Abstract
In this issue of Molecular Cell, Miotto and Struhl (2010) suggest that replication licensing, the loading of Mcm2-7 onto DNA, is promoted by Hbo1 acetylating histone H4 at replication origins, providing a molecular view of how chromatin status influences origin usage.
During late mitosis and G1 of the cell cycle, Mcm2-7 helicase is loaded onto chromatin to license origins of replication (Blow and Dutta, 2005). In order to prevent DNA from re-replicating during a single S phase, the ability to load further Mcm2-7 onto origins must cease before cells enter S phase. One of the main mechanisms that controls this in higher eukaryotes is the down-regulation of Cdt1 which plays an essential role in loading Mcm2-7 onto origins. Cdt1 activity is blocked in S phase and G2 by a combination of degradation and binding of the geminin repressor. Previous work has shown that the process of DNA licensing is stimulated by Hbo1, a histone acetyl transferase (HAT) that binds directly to Cdt1 (Iizuka et al., 2006; Miotto and Struhl, 2008). Recent work from Miotto & Struhl in this issue of Molecular Cell (Miotto and Struhl, 2010) investigates how Hbo1 stimulates licensing, and provides strong evidence that it is mediated by acetylation of histone H4 tails at replication origins (Figure 1). This Cdt1-dependent H4 acetylation is inhibited by the binding of geminin to Cdt1. It has been known for many years that histone modifications affect the function of replication origins, however, the current work provides one of the first mechanistic examples of the way that this might occur. It also provides further insight into the complex regulatory network ensuring the precise duplication of chromosomal DNA.
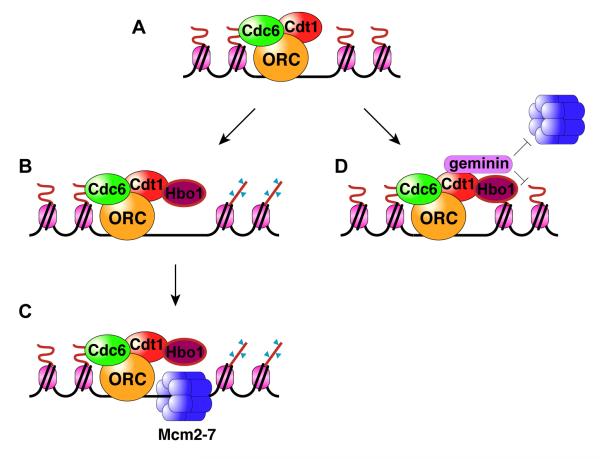
Figure 1. Regulation of replication licensing by Hbo1.
(A) From late M- to G1-phase ORC, Cdc6 and Cdt1 sequentially load onto chromatin to mark origins of replication.
(B) Cdt1-assisted binding of Hbo1 to origins results in acetylation of histone H4 at origins.
(C) The presence of ORC-Cdc6-Cdt1 and acetylated histones facilitate the loading of Mcm2-7 complex.
(D) Geminin binds to Cdt1 during S- and G2-phases of cell cycle and blocks its interaction with Mcm2-7 as well as inhibiting Hbo1 mediated H4 acetylation thus preventing re-loading of Mcm2-7 once replication is initiated.
Miotto and Struhl (2010) provide a number of lines of evidence that a key function of Hbo1 in origin licensing is mediated by H4 acetylation. Catalytically inactive Hbo1 binds to origins but fails to promote the loading of Mcm2-7. Consistent with Hbo1 being the major HAT activity for histone H4, they also showed that the knockdown of Hbo1 significantly reduces H4 acetylation at origins. Peak H4 acetylation at origins is at the G1/S boundary, which corresponds to the time of maximal binding of Mcm2-7 and Hbo1 to origins. In order to demonstrate in vivo substrate specificity of Hbo1 towards H4, Miotto and Struhl show that over-expression of Hbo1 along with Jade-1, a co-factor for Hbo1 that increases its association with chromatin, results in hyper-acetylation of H4 and increases Mcm2-7 loading. In addition, inhibition of Hbo1-induced H4 acetylation by co-expression of the histone-binding domain of Set8 decreased Mcm2-7 loading onto chromatin. Finally, inhibition of Cdt1 by geminin decreased Hbo1-dependent H4 acetylation. Although these experiments provide strong support for the idea that Hbo1 promotes Mcm2-7 loading by acetylating histone H4, they are not definitive and it remains possible that Hbo1 has some important non-histone substrates that promote licensing. Indeed, in vitro acetylation of other replication proteins by Hbo1 has been reported (Iizuka et al., 2006). It is also possible that H4 acetylation at origins involves other HAT activities other than Hbo1, given that there is constitutive H4 acetylation at origins in transcriptionally active chromatin and weak acetylation of H4 when Hbo1 had been inhibited by geminin.
