Abstract
Proteasome inhibitors are emerging as a new class of cancer therapeutics, and bortezomib has shown promise in the treatment of multiple myeloma and mantle cell lymphoma. However, bortezomib has failed to have an effect in preclinical models of glioma. NPI-0052 is a new generation of proteasome inhibitors with increased potency and strong inhibition of all three catalytic activities of the 26S proteasome. In this article, we test the antitumor efficacy of NPI-0052 against glioma, as a single agent and in combination with temozolomide and radiation using five different glioma lines. The intrinsic radiation sensitivities differed for all the lines and correlated with their PTEN expression status. In vitro, NPI-0052 showed a dose-dependent toxicity, and its combination with temozolomide resulted in radiosensitization of only the cell lines with a mutated p53. The effect of NPI-0052 as a single agent on glioma xenografts in vivo was only modest in controlling tumor growth, and it failed to radiosensitize the glioma xenografts to fractionated radiation. We conclude that NPI-0052 is not a suitable drug for the treatment of malignant gliomas despite its efficacy in other cancer types.
Introduction
Malignant gliomas are among the most aggressive solid tumors in humans. After debulking surgery and radiotherapy, the median survival is approximately 12 months. The only drug shown to be effective in combination with radiotherapy is temozolomide. However, when combined with radiotherapy after surgery, the increase in median survival is only 2 months [1]. These disappointing results have motivated the search for novel treatment options and drug combinations.
The 26S proteasome is a multicatalytic protease complex with at least three distinct proteolytic activities (chymotryptic, tryptic, caspase-like). It degrades almost all short-lived and almost all long-lived proteins in eukaryote cells in an ubiqutin-dependent fashion [2–4]. This protease is a key regulatory hub for many signal transduction pathways altered in cancer and is involved in cell death and DNA repair [5]. Consequently, specific inhibitors of this protease were introduced into cancer treatments, and bortezomib, a specific inhibitor of the chymotryptic activity of the proteasome has been approved for the treatment of patients with multiple myeloma or mantle cell lymphoma [6,7]. Although bortezomib has excellent anti-tumor activity in preclinical in vitro and in vivo models, it has shown poor clinical efficacy against solid cancers as a single agent or in combination with established chemotherapeutic drugs [8–10]. A recent phase 1 trial in glioma combining bortezomib with temozolomide and radiation in glioma did not show any additional benefit for patients treated with bortezomib [10].
NPI-0052 (salinosporamide A) is a novel proteasome inhibitor [11] that targets all three activities of the 26S proteasome [12], thus making it a more effective inducer of cancer cell death than bortezomib [13]. Therefore, clinical trials using NPI-0052 have been initiated for the treatment of multiple myeloma patients [14]. Given that bortezomib has failed to demonstrate a beneficial effect in gliomas [10], we tested if the enhanced potency of NPI-0052 translated into antitumor activity, as a single agent or in combination with temozolomide and radiation, the current standard therapy for this disease [15]. We hypothesized that NPI-0052 combined with radiation and temozolomide would be effective against glioma cells in vitro and in vivo. However, although NPI-0052 induced cell death in vitro, it failed to consistently radiosensitize glioma cells in vitro and did not synergize with radiation in vivo.
Materials and Methods
Cell Culture
The U87MG glioma cell line and the GBM-ES and GBM-RW primary glioma cultures were a kind gift from Dr P. Michel (Department of Pathology, UCLA). The GBM-2345 and GBM-177 primary glioma lines were a kind gift from Dr Kornblum (Department of Molecular and Medical Pharmacology, David Geffen School of Medicine at UCLA). All the cells were cultured in log-growth phase in Dulbecco's modified Eagle medium (DMEM)-F12 (Invitrogen, Grand Island, NY) (supplemented with 10% heat-inactivated fetal bovine serum (Sigma, St. Louis, MO), penicillin (100 U/ml), and streptomycin (100 µg/ml) cocktail) and were grown at 37°C in a humidified atmosphere (5% CO2). To obtain single-cell suspensions for further assays, cells were dissociated with trypsin-EDTA (Invitrogen), pelleted by centrifugation, and resuspended in DMEM-F12 medium and replated.
