Abstract
Context:
Most newly diagnosed prostate cancer is clinically localized, and major treatment options include surgery, radiation, or conservative management. Although conservative management can be a reasonable choice, there is little contemporary PSA era data on outcomes with this approach.
Objective:
To evaluate the outcomes of clinically localized prostate cancer managed without initial attempted curative therapy in the PSA era.
Design:
A population-based cohort study with a median follow-up 8.3 years (through December 31, 2007). Competing risk analyses were performed to assess outcomes.
Setting:
Areas covered by the Surveillance, Epidemiology and End Results (SEER) program.
Participants:
Men diagnosed with stage T1/T2 prostate cancer after age 65 between 1992 and 2002 managed without surgery or radiation within 6 months of cancer diagnosis.
Main Outcome Measure:
10-year overall survival, cancer-specific survival, and major cancer related interventions.
Results:
With a median age of 78 years at cancer diagnosis, ten-year prostate cancer-specific mortality was 8.3% (95% CI 4.2% – 12.8%), 9.1% (95% CI 8.3% - 10.1%), and 25.6% (95% CI 23.7% - 28.3%) for men with well-, moderately-, and poorly-differentiated tumors, respectively. The corresponding 10-year risks of dying of competing causes were 59.8% (95% CI 53.2% - 67.8%), 57.2% (95% CI 52.6% - 63.9%) and 56.5% (95% CI 53.6% - 58.8%), respectively. Ten-year disease specific mortality for men aged 66-74 years diagnosed with moderately-differentiated disease was 60% - 74% lower than earlier studies: 6% (95% confidence interval [CI] 4% - 8%) in the contemporary PSA era (1992-2002) compared to results of previous studies (15% - 23%) in earlier eras (1949-1992). Improved survival was also observed in poorly-differentiated disease. The use of chemotherapy (1.6%), or major interventions for spinal cord compression (0.9%), was uncommon.
Conclusion:
Results following conservative management of clinically localized prostate cancer diagnosed in 1992 – 2002 are better than outcomes among patients diagnosed in the 1970s and 1980s. This may be due, in part, to additional lead time, overdiagnosis related to PSA testing, grade migration, or advances in medical care.
Keywords: prostatic neoplasm, survival, population-based study
Introduction
Among men, prostate cancer is the most common non-skin cancer and the second most common cause of cancer death in the United States.1 When diagnosed, prostate cancer is contained within the prostate in approximately 85% of cases,2 and standard treatment options usually include surgery, radiation, or conservative management (active surveillance or deferral of treatment until necessitated by disease signs or symptoms).
For men <65 years of age with clinically localized prostate cancer, results of a large, randomized clinical trial have demonstrated that surgery improves survival compared with conservative management.3 The majority of men diagnosed with localized prostate cancer, however, are over age 65 years.4 Although not specifically designed to address age effects, this same clinical trial3 was unable to demonstrate a survival benefit for surgery among older men.3, 5 Coupled with data showing that the lifetime risk of being diagnosed with prostate cancer is ~17%, while the corresponding risk of dying of this disease is only ~3%,6 the evidence suggests that conservative management may be an important treatment consideration for the sizable majority of men diagnosed with localized prostate cancer.
Despite its potential as a reasonable treatment choice, however, conservative management has been utilized in only ~10% of patients,7 perhaps because of a limited understanding of, and contemporary data on, the anticipated course and outcomes of this approach. For example, most long-term data on conservative management have been acquired either in earlier eras when PSA testing was not performed, or from areas where PSA testing was uncommon,8-11 and cancers diagnosed in the contemporary PSA era have been shown to be significantly different from those found in earlier eras.2
This lack of reliable contemporary information makes it difficult for patients and their physicians to anticipate outcomes, make informed treatment decisions, and interpret the results of maturing clinical trials (often started in earlier eras) that compare outcomes to conservative management. We assembled a large population-based cohort of 14,516 men with localized T1/T2 prostate cancer in order to provide data on the results of conservatively managed localized prostate cancer diagnosed in the contemporary PSA era.
Methods
Data sources
Data were obtained from Medicare insurance program files linked to the population-based Surveillance, Epidemiology and End Results (SEER) cancer registries, which are 98% complete for case ascertainment.12 The SEER regions encompassed approximately 14% of the US population before 2000 and 25% thereafter.12 The Medicare database covers approximately 97% of US persons aged ≥65 years. Linkage to the SEER database is complete for approximately 93% of the patients.12 This study was approved by the University of Medicine and Dentistry of New Jersey institutional review board (IRB), as well as the SEER program, and the Center for Medicare & Medicaid Services. Informed consent was waived by the IRB because the data did not contain personal identifiers.
Cancer stage and grade for each case were abstracted from SEER data files. Gleason 2-4, 5-7, and 8-10 cancers were characterized as well-, moderately-, and poorly-differentiated cancers, respectively. Information regarding treatment was obtained from both SEER and Medicare files. A Charlson co-morbidity score was derived from Medicare claims during the year prior to prostate cancer diagnosis using a validated algorithm.13 Race was self-determined by the patients. Outcomes in the pre-PSA era (Table 3) were obtained from published literature except for the study by Albertsen et al.,10 where age-specific data was obtained directly from the authors. In this study, the pre-PSA era refers to the period before 1988. The contemporary PSA era refers to outcomes among patients diagnosed in 1992 or thereafter.
Table 3.