The work of Miotto and Struhl leaves open the question of what H4 acetylation actually does in promoting origin licensing. The licensing reaction involves the sequential binding of the origin recognition complex (ORC), Cdc6 and Cdt1, which then promote the loading of Mcm2-7 (Figure 1A). Origin licensing has been reconstituted with purified proteins on Xenopus sperm chromatin, where it does not appear to require Hbo1 (Gillespie et al., 2001). But Izuka et al. (2006) have shown that Mcm2-7 fails to load onto chromatin in Hbo1 immunodepleted Xenopus extracts, and this can be reversed by addition of further Cdt1. A likely explanation is that Hbo1 is not an obligatory component of the reaction but instead promotes the efficiency of Mcm2-7 loading onto chromatin templates (Figure 1B, C). H4 acetylation might loosen up compacted chromatin and make it more accessible for loading of MCM helicase complex as tetra-acetylated H4 is known to promote histone octamer eviction (Ferreira et al., 2007). Alternatively it may also serve as a molecular tag to which Mcm2-7 might preferentially be recruited.
Cdt1 must be downregulated by geminin to prevent re-replication of DNA in S phase and G2. Previous work has shown that geminin binds tightly to Cdt1 and prevents it from promoting Mcm2-7 loading (Blow and Dutta, 2005). Geminin directly prevents Cdt1 from binding to Mcm2-7 subunits, possibly as a consequence of the formation of a catalytically inactive 2:4 Cdt1:geminin hexamer (De Marco et al., 2009). In a reconstituted system involving the licensing of Xenopus sperm chromatin with purified proteins, geminin still efficiently inhibited origin licensing although Hbo1 was not present (Gillespie et al., 2001). This suggests that geminin can inhibit Cdt1 independently of its ability to inhibit Hbo1 HAT activity, and that inhibition of Hbo1 HAT activity is a new function of geminin (Figure 1D). It is unlikely to play a major role in preventing re-replication of DNA, as Hbo1 over-expression on its own does not cause re-replication, whereas loss of geminin is sufficient to cause re-replication in many cell types (Blow and Dutta, 2005). However, over-expression of Hbo1 increases the amount of re-replication that occurs when Cdt1 is over-expressed, suggesting that inhibition of Hbo1 by geminin can play a supporting role in preventing re-replication.
Another attractive possibility is that Hbo1 plays a role in determining origin usage during G1 by regulating the licensing of different chromatin regions. There is considerable plasticity of replication origin usage in higher eukaryotes, with major changes existing between different cell types and at different stages of development. As ORC in higher eukaryotes does not display strong sequence specificity in its binding to DNA and associates with non-origin sequences, Hbo1 binding and acetylation of histones in its vicinity could represent a novel way for origins to be selected. Consistent with this idea, artificial tethering of the Drosophila Hbo1 homolog HAT1 to an origin stimulated its origin activity (Aggarwal and Calvi, 2004). This regulation could be of importance in cancer cells, as Hbo1 is overexpressed in a range of primary cancers and cancer cell lines (Iizuka et al., 2009).
The new work of Miotto and Struhl (2010) provides some of the first information about how histone modification affects replication origins. The data suggest a new role for the Cdt1 repressor geminin in regulating Hbo1 mediated H4 acetylation at origins and opens up further interesting questions about how histone modification and higher order chromatin structure is involved in DNA replication.
References
- Aggarwal BD, Calvi BR. Nature. 2004;430:372–376. doi: 10.1038/nature02694. [DOI] [PubMed] [Google Scholar]
- Blow JJ, Dutta A. Nat Rev Mol Cell Biol. 2005;6:476–486. doi: 10.1038/nrm1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco V, Gillespie PJ, Li A, Karantzelis N, Christodoulou E, Klompmaker R, van Gerwen S, Fish A, Petoukhov MV, Iliou MS, et al. Proc Natl Acad Sci U S A. 2009;106:19807–19812. doi: 10.1073/pnas.0905281106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira H, Flaus A, Owen-Hughes T. J Mol Biol. 2007;374:563–579. doi: 10.1016/j.jmb.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie PJ, Li A, Blow JJ. BioMed Central Biochemistry. 2001;2:15. doi: 10.1186/1471-2091-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka M, Matsui T, Takisawa H, Smith MM. Mol Cell Biol. 2006;26:1098–1108. doi: 10.1128/MCB.26.3.1098-1108.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka M, Takahashi Y, Mizzen CA, Cook RG, Fujita M, Allis CD, Frierson HF, Jr., Fukusato T, Smith MM. Gene. 2009;436:108–114. doi: 10.1016/j.gene.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto B, Struhl K. Genes Dev. 2008;22:2633–2638. doi: 10.1101/gad.1674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto B, Struhl K. Mol Cell. 2010 doi: 10.1016/j.molcel.2009.12.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]