Western Blot Analysis
The proteins were separated by SDS-PAGE and then transferred to polyvinylidene fluoride membranes (BioRad, Hercules, CA). Blots were blocked and then probed with antibodies against epidermal growth factor receptor (EGFR, 1:1000 dilution; Abcam, Cambridge, MA), p53 (1:200 dilution; EMD Biosciences, Gibbstown, NJ), O6-methylguanine-DNA methyltransferase (MGMT, 1:500 dilution; Abcam), and phosphatase and tensin homolog (PTEN, 1:500 dilution; Abcam). After washing, the blots were incubated with horse-radish peroxidase-conjugated secondary antibodies (GE Healthcare, Piscataway, NJ) and visualized by ECL Plus Membrane Blot Analysis detection system (GE Healthcare).
Clonogenic Survival Assays
For clonogenic assays, cells derived from monolayer cultures were treated with medium containing either 100 nM NPI-0052 (kind gift of Michael Palladino, Nereus Pharmaceuticals), 10 µM temozolomide (Sigma), or a combination of both, and placed at 37°C in a humidified atmosphere (5% CO2) for 3 hours. After 3 hours, the medium was removed, and the cells were enzymatically dissociated with trypsin-EDTA to produce a single-cell suspension. The cells were counted, diluted into the desired seeding concentration, and immediately irradiated at room temperature with a cesium (Cs) 137 laboratory irradiator (Mark I, JL Shephard, San Fernando, CA) at a dose rate of 4.95 Gy/min for the time required to generate a dose curve of 0, 2, 4, 6, and 8 Gy. Corresponding controls were sham-irradiated. Colony-forming assays were performed immediately after irradiation by plating cells into triplicate 100-mm culture dishes. After 10 to 14 days, cells were fixed with 75% ethanol and stained with 1% crystal violet, and colonies containing more than 50 cells were counted. To generate a radiation survival curve, the surviving fraction at each radiation dose was normalized to that of the sham-irradiated control, and curves were fitted using a linear-quadratic model (surviving fraction = e(-αdose - βdose2) [24]. Three independent experiments were performed, each in triplicates.
Methylthiazolyldiphenyl-Tetrazolium Bromide Assay
A total of 1000 cells derived from the U87MG cell line or each of the primary GBM lines were plated into white 96-well plates in 50 µl of DMEM-F12 medium per well. The cells were allowed to adhere overnight. The next day, the proteasome inhibitor NPI-0052, dissolved in DMSO and diluted in DMEM-F12, was added at the indicated concentrations. The control wells were treated with DMEM-F12 medium with DMSO. After 7 to 8 days of incubation with the drug, 20 µl of methylthiazolyldiphenyl-tetrazolium bromide (MTT) reagent (5 mg/ml in PBS; Sigma) was added to each well. Four hours later, 50 µl of 20% SDS/0.01% HCl solution was added to each well. The absorbance at 570 nm was measured immediately using a fluorescence plate reader (SpectraMax M5).
Animals
Nude (nu/nu), 6- to 8-week-old female mice originally from The Jackson Laboratories (Bar Harbor, ME), were rederived, bred, and maintained in a defined flora environment in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited Animal Facilities, of the Department of Radiation Oncology, University of California (Los Angeles, CA), in accordance with all local and national guidelines for the care of animals. U87MG cells derived from monolayer cultures were injected subcutaneously into the thighs of nude mice (106 cells per inoculum). The tumor growth was monitored on a weekly basis, and when the average tumor size reached approximately 100 mm3, the mice were randomly assigned to four treatment groups: nontreated (NT), fractionated radiation (5 x 3 Gy), proteasome inhibitor (NPI-0052), and combination treatment (5 x 3 Gy + NPI-0052). The mice treated with NPI-0052 were administered NPI-0052 (0.25 mg/kg) or vehicle alone (1% DMSO in PBS) intraperitoneally 3 hours before radiation treatment and on days 1, 3, and 5 of radiation treatment. The groups treated with fractionated radiation were irradiated with 3 Gy for five consecutive days using a cobalt 60 source (dose rate, 0.6 Gy/min). The thighs of anesthetized mice bearing the tumor were placed in a 5 x 5-cm radiation field of the cobalt 60 source while the rest of the body was shielded.