Ten-Year Competing Risk of Dying of Prostate Cancer according to Cancer Guide and Age for Men Aged 65-74 Years without Initial Attempted Curative Therapy
| Cancer Grade |
|||||
|---|---|---|---|---|---|
| Moderately-Differentiated (Gleason 5-7) % (95% CI) |
Poorly-Differentiated (Gleason 8-10) % (95% CI) |
||||
| Age at diagnosis | |||||
| Year of cancer diagnosis |
65–69 | 70–74 | 65–69 | 70–74 | |
| Current studya Stage T1 |
1992 – 2002 | 2 (1 – 4) |
5 (3 – 7) |
---- | ---- |
|
| |||||
| Current studya Stage T1 or T2 |
1992 – 2002 | 6 (4 – 8) |
6 (5 – 8) |
38 (27 – 54) |
25 (20 – 31) |
|
| |||||
| Albertsen, 2005b | 1971 – 1984 | 21 (10 – 32) |
23 (13 – 34) |
61 (42 – 79) |
50 (31 – 69) |
|
| |||||
| Johansson, 2004c | 1977 – 1984 | 15 (4 – 26) |
15 (4 – 26) |
---- | ---- |
|
| |||||
| Lu-Yao, 1997d | 1983 – 1992 | 23 (20 – 26) |
23 (20 – 26) |
55 (49 – 60) |
55 (49 – 60) |
|
| |||||
| Chodak, 1994e | 1949 – 1989 | 16 (11 – 20) |
16 (11 – 20) |
66 (50 – 81) |
66 (50 – 81) |
|
| |||||
| Adolfsson, 1992f | 1978 – 1982 | 16 (9 – 23) |
16 (9 – 23) |
---- | ---- |
Current study, patients aged 66-69 and 70-74 years. Comparison limited to men aged 66-74 years because most other studies had a median age ~70 and had very limited data on men over age 75.
Authors10 provided age-specific and Gleason score-specific data to calculate weighted averages for moderately-differentiated cancer. Confidence intervals were estimated using a parametric bootstrap with 100 replications based on the mortality estimates provided by the authors.
Age-specific data not available. Data taken from Table 2, moderately-differentiated cancer.37 Mean age not provided. Data for men with poorly-differentiated cancer not presented due to small sample size.
Mean age for moderately- and poorly-differentiated cancer was 71 and 72 years, respectively. Data taken from Table 2, intention to treat analyses.38
Age-specific data not available. Data26 taken from Table 5 for well- or moderately-differentiated cancer (men aged >61 years), and poorly-differentiated cancer (mean age 70).
Age-specific data not available.36 Mean age 68 years. All patients had well- or moderately-differentiated cancer.
Study participants
The study cohort consisted of men aged >65 years who were SEER residents and diagnosed with stage T1-T2 cancer between 1992 and 2002 (N = 89,877). This ensured that every patient had at least 12-months of Medicare claims data to assess their comorbidity status prior to cancer diagnosis (1991 was the first year Medicare claims data were available for all cancer cases). Men who died within 180 days of diagnosis (N=1,761), or who received attempted curative therapy such as prostatectomy or radiation within 180 days of diagnosis were excluded (N =31,485). Patients who had other cancers diagnosed either before or after prostate cancer were excluded (N=3,965) to ensure that all cancer therapies were for prostate cancer. Men who did not have both Medicare Part A and Part B as their primary health insurance coverage during the study period were excluded (N= 34,777) because their cancer treatment history might be incomplete. Men with missing data (N=2,995), an unknown cancer grade (N=255), or who received androgen deprivation therapy (ADT) prior to diagnosis (N=123) were also excluded. In this study, we classified T1c cancer as PSA screen-detected cancer and the rest as non-screen detected cancer. Results (both mortality and secondary cancer therapies) remained similar when patients receiving attempted curative therapy more than 180 days after diagnosis were excluded.
Outcomes assessment
Overall and prostate cancer-specific survival was available through December 31, 2007 and December 31, 2005, respectively. Underlying causes of death were obtained from the SEER database. Previous studies have shown high agreement (87-92%) between cause of death in the SEER database and that determined through medical record review.14, 15 Follow-up cancer therapies were identified from SEER and Medicare claims data through the end of 2005.
External beam radiation that consisted of <20 visits within a 6 week period was considered palliative, whereas brachytherapy or external beam radiation delivered over 20 visits within 6 weeks was considered attempted curative therapy. Chemotherapy use was identified through previously published algorithms (kappa ≥0.73 compared to medical record review).16 A validated algorithm was used to identify androgen deprivation therapy.17, 18 We developed and validated a new algorithm to identify palliative surgery or radiation for spinal cord compression, impending cord compression, or painful metastasis based on chart review.
Statistical analyses
The primary study endpoints were time to death from prostate cancer and time to death from other causes, stratified by patient age, cancer grade, and stage at diagnosis. Our study had >95% power to detect a change of ten percentage points in the prostate-cancer death rate estimates compared to previously reported rates. For the analysis of competing risks, we tabulated the numbers of men with each of the three outcomes of interest (alive, dead from prostate cancer, and dead from other causes) for each of the age-grade-stage combinations (Table 2). Results for men with T1 and T2 well-differentiated cancers were combined because of limited sample sizes.
Table 2.
