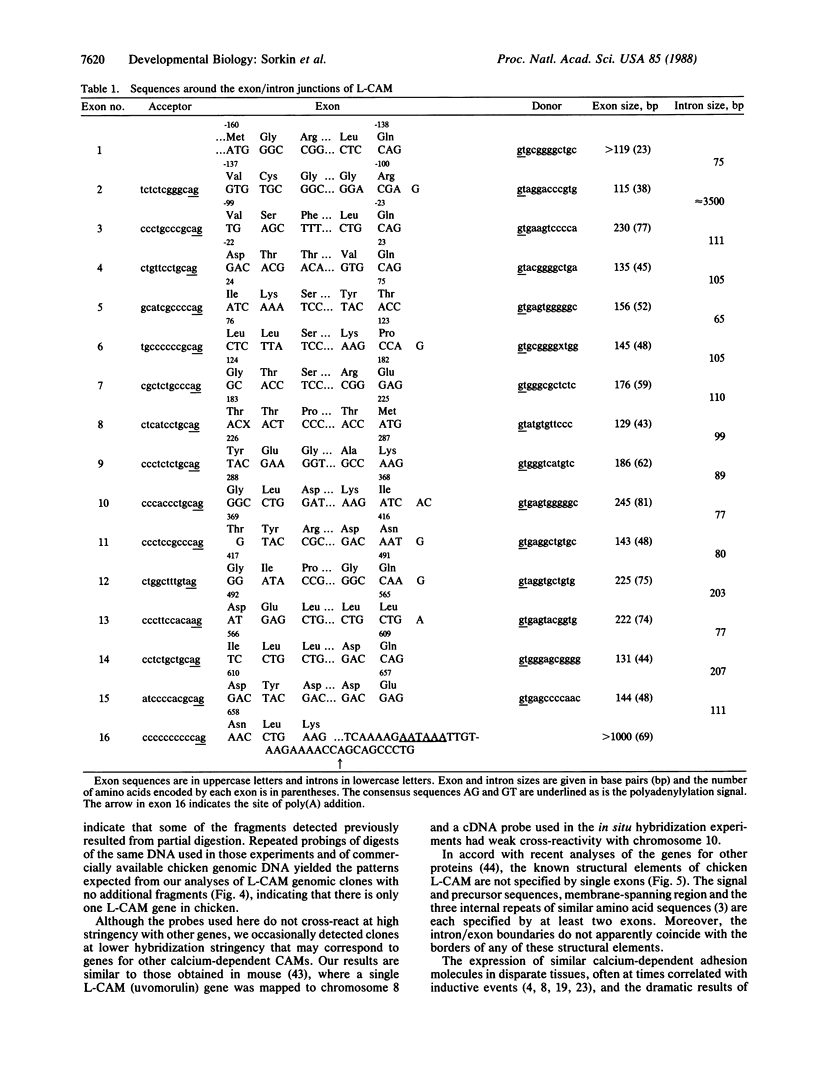
The liver cell adhesion molecule, L-CAM, mediates calcium-dependent cell-cell adhesion in early embryos and in nonneural epithelia in adult tissues. Earlier studies of cDNAs for chicken L-CAM established the amino acid sequence of the mature protein. The sequence has now been extended in the 5' direction through the precursor and signal sequences and past a consensus translation initiation site. The combined cDNAs were used to isolate genomic clones covering the entire L-CAM coding sequence. The structural gene for chicken L-CAM contains 16 exons ranging in size from 115 to over 1045 base pairs with an average size of 222 base pairs. Single exons do not correspond to known structural elements such as the signal sequence, precursor segment, internal repeats, or membrane-spanning region of L-CAM. Hybridization of restriction digests of chicken genomic DNA with cDNA and genomic probes indicated that there is a single L-CAM gene in the chicken. In contrast to genes for other cell-cell or cell-substrate adhesion molecules, there is no evidence for alternative splicing of exons in this gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crossin K. L., Chuong C. M., Edelman G. M. Expression sequences of cell adhesion molecules. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6942–6946. doi: 10.1073/pnas.82.20.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham B. A., Hemperly J. J., Murray B. A., Prediger E. A., Brackenbury R., Edelman G. M. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987 May 15;236(4803):799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Leutzinger Y., Gallin W. J., Sorkin B. C., Edelman G. M. Linear organization of the liver cell adhesion molecule L-CAM. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5787–5791. doi: 10.1073/pnas.81.18.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky C. H., Richa J., Solter D., Knudsen K., Buck C. A. Identification and purification of a cell surface glycoprotein mediating intercellular adhesion in embryonic and adult tissue. Cell. 1983 Sep;34(2):455–466. doi: 10.1016/0092-8674(83)90379-3. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Gallin W. J., Delouvée A., Cunningham B. A., Thiery J. P. Early epochal maps of two different cell adhesion molecules. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4384–4388. doi: 10.1073/pnas.80.14.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Morphoregulatory molecules. Biochemistry. 1988 May 17;27(10):3533–3543. doi: 10.1021/bi00410a001. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Murray B. A., Mege R. M., Cunningham B. A., Gallin W. J. Cellular expression of liver and neural cell adhesion molecules after transfection with their cDNAs results in specific cell-cell binding. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8502–8506. doi: 10.1073/pnas.84.23.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eistetter H. R., Adolph S., Ringwald M., Simon-Chazottes D., Schuh R., Guénet J. L., Kemler R. Chromosomal mapping of the structural gene coding for the mouse cell adhesion molecule uvomorulin. Proc Natl Acad Sci U S A. 1988 May;85(10):3489–3493. doi: 10.1073/pnas.85.10.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gallin W. J., Chuong C. M., Finkel L. H., Edelman G. M. Antibodies to liver cell adhesion molecule perturb inductive interactions and alter feather pattern and structure. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8235–8239. doi: 10.1073/pnas.83.21.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin W. J., Edelman G. M., Cunningham B. A. Characterization of L-CAM, a major cell adhesion molecule from embryonic liver cells. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1038–1042. doi: 10.1073/pnas.80.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin W. J., Prediger E. A., Edelman G. M., Cunningham B. A. Isolation of a cDNA clone for the liver cell adhesion molecule (L-CAM). Proc Natl Acad Sci U S A. 1985 May;82(9):2809–2813. doi: 10.1073/pnas.82.9.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin W. J., Sorkin B. C., Edelman G. M., Cunningham B. A. Sequence analysis of a cDNA clone encoding the liver cell adhesion molecule, L-CAM. Proc Natl Acad Sci U S A. 1987 May;84(9):2808–2812. doi: 10.1073/pnas.84.9.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B., Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol. 1986 Feb;102(2):457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K., Nose A., Nagafuchi A., Takeichi M. Cloning and expression of cDNA encoding a neural calcium-dependent cell adhesion molecule: its identity in the cadherin gene family. J Cell Biol. 1988 Mar;106(3):873–881. doi: 10.1083/jcb.106.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K., Okada T. S., Takeichi M. A monoclonal antibody disrupting calcium-dependent cell-cell adhesion of brain tissues: possible role of its target antigen in animal pattern formation. Proc Natl Acad Sci U S A. 1985 May;82(9):2789–2793. doi: 10.1073/pnas.82.9.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyafil F., Morello D., Babinet C., Jacob F. A cell surface glycoprotein involved in the compaction of embryonal carcinoma cells and cleavage stage embryos. Cell. 1980 Oct;21(3):927–934. doi: 10.1016/0092-8674(80)90456-0. [DOI] [PubMed] [Google Scholar]
- Imhof B. A., Vollmers H. P., Goodman S. L., Birchmeier W. Cell-cell interaction and polarity of epithelial cells: specific perturbation using a monoclonal antibody. Cell. 1983 Dec;35(3 Pt 2):667–675. doi: 10.1016/0092-8674(83)90099-5. [DOI] [PubMed] [Google Scholar]
- Jones F. S., Burgoon M. P., Hoffman S., Crossin K. L., Cunningham B. A., Edelman G. M. A cDNA clone for cytotactin contains sequences similar to epidermal growth factor-like repeats and segments of fibronectin and fibrinogen. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2186–2190. doi: 10.1073/pnas.85.7.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R., Babinet C., Eisen H., Jacob F. Surface antigen in early differentiation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4449–4452. doi: 10.1073/pnas.74.10.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Vibe-Pedersen K., Baralle F. E. Human fibronectin: cell specific alternative mRNA splicing generates polypeptide chains differing in the number of internal repeats. Nucleic Acids Res. 1984 Jul 25;12(14):5853–5868. doi: 10.1093/nar/12.14.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levi G., Crossin K. L., Edelman G. M. Expression sequences and distribution of two primary cell adhesion molecules during embryonic development of Xenopus laevis. J Cell Biol. 1987 Nov;105(5):2359–2372. doi: 10.1083/jcb.105.5.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mege R. M., Matsuzaki F., Gallin W. J., Goldberg J. I., Cunningham B. A., Edelman G. M. Construction of epithelioid sheets by transfection of mouse sarcoma cells with cDNAs for chicken cell adhesion molecules. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7274–7278. doi: 10.1073/pnas.85.19.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. A., Hemperly J. J., Gallin W. J., MacGregor J. S., Edelman G. M., Cunningham B. A. Isolation of cDNA clones for the chicken neural cell adhesion molecule (N-CAM). Proc Natl Acad Sci U S A. 1984 Sep;81(17):5584–5588. doi: 10.1073/pnas.81.17.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A., Shirayoshi Y., Okazaki K., Yasuda K., Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987 Sep 24;329(6137):341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- Nose A., Nagafuchi A., Takeichi M. Isolation of placental cadherin cDNA: identification of a novel gene family of cell-cell adhesion molecules. EMBO J. 1987 Dec 1;6(12):3655–3661. doi: 10.1002/j.1460-2075.1987.tb02698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A., Takeichi M. A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol. 1986 Dec;103(6 Pt 2):2649–2658. doi: 10.1083/jcb.103.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G. C., Edelman G. M., Cunningham B. A. Organization of the neural cell adhesion molecule (N-CAM) gene: alternative exon usage as the basis for different membrane-associated domains. Proc Natl Acad Sci U S A. 1987 Jan;84(1):294–298. doi: 10.1073/pnas.84.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyriéras N., Hyafil F., Louvard D., Ploegh H. L., Jacob F. Uvomorulin: a nonintegral membrane protein of early mouse embryo. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6274–6277. doi: 10.1073/pnas.80.20.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer T. H., Jr, Tarentino A. L. Facile cleavage of complex oligosaccharides from glycopeptides by almond emulsin peptide: N-glycosidase. J Biol Chem. 1981 Oct 25;256(20):10243–10246. [PubMed] [Google Scholar]
- Ringwald M., Schuh R., Vestweber D., Eistetter H., Lottspeich F., Engel J., Dölz R., Jähnig F., Epplen J., Mayer S. The structure of cell adhesion molecule uvomorulin. Insights into the molecular mechanism of Ca2+-dependent cell adhesion. EMBO J. 1987 Dec 1;6(12):3647–3653. doi: 10.1002/j.1460-2075.1987.tb02697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery J. P., Delouvée A., Gallin W. J., Cunningham B. A., Edelman G. M. Ontogenetic expression of cell adhesion molecules: L-CAM is found in epithelia derived from the three primary germ layers. Dev Biol. 1984 Mar;102(1):61–78. doi: 10.1016/0012-1606(84)90175-1. [DOI] [PubMed] [Google Scholar]
- Traut T. W. Do exons code for structural or functional units in proteins? Proc Natl Acad Sci U S A. 1988 May;85(9):2944–2948. doi: 10.1073/pnas.85.9.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennström B., Bishop J. M. Isolation and characterization of chicken DNA homologous to the two putative oncogenes of avian erythroblastosis virus. Cell. 1982 Jan;28(1):135–143. doi: 10.1016/0092-8674(82)90383-x. [DOI] [PubMed] [Google Scholar]
- Volk T., Geiger B. A 135-kd membrane protein of intercellular adherens junctions. EMBO J. 1984 Oct;3(10):2249–2260. doi: 10.1002/j.1460-2075.1984.tb02123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida-Noro C., Suzuki N., Takeichi M. Molecular nature of the calcium-dependent cell-cell adhesion system in mouse teratocarcinoma and embryonic cells studied with a monoclonal antibody. Dev Biol. 1984 Jan;101(1):19–27. doi: 10.1016/0012-1606(84)90112-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]