Results
NPI-0052 Induces Cell Death in Established and Primary Glioma Cell Lines
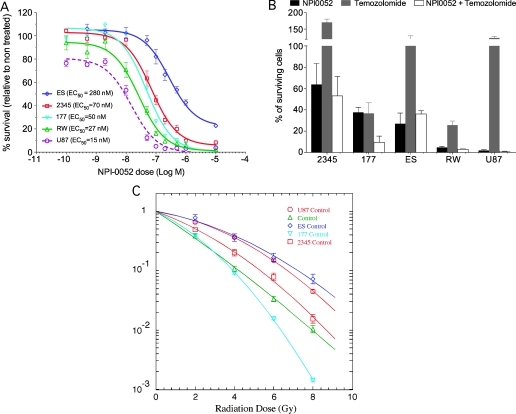
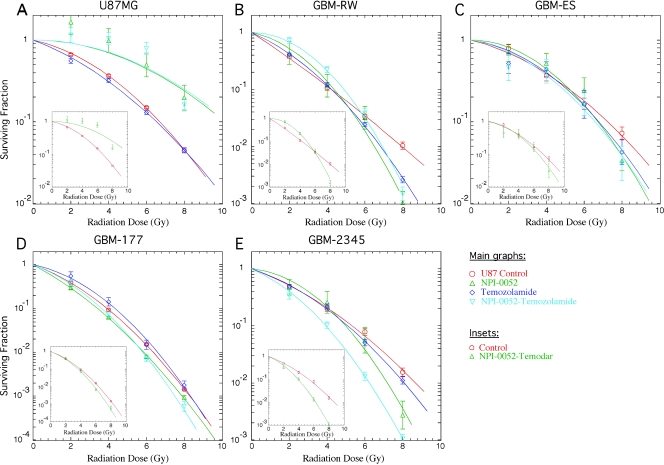
NPI-0052 showed a dose-dependent killing of one established glioma cell line, U87MG, and four primary glioma lines, GBM-177, GBM-2345, GBM-ES, and GBM-RW, in vitro (Figure 1A). In MTTassays, all of the cell lines had comparable half maximal effective concentration (EC50) values ranging from 15 (U87MG) to 70 nM (GBM-2345), except for the GBM-ES glioma line, which had a significantly higher EC50 (280 nM). Because radiation is the primary treatment modality for GBM, we wanted to test the effect of NPI-0052 on glioma lines in combination with radiation, and given that postoperative radiotherapy in GBM patients is often accompanied and followed by treatment with temozolomide, we explored a potential synergizing effect of NPI-0052 with temozolomide and radiation. First, all the cell lines were treated for 3 hours with 100 nM NPI-0052 or 10 µM temozolomide, as a single agent or in combination, and clonogenic survival assays were performed. Also, to characterize their relative intrinsic radiosensitivity, all the cell lines were irradiated with single doses of radiation, and clonogenic survival assays were performed. The acute toxicity to the 3-hour treatment with NPI-0052 and temozolomide, as well as the relative radiosensitivity of all the cell lines tested, is shown in Figure 1, B and C. Two cell lines (GBM-177 and GBM-RW) were sensitive to temozolomide in the acute toxicity assays, where killing by NPI-0052 dominated (Figure 1B). Sensitivity to temozolomide seemed to correlate with the higher intrinsic radiosensitivity of these cell lines (Figure 1, B and C). Combining temozolomide and/or NPI-0052 treatments with irradiation showed that, as single agents, they had little or no radiosensitization effect (Figure 2, A–E); however, there seemed to be an inverse relationship between the level of sensitivity to NPI-0052 and the response to combined treatment (Figure 2, A–E). The combination treatment resulted in a significant radiosensitizing effect of GBM-2345 cell line (Student's paired t test: at 2 Gy, P = .01; at 4 Gy, P < .01) and a radioprotective effect on U87MG (Student's paired t test: at 2 Gy, P < .01; at 4 Gy, P < .01; Figure 2, A and E). The other cell lines did not reach statistical significance for the radiosensitizing effect of the combination treatment (Figure 2, B–D). However, there seemed to be a trend between the acute toxicity of NPI-0052 treatment and the radiosensitizing effect of the combination treatment—the lower the sensitivity of a cell line to the NPI-0052 treatment, the higher the radiosensitizing effect of the combination treatment (Figures 1, A and B, and 2, A–E, inserts).
Figure 1.