Sample Sizes by Age and Vital Status as of December 31, 2005 among 14,516 Patients with Clinically Localized Prostate Cancera
| Age at diagnosis, years |
|||||
|---|---|---|---|---|---|
| Characteristic | 66-69 | 70-74 | 75-79 | 80+ | All ages |
| N (%) | N (%) | N (%) | N (%) | N (%) | |
|
Well-differentiated (Gleason 2-4), stages I & II
| |||||
| Overall sample size | 27 (100) | 53 (100) | 63 (100) | 79 (100) | 222 (100) |
|
| |||||
| Died of prostate cancer | NA | NA | 8 (13) | 8 (10) | 17 (8) |
|
| |||||
| Died of other cause | NA | NA | 37 (59) | 62 (78) | 141 (64) |
|
| |||||
| Alive | 16 (59) | 21 (40) | 18 (29) | 9 (11) | 64 (29) |
|
| |||||
|
Moderately-differentiated (Gleason 5-7), T1a & T1b
| |||||
| Overall sample size | 345 (100) | 713 (100) | 946 (100) | 1,231 (100) | 3,235 (100) |
|
| |||||
| Died of prostate cancer | NA | 21 (3) | 40 (4) | 79 (6) | 143 (4) |
|
| |||||
| Died of other cause | NA | 252 (35) | 411 (43) | 738 (60) | 1,485 (46) |
|
| |||||
| Alive | 258 (75) | 440 (62) | 495 (52) | 414 (34) | 1,607 (50) |
|
| |||||
|
Moderately-differentiated (Gleason 5-7), T1c
| |||||
| Overall sample size | 415 (100) | 818 (100) | 1,127 (100) | 1,199 (100) | 3,559 (100) |
|
| |||||
| Died of prostate cancer | 9 (2) | 26 (3) | 64 (6) | 75 (6) | 174 (5) |
|
| |||||
| Died of other cause | 66 (16) | 190 (23) | 311 (28) | 501 (42) | 1,068 (30) |
|
| |||||
| Alive | 340 (82) | 602 (74) | 752 (67) | 623 (52) | 2,317 (65) |
|
| |||||
|
Moderately-differentiated (Gleason 5-7), T2
| |||||
| Overall sample size | 431 (100) | 891 (100) | 1,336 (100) | 1,536 (100) | 4,194 (100) |
|
| |||||
| Died of prostate cancer | 32 (7) | 54 (6) | 105 (8) | 160 (10) | 351 (8) |
|
| |||||
| Died of other cause | 94 (22) | 277 (31) | 498 (37) | 756 (49) | 1,625 (39) |
|
| |||||
| Alive | 305 (71) | 560 (63) | 733 (55) | 620 (40) | 2,218 (53) |
|
| |||||
|
Poorly-differentiated (Gleason 8-10), T1a & T1b
| |||||
| Overall sample size | 32 (100) | 80 (100) | 164 (100) | 370 (100) | 646 (100) |
|
| |||||
| Died of prostate cancer | 9 (28) | 23 (29) | 41 (25) | 106 (29) | 179 (28) |
|
| |||||
| Died of competing cause | 12 (38) | 27 (34) | 72 (44) | 193 (52) | 304 (47) |
|
| |||||
| Alive | 11 (34) | 30 (38) | 51 (31) | 71 (19) | 163 (25) |
|
| |||||
|
Poorly-differentiated (Gleason 8-10), T1c
| |||||
| Overall sample size | 70 (100) | 160 (100) | 294 (100) | 41 (100) | 934 (100) |
|
| |||||
| Died of prostate cancer | 6 (9) | 32 (20) | 49 (17) | 66 (16) | 153 (16) |
|
| |||||
| Died of competing cause | 15 (21) | 48 (30) | 96 (33) | 179 (44) | 338 (36) |
|
| |||||
| Alive | 49 (70) | 80 (50) | 149 (51) | 165 (40) | 443 (47) |
|
| |||||
|
Poorly-differentiated (Gleason 8-10), T2
| |||||
| Overall sample size | 130 (100) | 290 (100) | 446 (100) | 860 (100) | 1,726 (100) |
|
| |||||
| Died of prostate cancer | 38 (29) | 51 (18) | 98 (22) | 171 (20) | 358 (21) |
|
| |||||
| Died of competing cause | 30 (23) | 108 (37) | 184 (41) | 438 (52) | 760 (44) |
|
| |||||
| Alive | 62 (48) | 131 (45) | 164 (37) | 251 (30) | 608 (35) |
Abbreviations: NA, not available. SEER-Medicare privacy rules prohibit disclosure of numbers <5 in any specific cell.
Data are presented as No. SEER clinical extension information was used to determine cancer stage (T1, T2).
For Table 3, confidence intervals (CIs) for the current study were based on 95% percentiles of 1,000 bootstrap replications of the competing risks model for death from either prostate cancer or other causes.{Efron, 1994 #1029} Confidence intervals for Albertsen et al. were estimated using a Weibull survival model that matched the 5- and 10-year prostate cancer death rates reported in that paper. The rest of the 95% CIs were extracted from published literature. Our study, with much larger sample sizes than the Albertsen et al. study,10 had more than 95% power to detect a change of ten percentage points in the prostate-cancer death rate estimates over Albertsen's reported rates for moderately differentiated disease, and more than 80% power for poorly differentiated disease, based on simulations with 1,000 replications. For Table 4, 95% CIs were based on percentiles of 1,000 bootstrap replications of the competing risks model for the three outcomes.
Table 4.