NPI-0052 and temozolomide toxicity to glioma cells. (A) U87MG established cell line and four primary glioma cell lines (GBM-ES, GBM-2345, GBM-177, GBM-RW) were grown as a monolayer in serum conditions. On the day of the experiment, the cells were seeded into 96-well plates at 1000 cells per well. The cells were allowed to adhere overnight. The next day, the cells were treated with the indicated concentrations of NPI-0052. At 7 to 8 days later, MTT assays were performed. (B and C) All four cell lines were treated with either 100 nM NPI-0052 or 10 µM temozolomide 3 hours before being irradiated. The drug was then washed, cells were removed, irradiated with the indicated doses, and plated at clonal densities. Approximately 3 weeks later, cell colonies were stained and counted. Acute toxicity of NPI-0052 and temozolomide for all the cell lines was determined (B), and their relative intrinsic radiation sensitivity was compared (C).
Figure 2.
Effect of NPI-0052 and temozolomide treatment on radiation sensitivity of glioma cells. (A–E) Each cell line was grown as a monolayer in serum conditions. On the day of the experiment, each cell line was treated with 100 nM of NPI-0052 and/or 10 µM temozolomide 3 hours before radiation treatment. Cells were then trypsinized, counted, irradiated with the indicated doses, and plated at the appropriate numbers for clonogenic survival assays.
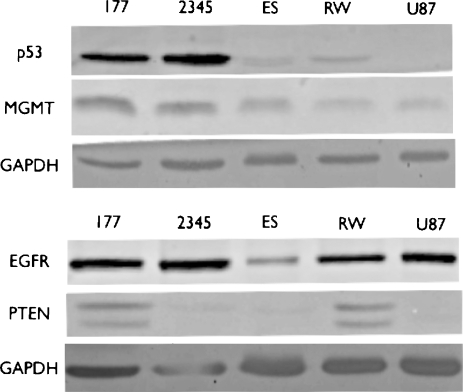
To investigate if the variable response of these cell lines to the different treatments is reflected in their molecular phenotype, we performed Western blots to analyze the expression of EGFR, PTEN, MGMT, and p53 proteins in each cell line (Figure 3), which reflect the key pathways altered in glioma [16]. MGMT protein levels were comparable in all the cell lines, thus different sensitivities to temozolomide treatment in these cell lines were not reflected by MGMT protein levels, neither did they correlate with EGFR expression; the two cell lines (GBM-177 and GBM-RW) that were sensitive to temozolomide (Figure 1B) did express PTEN (Figure 3). These are the most radiosensitive cell lines.
Figure 3.
Molecular profile of the glioma cell lines. The established glioma cell line U87MG and the four primary glioma lines, GBM-177, GBM-2345, GBM-ES, and GBM-RW, were grown as monolayers in serum conditions. The cells were removed, lysed, and analyzed for the expression of p53, MGMT, EGFR, and PTEN proteins through Western blots.
There seemed to be a correlative trend between the p53 status of the glioma cell line (Figure 3) and the radiosensitizing effect of the combination treatment. The cell lines with wtp53 protein (GBM-ES, GBM-RW, and U87MG) were not affected by the combination treatment or, in the case of U87MG, were actually strongly radioprotected by this treatment (Figures 2, A–C, and 3). In contrast, the GBM-2345 cell line, which contained higher levels of mutated p53 protein, was significantly radiosensitized by the combination treatment (Student's paired t test: at 2 Gy, P = .01; at 4 Gy, P < .01). The GBM-177 cell line, which showed lower levels of the mutant p53 protein (compared with GBM-2345), did not reach statistical significance for the radiosensitizing effect of the combination treatment; however, there seemed to be a trend toward radiosensitization (Figures 2, D and E, and 3).
NPI-0052 Does Not Radiosensitize Glioma Xenografts In Vivo
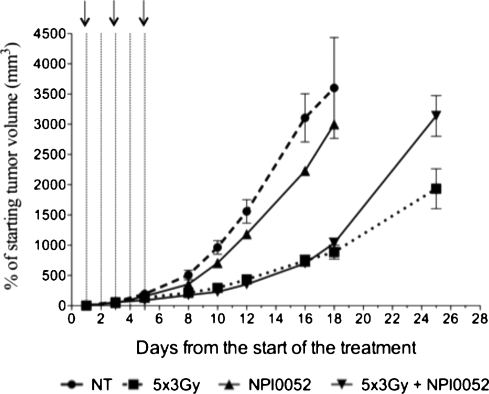
The effect of NPI-0052 in vivo as a single agent or in combination with fractionated radiation (5 x 3 Gy) was determined using U87MG xenografts on immunodeficient mice. This cell line was chosen because treatment with NPI-0052, as a single agent or in combination with temozolomide, resulted in significant radioprotection (Figure 2A). To be able to continuously monitor tumor growth, we implanted U87MG cells (1 x 106 cells/tumor) subcutaneously on the thighs of nude mice. The tumor-bearing mice were treated intra-peritoneally (i.p.) with 0.25 mg/kg of NPI-0052 [17] 3 hours before radiation treatment. Radiation treatment was administered in doses of 3 Gy for five consecutive days, whereas the NPI-0052 was administered on days 1, 3, and 5 of radiation treatment. When NPI-0052 was used as a single agent, it delayed U87MG glioma tumor growth only moderately (Figure 4). The combination of NPI-0052 with fractionated radiation initially had no effect on tumor growth when compared with fractionated radiation alone; however, approximately 2 weeks after the end of the treatment, the group treated with the combination regimen did worse than the group treated with fractionated radiation alone, possibly indicating a radioprotective effect of the NPI-0052 in this glioma xenograft model (Figure 4). The treatment dose of 0.25 mg/kg of NPI-0052 resulted in a significant toxicity in vivo; however, the mice recovered quickly after the administration of the three NPI-0052 doses (data not shown). It should be noted that the combination treatment with NPI-0052 and temozolomide was too toxic to the mice (data not shown); therefore, this combination treatment was not tested in combination with fractionated radiation.
Figure 4.
In vivo treatment of U87MG xenografts with NPI-0052 and radiation. Nude mice with U87MG sub-c tumors were treated with fractionated radiation (5 x 3 Gy). Three hours before radiation treatment, NPI-0052 was administered at 0.25 mg/kg on days 1, 3, and 5 of the radiation treatment (black arrows). Doted lines denote fractionated radiation schedule (each line symbolizes a dose of 3 Gy). This is a representative of three independent experiments (n = 5).
We first used the sub-c glioma xenograft model where the blood-brain barrier does not represent an obstacle to the drug uptake by the glioma cells. Given the lack of therapeutic effect of NPI-0052 alone, or in combination with fractionated radiation in this model, it did not justify the confirmation of these results in an orthotopic glioma model where the blood-brain barrier could still be a potential concern.
Discussion
We previously reported that proteasome inhibitors (MG132 and bortezomib) effectively induced cell death in a wide variety of preclinical cancer models and that the surviving cells were sensitized to ionizing radiation [18–22]. Surprisingly, bortezomib, another inhibitor of the 26S proteasome, has so far not shown significant clinical antitumor activity in solid cancers as a single agent or in combination with other chemotherapeutic drugs [23–25] and failed to synergize with radiation and temozolomide in patients with glioma [10].
Treatment of four primary and one established glioma lines with NPI-0052 resulted in a dose-dependent induction of cell death with the primary GBM lines (GBM-177, GBM-2345, GBM-ES,GBM-RW) possessing higher EC50 values for NPI-0052 than the established glioma cell line (U87MG; Figure 1A). A 3-hour treatment with the drug resulted in a significant reduction in clonogenic survival of all the cell lines tested (Figure 1B). Overall, the extent of cell killing in the four primary cell lines and U87MG cells compared well to the effect of bortezomib in other glioma cell lines [26]. The intrinsic radiation sensitivity of all primary cell lines in vitro varied approximately two-fold (assessed at 10% survival; Figure 1C). Temozolomide treatment only affected the clonogenic survival of GBM-177 and GBM-RW-primary glioma lines (Figure 1B), which did not differ in MGMT expression but differed in p53 status and expressed PTEN protein. This was in agreement with a previous study reporting that the effect of temozolomide on GBM cells was not determined by the MGMT status of the cell alone [27]. Furthermore, a recent report demonstrating an enrichment of the therapy-resistant side population of cells by temozolomide [28] does not explain our findings because the interval between drug treatment and plating of the cells was too short to allow for any selection but was in agreement with our observation that PTEN-deleted GBMs were insensitive to temozolomide. Interestingly, the two temozolomide-sensitive cell lines were also characterized by the lowest relative intrinsic radiation resistance compared with the other cell lines, with GBM-ES and U87MG being the most radiation resistant cell lines. GBM-ES and U87MG cells showed a shoulder of the survival curve, which indicated an increased ability to repair DNA damage. PTEN protein expression levels also correlated with the relative radioresistance of these cell lines. The two intrinsically radiation-sensitive cell lines, GBM-177 and GBM-RW, expressed PTEN protein, whereas the other three cell lines did not. This is in agreement with other reports demonstrating that loss of PTEN expression correlates with radioresistance [29,30].