Ten-year Cumulative Risk of Selected Secondary Cancer Treatments after Diagnosis in Patients with Localized Prostate Cancer without Initial Attempted Curative Therapy
| Cancer grade | Moderately-differentiated Gleason 5-7, % (95% CI) c | |||||||
|---|---|---|---|---|---|---|---|---|
| Age at diagnosis, years | 66-74 | ≥ 75 | ||||||
| Screen status | Screen-detected (T1c) | Non-screen detected | Screen-detected (T1c) | Non-screen detected | ||||
| No. Person at Risk | N = 1,233 | N = 2,377 | N = 2,324 | N = 5,047 | ||||
| Treatments | Event Number |
10-year Risk* (95% CI) |
Event Number |
10-year Risk* (95% CI) |
Event Number |
10-year Risk* (95% CI) |
Event Number |
10-year Risk* (95% CI) |
| Attempted curative therapya | 257 | 23.7% (21.2 – 26.9) |
257 | 13.0% (11.2 – 14.3) |
113 | 5.9% (4.9 – 7.1) |
119 | 2.9% (2.3 – 3.6) |
| Androgen deprivation therapy | 590 | 60.3% (56.7 – 64.4) |
962 | 51.4% (47.9 – 53.6) |
1,457 | 73.8% (67.4 – 77.9) |
2,477 | 58.2% (55.3 – 60.3) |
| Palliative radiation,b chemotherapy, or spinal surgery or radiation |
109 | 10.9% (9.2 – 13.3) |
168 | 9.3% (7.6 – 10.8) |
83 | 5.0% (3.7 – 6.6) |
160 | 4.1% (3.6 – 4.8) |
| Cancer grade | Poorly-differentiated Gleason 8-10, % (95% CI) c | |||||||
| Age at diagnosis, years | 66-74 | ≥ 75 | ||||||
| Screen status | Screen-detected (T1c) | Non-screen detected | Screen-detected (T1c) | Non-screen detected | ||||
| No. Person at Risk | N = 230 | N = 532 | N = 703 | N = 1,839 | ||||
| Treatments | Event Number |
10-year Risk* (95% CI) |
Event Number |
10-year Risk* (95% CI) |
Event Number |
10-year Risk* (95% CI) |
Event Number | 10-year Risk* (95% CI) |
| Attempted curative therapya | 24 | 14.1% (9.5 – 20.1) |
64 | 14.5% (11.4 – 17.9) |
37 | 6.3% (4.7 – 8.5) |
46 | 3.0% (2.3 – 4.0) |
| Androgen deprivation therapy | 157 | 81.8% (75.1 – 97.4) |
387 | 80.7% (68.4 – 89.5) |
580 | 83.1% (74.9 – 94.7) |
1,442 | 83.7% (77.8 – 86.6) |
| Palliative radiation,b chemotherapy, or spinal surgery or radiation |
35 | 30.1% (16.1 – 51.1) |
78 | 17.0% (13.2 – 20.2) |
49 | 8.1% (6.1 – 10.4) |
106 | 6.9% (5.6 – 9.0) |
The cumulative risks were derived from competing risk models and maybe different from raw rate (Event No. / No. person at risk)
Radical prostatectomy, or at least 20 visits for radiation therapy during a 6-week period, and/or brachytherapy
Less than 20 visits for radiation therapy during a 6-week period. Due to small numbers of chemotherapy and spinal surgery, these categories are not presented separately
Confidence intervals were estimated using a bootstrap with 1,000 replications.
Estimates of competing risks and P values were computed using cumulative incidence functions (Figure, Table 4).19 For the analysis of competing risks for secondary cancer therapy listed in Table 4, we computed the competing risks of each outcome independently, with death treated as a competing risk, since one individual could have had more than one secondary treatment. To provide more stable estimates of the survival curves, we used a nearest neighbor hazard smoother with an Epanechnikov kernel20 as implemented in the R statistical system (R Foundation for Statistical Computing, Vienna, Austria). All P-values were two-sided. P-values <0.05 were considered statistically significant.
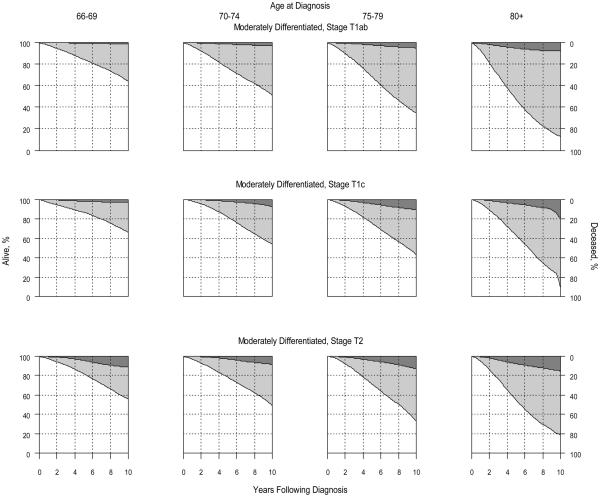
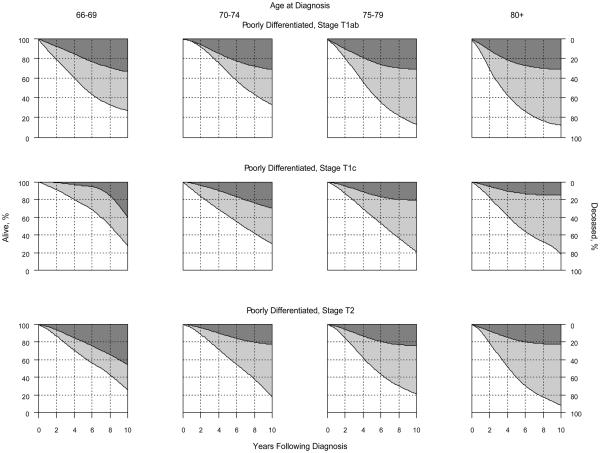
Figure.
Competing Risk of Death by Age at Diagnosis, Cancer Stage, and Grade†
Panel A: Moderately-Differentiated (Gleason 5-7) Cancer
Panel B: Poorly-Differentiated (Gleason 8-10) Cancer
† Darkly shaded areas represent prostate cancer-specific mortality; lightly shaded areas represent mortality due to competing causes; non-shaded areas represent the probability of being alive. Results for well-differentiated disease are not shown because estimates were unstable due to limited sample sizes.
Results
Baseline characteristics of the study population are summarized in Table 1. Most tumors had Gleason scores of 5-7 (76%) and 4,493 (31%) men had screen detected cancer (T1c). Palpable disease (T2) at diagnosis was present in 42% of the cases, and most men (~70%) did not have significant comorbid conditions. The median age at diagnosis was 78 years and median follow-up was 8.3 years.
Table 1.
Characteristics of 14,516 Men without Initial Attempted Curative Therapy for Clinically Localized (T1/T2) Prostate Cancera
| Characteristics | Result N(%) |
|---|---|
| Age at diagnosis, median (IQR), y | 78 (73 - 82) |
| Follow-up, median (IQR), months | 100 (77 - 137) |
| Black race | 1,577 (10.9) |
| Married at diagnosis | 9,070 (62.5) |
| Urban residence | 12,553 (86.5) |
| Zip code-level income, median (IQR), US $ | 42,924 (33,677–57,315) |
| SEER regions | |
| Northeast | 1,302 (9.0) |
| North-central | 3,930 (27.1) |
| West | 8,807 (60.7) |
| South | 477 (3.3) |
| Cancer grade | |
| Well-differentiated (Gleason 2-4) | 222 (1.5) |
| Moderately-differentiated (Gleason 5-7) | 10,988 (75.7) |
| Poorly-differentiated (Gleason 8-10) | 3,306 (22.8) |
| Clinical stage | |
| T1a, T1b | 3,972 (27.4) |
| T1c | 4,493 (31.0) |
| T2 | 6,051 (41.7) |
| Comorbidity | |
| Charlson comorbidity score 0 | 10,127 (69.8) |
| Charlson comorbidity score 1 | 2,807 (19.3) |
| Charlson comorbidity score ≥2 | 1,582 (10.9) |
| Year of diagnosis | |
| 1992 – 1996 | 4,623 (31.9) |
| 1997 - 2002 | 9,893 (68.2) |
| Use of primary androgen deprivation therapy | 6,041 (41.6) |
| Vital status at last follow-up | |
| Alive as of Dec 31, 2007 | 5,814 (40.1) |
Abbreviations: IQR, interquartile range; SEER, Surveillance, Epidemiology, and End Results.
Data are presented as No. (%) unless otherwise specified. Race was self-determined by the patients. SEER clinical extension information was used to determine cancer stage (T1a, T1b, T1c, T2). Charlson comorbidity score was derived from Medicare claims during the year before prostate cancer diagnosis by using a validated algorithm.13
At the end of the study period, most men were either alive or had died of other causes (Table 2). Among the 222 patients diagnosed with well-differentiated prostate cancer, 15 died of prostate cancer and 133 died of other causes during the first 10 years. Among the 10,988 patients diagnosed with moderately differentiated prostate cancer, 642 died of prostate cancer and 5,005 died of other causes during the first 10 years. Among the 3,306 patients diagnosed with poorly differentiated prostate cancer, 684 died of prostate cancer and 1,652 died of other causes during the first 10 years. With a median age of 78 years at cancer diagnosis, ten-year prostate cancer-specific mortality was 8.3% (95% confidence interval [CI] 4.2% – 12.8%), 9.1% (95% CI 8.3% - 10.1%), and 25.6% (95% CI 23.7% - 28.3%) for men with well-, moderately-, and poorly-differentiated tumors, respectively. The corresponding 10-year risks of dying of causes other than prostate cancer were 59.8% (95% CI 53.2% - 67.8%), 57.2% (95% CI 52.6% - 63.9%) and 56.5% (95% CI 53.6% - 58.8%), respectively. The Figure illustrates the competing risk of death according to age at diagnosis, cancer stage and grade. Results for well-differentiated tumors were not shown because sample sizes were too small for reliable estimates. When the analyses were restricted to men without androgen deprivation therapy within 6 months of cancer diagnosis, the results were comparable or even more favorable than those shown in the Figure.
Survival results in our contemporary PSA era study cohort were more favorable than results previously reported. For example, in the current study, 10-year prostate cancer-specific mortality was 6% (95% confidence interval [CI] 4% - 8%) in the contemporary PSA era (1992-2002) compared to results of previous studies (15% - 23%) in earlier eras (1949-1992) for men aged 65 - 74 years diagnosed with moderately-differentiated disease (Table 3) Improvement in survival among men with older age or poorly-differentiated disease was also observed.