Regardless of their intrinsic radiosensitivity, p53, MGMT, or PTEN status, temozolomide did not sensitize any of the GMB cell lines to radiation. This was in disagreement with previous studies showing no radiosensitization of GBM cells by temozolomide [27,31]. In these studies, cells were exposed to temozolomide for long periods (24–96 hours), whereas we pretreated the cells with temozolomide for only 3 hours before irradiation. Therefore, it is possible that longer incubation with temozolomide would also radiosensitize the GBM cell lines used in our study. However, given the short half-life of temozolomide of 1.9 hours [32], we felt that our approach was closer to the clinical situation in which temozolomide plasma level will peak after approximately an hour [32].
In clonogenic survival assays, U87MG cells were protected from radiation by NPI-0052, whereas this drug had only little, if any, effect on the radiosensitivity of all other GBM cell lines. The mechanisms by which proteasome inhibitors can sensitize cancer cells to radiation are still not well understood. However, the extent of sensitization or even protection of cells by proteasome inhibitors from cytotoxic agents may depend on the sequence of application [33] and the microenvironment [19].
Combination of NPI-0052 and temozolomide treatment failed also to radiosensitize U87MG, GBM-ES, GBM-RW, or GBM-177 cells, but did radiosensitize GBM-2345 cells (Figure 2, A–E). GBM-2345 cells have mutated p53 protein and may thus not be arrested in the G1 phase of the cell cycle where nonhomologous end joining dominates as DNA double-strand repair mechanism. Treatment with temozolomide inhibits homologous recombination and thus may only be effective if a cell relies on homologous recombination to repair DNA damage [34]. In contrast, U87MG, GBM-RW, and GBM-ES have a WT p53 and are able to arrest in G1, perform nonhomologous end joining, and repair their DNA damage, and therefore, temozolomide is ineffective in these cells. Interestingly, GBM-177 cells, which also have mutated p53, but express PTEN protein, were also not sensitized by the combination treatment.
In vivo, the 0.25-mg/kgNPI-0052 dose chosen to be administered i.p. in these studies is higher than the dose used in other studies where this drug is also administered i.p. Chauhan [17] used a dose of 0.15 mg/kg of NPI-0052 to test its effect in a multiple myeloma model; however, they administered this dose twice a week for 5 weeks, whereas our higher dose of 0.25 mg/kg was only administered a total of three times, thus justifying the use of a higher dose in hopes of a more optimal effect. However, NPI-0052 showed a moderate effect on the growth of U87MG glioma xenografts, and in combination with radiation, it decreased the efficacy of radiation, which reflected the in vitro situation [35]. Overall, our results indicate that whereas gliomas in general were sensitive to NPI-0052.induced cell death, its combination with radiation may have only little effect in a subgroup of gliomas, whereas others may even be radioprotected by this class of drugs. These results and a recent study in which resistance of glioma stem cells to proteasome inhibitors was reported [36], led us to conclude that proteasome inhibitors have only limited, if any, use in the radiotherapy for gliomas and the increase in overall toxicity may not be justified by the survival benefit expected from such combination treatment.
Footnotes
E.V. was supported by a training grant from the National Institute of Biomedical Imaging and BioEngineering (2 T32 EB002101-31).