Table 4 summarizes 10-year cumulative risks of various secondary cancer therapies based on the analyses of competing risks. Overall, androgen deprivation therapy (ADT) use was high: about 60 - 83% within 10 years. Forty-one percent of our cohort received androgen deprivation therapy within 6 months of cancer diagnosis; among the remaining cohort members, 28.4% and 45.4% received androgen deprivation therapy for moderately- and poorly differentiated cancer within 10 years. Relatively few patients received chemotherapy (N= 237, 1.6%), or underwent spinal surgery or radiation for metastatic disease (N=134, 0.9%). Ten-year cumulative risks of palliative therapy (palliative radiation, chemotherapy, or spinal surgery or radiation) were 4.1% and 6.9% among older patients (≥75 years) with non-screen detected moderately- and poorly-differentiated cancer, respectively. Younger age was associated with higher use of palliative therapy (P<0.001). Outcomes were similar when analyses were restricted to relatively healthy men (comorbidity score zero).
Discussion
The appropriate treatment of men with clinically localized prostate cancer diagnosed in the PSA era has been a subject of great controversy. For the majority of older men (aged ≥65 years) who are diagnosed with localized disease, randomized clinical trial data have not been able to demonstrate a survival benefit for surgery,3 or any other approach compared to conservative management.21 Despite these data raising the possibility that conservative management may be a reasonable treatment choice, little data exist that describe outcomes following conservative management in the contemporary PSA era.22-25
To address this lack of data, we examined 14,516 men with localized T1/T2 prostate cancer without initial attempted curative therapy and found that ten-year prostate cancer specific mortality declined by more than 60% compared to previous studies (Table 3). We also found that for the majority of men managed without initial attempted curative therapy (ie., those >65 years old with moderately-differentiated cancer), only a limited proportion (4-11%) used palliative radiation therapy, chemotherapy, or treatments for spinal cord compression over the ensuing 10 years following diagnosis. In contrast, use of androgen deprivation therapy was quite common.
The substantial improvement in survival we observed in our study compared with previous reports10, 11, 26 might be explained, in part, by additional lead time, overdiagnosis related to PSA testing, or grade migration, among other factors.27 PSA testing identifies disease 6-13 years before it presents clinically.28 Contemporary patients identified through such testing would be expected to live at least 6-13 years longer because of this lead time.28 In addition, previously documented systematic upgrading of modern tumors compared to earlier eras29 makes more recently graded tumors appear to have a more benign course, resulting in longer survivals.27 Finally, it is also possible that advancements in medical care might have led to improved outcomes. The net overall effect is that outcomes following conservative management are now significantly better than those reported in previous eras; therefore, physicians and their patients may need to reconsider this management option, particularly in light of randomized trial data from the pre-PSA era suggesting little if any benefit to more aggressive intervention.
Our documentation of a major improvement in conservative management outcomes is important, not only because it provides updated information for physicians and patients, but also because the results may color the interpretation of maturing randomized clinical trials. For example, in the widely cited Scandinavian randomized study of prostatectomy vs. conservative management, disease-specific survival in the conservative management arm (~85% at 10-years)3 was found to be very similar to that documented in several observational cohort studies of conservative management from the same pre- or early PSA era (~87%,26 ~86%,30 and ~83%31). The use of radical prostatectomy resulted in a ~5.3% absolute percentage point increase (to ~90%) in cancer-specific survival in this study.
The results of our study, however, demonstrated that 10-year cancer-specific survival with conservative management has now increased from ~83-87% in the pre- or early PSA era to ~94% in the PSA era, which is now beyond the ~90% 10-year cancer-specific survival rate for a similar population of men treated with prostatectomy in the pre- or early PSA era Scandinavian trial (ie., those aged 66-74 years with moderately-differentiated cancer) (Table 3). The room available for additional improvement when 10-year cancer-specific survival is already ~94% with conservative management may be limited, and the absolute benefit of surgery in the Scandinavian trial may be difficult to reproduce in similar studies like the U.S. Prostate Cancer Intervention Versus Observation Trial (PIVOT), where most men were diagnosed through PSA screening.32 Nonetheless, the only true way to determine if this will be the case is to await the results of contemporary randomized studies like PIVOT, and it is not our intent to suggest that benefit for the majority of men with localized prostate cancer (ie., those ≥65 years old) can be excluded based on our results and those of the Scandinavian study.3 On the other hand, for men with poorly-differentiated disease managed conservatively, the 10-year cancer-specific survival was substantially lower (~58-74%) than reported in the Scandinavian trial and, therefore, the potential for benefit with attempted curative therapy may be greater in these men.
Our study had some limitations. The men in our study, like the majority of prostate cancer patients, were ≥65 years of age and our results might not apply to younger patients. In addition, we were limited to data available in the SEER registries. For example, PSA values at diagnosis were not collected during the study period and Gleason 5, 6, and 7 tumors were grouped together as moderately-differentiated disease. Consequently, the results for moderately-differentiated disease as a whole may overestimate survival for Gleason 7 tumors and underestimate survival for Gleason 5 tumors. In addition, there may be unmeasured patient or disease characteristics beyond age, tumor stage and tumor grade, unique to patients selecting conservative management that impact results so that they may not apply to patients with more aggressive disease characteristics not captured in the database. Another limitation is the length of follow-up. Because of the protracted nature of the disease, longer follow-up data are needed for men with a life expectancy greater than 10 years.
Finally, as in other observational and randomized trials and studies, the secondary endpoints were supportive, exploratory, and less robust than the primary endpoints. For example, although the Medicare database is generally able to capture the initiation of secondary therapy accurately (surgery, radiation, ADT, and chemotherapy, etc.), the actual accuracy may vary somewhat from procedure to procedure and, therefore, comparisons between rates of secondary therapies may be less exact.12, 16, 33 In addition, the Medicare database does not consistently capture the use of oral agents, such as the antiandrogens, that may be used for ADT. In the case of antiandrogens, however, data from the CaPSURE database34 have shown that the use of antiandrogens as sole treatment for localized prostate cancer is uncommon (~2%) and, therefore, it is unlikely that the use of hormonal therapy would be significantly underestimated. Irrespective of the strengths and limitations of each secondary endpoint, however, it is important to recognize that the purpose of these additional analyses was to provide additional insight and context for the interpretation of the primary endpoints of cancer-specific and overall survival, and not necessarily for these endpoints to stand alone as definitive conclusions.
In addition to the study’s limitations, there were also some important strengths. The study was population-based, and all-inclusive in the regions studied, rather than limited to specific institutions or networks. Consequently, the results are more likely to apply more broadly. In addition, the study was much larger than previous studies and, therefore, provided more stable estimates on which to base future clinical decisions. In particular, conservative management is often an especially relevant treatment choice for men aged ≥75 years. However, data on this older population are rare and this group is often excluded or underrepresented in randomized trials. Our study, with more than 10,000 men aged ≥75 years, provided crucial information to fill this important knowledge gap.
In summary, our findings suggest that outcomes following conservative management of contemporary PSA era patients with localized prostate cancer are substantially more favorable than in studies from earlier eras, and patients with well- or moderately-differentiated disease managed conservatively are generally even more likely to die of causes other than prostate cancer.9, 10, 26, 30 Considering favorable 10-year outcomes following conservative management, men with a life expectancy less than 10-years may wish to consider an active surveillance/watchful waiting protocol as an alternative to immediate attempted curative therapy.10, 25, 26, 30, 35
ACKNOWLEDGEMENTS
The authors acknowledge the efforts of the Applied Research Branch, Division of Cancer Prevention and Population Science, National Cancer Institute (NCI), the Office of Information Services, and the Office of Strategic Planning, Center for Medicare and Medicaid Services (CMS); Information Management Services (IMS), Inc.,and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The study was supported by the following grants and awards: award DAMD17-01-1-0755 from the US Army Medical Research Acquisition Activity, Fort Detrick, MD, DOD award W81XWG-05-1-0235, Ohl Foundation, NCI grant # R01 CA116399, and CINJ core grant NCI CA-72720-10. We thank Thanusha Puvananayagam, MPH, an assistant staff with salary support from the Cancer Institute of New Jersey, for outstanding administrative and technical assistance.
ROLE OF SPONSORS:
The Department of Defense, Ohl Foundation, and National Cancer Institute provided funding for this study. The Department of Defense and Ohl Foundation did not play any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The National Cancer Institute did not play any role in the design and conduct of the study; analysis and interpretation of the data; and preparation of the manuscript, but did collect and manage the data as well as review and approve the manuscript.
Footnotes
Publisher's Disclaimer: This study utilizes the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The content of the information does not necessarily reflect the position or the policy of the Government or the employers, and no official endorsement should be inferred.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178:S14–9. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352:1977–84. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Broering JM, Litwin MS, et al. The contemporary management of prostate cancer in the United States: lessons from the cancer of the prostate strategic urologic research endeavor (CapSURE), a national disease registry. J Urol. 2004;171:1393–401. doi: 10.1097/01.ju.0000107247.81471.06. [DOI] [PubMed] [Google Scholar]
- 5.Walsh PC, DeWeese TL, Eisenberger MA. Clinical practice. Localized prostate cancer. N Engl J Med. 2007;357:2696–705. doi: 10.1056/NEJMcp0706784. [DOI] [PubMed] [Google Scholar]
- 6.Ries L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2004. National Cancer Institute. 2007 [Google Scholar]
- 7.Cooperberg MR, Grossfeld GD, Lubeck DP, Carroll PR. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003;95:981–9. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardie C, Parker C, Norman A, et al. Early outcomes of active surveillance for localized prostate cancer. BJU Int. 2005;95:956–60. doi: 10.1111/j.1464-410X.2005.05446.x. [DOI] [PubMed] [Google Scholar]