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 3.Spataro V, Norbury C, Harris AL. The ubiquitin-proteasome pathway in cancer. Br J Cancer. 1998;77:448–455. doi: 10.1038/bjc.1998.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller J, Gordon C. The regulation of proteasome degradation by multiubiquitin chain binding proteins. FEBS Lett. 2005;579:3224–3230. doi: 10.1016/j.febslet.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 5.Pajonk F, McBride WH. The proteasome in cancer biology and treatment. Radiat Res. 2001;156:447–459. doi: 10.1667/0033-7587(2001)156[0447:tpicba]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8:508–513. doi: 10.1634/theoncologist.8-6-508. [DOI] [PubMed] [Google Scholar]
- 7.Kane RC, Dagher R, Farrell A, Ko CW, Sridhara R, Justice R, Pazdur R. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res. 2007;13:5291–5294. doi: 10.1158/1078-0432.CCR-07-0871. [DOI] [PubMed] [Google Scholar]
- 8.Pedeboscq S, L'Azou B, Passagne I, De Giorgi F, Ichas F, Pometan JP, Cambar J. Cytotoxic and apoptotic effects of bortezomib and gefitinib compared to alkylating agents on human glioblastoma cells. J Exp Ther Oncol. 2008;7:99–111. [PubMed] [Google Scholar]
- 9.Labussiere M, Pinel S, Delfortrie S, Plenat F, Chastagner P. Proteasome inhibition by bortezomib does not translate into efficacy on two malignant glioma xenografts. Oncol Rep. 2008;20:1283–1287. [PubMed] [Google Scholar]
- 10.Kubicek GJ, Werner-Wasik M, Machtay M, Mallon G, Myers T, Ramirez M, Andrews D, Curran WJ, Jr, Dicker AP. Phase I trial using proteasome inhibitor bortezomib and concurrent temozolomide and radiotherapy for central nervous system malignancies. Int J Radiat Oncol Biol Phys. 2008;74:433–439. doi: 10.1016/j.ijrobp.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feling RH, Buchanan GO, Mincer TJ, Kauffman CA, Jensen PR, Fenical W. Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew Chem Int Ed Engl. 2003;42:355–357. doi: 10.1002/anie.200390115. [DOI] [PubMed] [Google Scholar]
- 12.Macherla VR, Mitchell SS, Manam RR, Reed KA, Chao TH, Nicholson B, Deyanat-Yazdi G, Mai B, Jensen PR, Fenical WF, et al. Structure-activity relationship studies of salinosporamide A (NPI-0052), a novel marine derived proteasome inhibitor. J Med Chem. 2005;48:3684–3687. doi: 10.1021/jm048995+. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz S, Krupnik Y, Keating M, Chandra J, Palladino M, McConkey D. The proteasome inhibitor NPI-0052 is a more effective inducer of apoptosis than bortezomib in lymphocytes from patients with chronic lymphocytic leukemia. Mol Cancer Ther. 2006;5:1836–1843. doi: 10.1158/1535-7163.MCT-06-0066. [DOI] [PubMed] [Google Scholar]
- 14.Chauhan D, Hideshima T, Anderson KC. A novel proteasome inhibitor NPI-0052 as an anticancer therapy. Br J Cancer. 2006;95:961–965. doi: 10.1038/sj.bjc.6603406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 16.McLendon R, Friedman A, Bigner D, VanMeir EG, Brat DJ, Mastrogianakis GM, Olson JJ, Mikkelsen T, Lehman N, Aldape K, et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chauhan D, Catley L, Li G, Podar K, Hideshima T, Velankar M, Mitsiades C, Mitsiades N, Yasui H, Letai A, et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from bortezomib. Cancer Cell. 2005;8:407–419. doi: 10.1016/j.ccr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Pajonk F, van Ophoven A, Weissenberger C, McBride WH. The proteasome inhibitor MG-132 sensitizes PC-3 prostate cancer cells to ionizing radiation by a DNA-PK-independent mechanism. BMC Cancer. 2005;5:76. doi: 10.1186/1471-2407-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pajonk F, Grumann T, McBride WH. The proteasome inhibitor MG-132 protects hypoxic SiHa cervical carcinomacells after cyclic hypoxia/reoxygenation from ionizing radiation. Neoplasia. 2006;8:1037–1041. doi: 10.1593/neo.06634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pajonk F, Himmelsbach J, Riess K, Sommer A, McBride WH. The human immunodeficiency virus (HIV)-1 protease inhibitor saquinavir inhibits proteasome function and causes apoptosis and radiosensitization in non-HIV-associated human cancer cells. Cancer Res. 2002;62:5230–5235. [PubMed] [Google Scholar]