- 9.Kattan MW, Cuzick J, Fisher G, et al. Nomogram incorporating PSA level to predict cancer-specific survival for men with clinically localized prostate cancer managed without curative intent. Cancer. 2008;112:69–74. doi: 10.1002/cncr.23106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293:2095–101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 11.Johansson J-E, Andren O, Andersson S-O, et al. Natural History of Early, Localized Prostate Cancer. JAMA. 2004;291:2713–9. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 12.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 13.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 14.Albertsen PC, Walters S, Hanley JA. A comparison of cause of death determination in men previously diagnosed with prostate cancer who died in 1985 or 1995. J Urol. 2000;163:519–23. [PubMed] [Google Scholar]
- 15.Penson DF, Albertsen PC, Nelson PS, Barry M, Stanford JL. Determining cause of death in prostate cancer: are death certificates valid? J Natl Cancer Inst. 2001;93:1822–3. doi: 10.1093/jnci/93.23.1822. [DOI] [PubMed] [Google Scholar]
- 16.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40:IV-55-61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 17.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–64. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 18.Oleynick JU, Albertsen PC, Barry M, Walker-Corkery E, Yao S, Lu-Yao GL. Utility of the SEER-Medicare data to identify medical androgen deprivation therapy. 2006 [Google Scholar]
- 19.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 20.Mueller H, Wang J. Hazard rates estimation under random censoring with varying kernels and bandwidths. Biometrics. 1994;50:61–76. [PubMed] [Google Scholar]
- 21.Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic Review: The Comparative Effectiveness and Harms of Treatments for Clinically Localized Prostate Cancer. Ann Intern Med. 2008 doi: 10.7326/0003-4819-148-6-200803180-00209. 0000605-200803180-00209. [DOI] [PubMed] [Google Scholar]
- 22.Dall'era MA, Cooperberg MR, Chan JM, et al. Active surveillance for early-stage prostate cancer: review of the current literature. Cancer. 2008;112:1650–9. doi: 10.1002/cncr.23373. [DOI] [PubMed] [Google Scholar]
- 23.Warlick C, Trock BJ, Landis P, Epstein JI, Carter HB. Delayed Versus Immediate Surgical Intervention and Prostate Cancer Outcome. J Natl Cancer Inst. 2006;98:355–7. doi: 10.1093/jnci/djj072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel MI, DeConcini DT, Lopez-Corona E, Ohori M, Wheeler T, Scardino PT. An analysis of men with clinically localized prostate cancer who deferred definitive therapy. J Urol. 2004;171:1520–4. doi: 10.1097/01.ju.0000118224.54949.78. [DOI] [PubMed] [Google Scholar]
- 25.Klotz L. Active surveillance for favorable risk prostate cancer: what are the results, and how safe is it? Semin Radiat Oncol. 2008;18:2–6. doi: 10.1016/j.semradonc.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Chodak GW, Thisted RA, Gerber GS, et al. Results of Conservative Management of Clinically Localized Prostate Cancer. N Engl J Med. 1994;330:242–8. doi: 10.1056/NEJM199401273300403. [DOI] [PubMed] [Google Scholar]
- 27.Jani AB, Johnstone PA, Liauw SL, Master VA, Brawley OW. Age and grade trends in prostate cancer (1974-2003): a Surveillance, Epidemiology, and End Results Registry analysis. Am J Clin Oncol. 2008;31:375–8. doi: 10.1097/COC.0b013e3181637384. [DOI] [PubMed] [Google Scholar]
- 28.Draisma G, Boer R, Otto SJ, et al. Lead Times and Overdetection Due to Prostate-Specific Antigen Screening: Estimates From the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95:868–78. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 29.Albertsen PC, Hanley JA, Barrows GH, et al. Prostate Cancer and the Will Rogers Phenomenon. J Natl Cancer Inst. 2005;97:1248–53. doi: 10.1093/jnci/dji248. [DOI] [PubMed] [Google Scholar]
- 30.Johansson JE, Holmberg L, Johansson S, Bergstrom R, Adami HO. Fifteen-year survival in prostate cancer. A prospective, population-based study in Sweden. JAMA. 1997;277:467–71. [PubMed] [Google Scholar]
- 31.Adolfsson J, Steineck G, 'whitmore WF. Recent results of management of paplable clinically localized prostate cancer. Cancer. 1993;72:310–22. doi: 10.1002/1097-0142(19930715)72:2<310::aid-cncr2820720203>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 32.Wilt TJ, Brawer MK, Barry MJ, et al. The Prostate cancer Intervention Versus Observation Trial:VA/NCI/AHRQ Cooperative Studies Program #407 (PIVOT): Design and baseline results of a randomized controlled trial comparing radical prostatectomy to watchful waiting for men with clinically localized prostate cancer. Contemp Clin Trials. 2008 doi: 10.1016/j.cct.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Virnig BA, Warren JL, Cooper GS, Klabunde CN, Schussler N, Freeman J. Studying radiation therapy using SEER-Medicare-linked data. Med Care. 2002;40:IV-49-54. doi: 10.1097/00005650-200208001-00007. [DOI] [PubMed] [Google Scholar]
- 34.Kawakami J, Cowan JE, Elkin EP, Latini DM, Duchane J, Carroll PR. Androgen-deprivation therapy as primary treatment for localized prostate cancer: data from Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) Cancer. 2006;106:1708–14. doi: 10.1002/cncr.21799. [DOI] [PubMed] [Google Scholar]
- 35.Carter HB, Kettermann A, Warlick C, et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J Urol. 2007;178:2359–64. doi: 10.1016/j.juro.2007.08.039. discussion 64-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adolfsson J, Carstensen J, Lowhagen T. Deferred treatment in clinically localised prostatic carcinoma. British Journal of Urology. 1992;69:183–7. doi: 10.1111/j.1464-410x.1992.tb15493.x. [DOI] [PubMed] [Google Scholar]
- 37.Johansson JE, Adami HO, Andersson SO, Bergstrom R, Holmberg L, Krusemo UB. High 10-year survival rate in patients with early, untreated prostatic cancer. JAMA. 1992;267:2191–6. [PubMed] [Google Scholar]
- 38.Lu-Yao GL, Yao SL. Population-based study of long-term survival in patients with clinically localised prostate cancer [see comments] Lancet. 1997;349:906–10. doi: 10.1016/S0140-6736(96)09380-4. [DOI] [PubMed] [Google Scholar]