- 21.Pajonk F, Pajonk K, McBride WH. Apoptosis and radiosensitization of hodgkin cells by proteasome inhibition. Int J Radiat Oncol Biol Phys. 2000;47:1025–1032. doi: 10.1016/s0360-3016(00)00516-2. [DOI] [PubMed] [Google Scholar]
- 22.Pervan M, Pajonk F, Sun J-R, Withers HR, McBride WH. Molecular pathways that modify tumor radiation response. Am J Clin Oncol. 2001;24:481–485. doi: 10.1097/00000421-200110000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Cresta S, Sessa C, Catapano CV, Gallerani E, Passalacqua D, Rinaldi A, Bertoni F, Viganò L, Maur M, Capri G, et al. Phase I study of bortezomib with weekly paclitaxel in patients with advanced solid tumours. Eur J Cancer. 2008;44:1829–1834. doi: 10.1016/j.ejca.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Dudek AZ, Lesniewski-Kmak K, Shehadeh NJ, Pandey ON, Franklin M, Kratzke RA, Greeno EW, Kumar P. Phase I study of bortezomib and cetuximab in patients with solid tumours expressing epidermal growth factor receptor. Br J Cancer. 2009;100:1379–1384. doi: 10.1038/sj.bjc.6605043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieu C, Chow L, Pierson AS, Eckhardt SG, O'Bryant CL, Morrow M, Tran ZV, Wright JJ, Gore L. A phase I study of bortezomib, etoposide and carboplatin in patients with advanced solid tumors refractory to standard therapy. Invest New Drugs. 2009;27:53–62. doi: 10.1007/s10637-008-9154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin D, Zhou H, Kumagai T, Liu G, Ong JM, Black KL, Koeffler HP. Proteasome inhibitor PS-341 causes cell growth arrest and apoptosis in human glioblastoma multiforme (GBM) Oncogene. 2005;24:344–354. doi: 10.1038/sj.onc.1208225. [DOI] [PubMed] [Google Scholar]
- 27.van Nifterik KA, van den Berg J, Stalpers LJ, Lafleur MV, Leenstra S, Slotman BJ, Hulsebos TJ, Sminia P. Differential radiosensitizing potential of temozolomide in MGMT promoter methylated glioblastoma multiforme cell lines. Int J Radiat Oncol Biol Phys. 2007;69:1246–1253. doi: 10.1016/j.ijrobp.2007.07.2366. [DOI] [PubMed] [Google Scholar]
- 28.Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomioka A, Tanaka M, De Velasco MA, Anai S, Takada S, Kushibiki T, Tabata Y, Rosser CJ, Uemura H, Hirao Y. Delivery of PTEN via a novel gene microcapsule sensitizes prostate cancer cells to irradiation. Mol Cancer Ther. 2008;7:1864–1870. doi: 10.1158/1535-7163.MCT-07-2198. [DOI] [PubMed] [Google Scholar]
- 30.Park JK, Jung HY, Park SH, Kang SY, Yi MR, Um HD, Hong SH. Combination of PTEN and gamma-ionizing radiation enhances cell death and G(2)/M arrest through regulation of AKT activity and p21 induction in non-small-cell lung cancer cells. Int J Radiat Oncol Biol Phys. 2008;70:1552–1560. doi: 10.1016/j.ijrobp.2007.11.069. [DOI] [PubMed] [Google Scholar]
- 31.van Rijn J, Heimans JJ, van den Berg J, van der Valk P, Slotman BJ. Survival of human glioma cells treated with various combination of temozolomide and x-rays. Int J Radiat Oncol Biol Phys. 2000;47:779–784. doi: 10.1016/s0360-3016(99)00539-8. [DOI] [PubMed] [Google Scholar]
- 32.Baker SD, Wirth M, Statkevich P, Reidenberg P, Alton K, Sartorius SE, Dugan M, Cutler D, Batra V, Grochow LB, et al. Absorption, metabolism, and excretion of 14C-temozolomide following oral administration to patients with advanced cancer. Clin Cancer Res. 1999;5:309–317. [PubMed] [Google Scholar]
- 33.Mortenson MM, Schlieman MG, Virudachalam S, Bold RJ. Effects of the proteasome inhibitor bortezomib alone and in combination with chemotherapy in the A549 non-small-cell lung cancer cell line. Cancer Chemother Pharmacol. 2004;54:343–353. doi: 10.1007/s00280-004-0811-4. [DOI] [PubMed] [Google Scholar]
- 34.Johannessen TC, Bjerkvig R, Tysnes BB. DNA repair and cancer stem-like cells—potential partners in glioma drug resistance? Cancer Treat Rev. 2008;34:558–567. doi: 10.1016/j.ctrv.2008.03.125. [DOI] [PubMed] [Google Scholar]
- 35.Furnari FB, Huang HJ, Cavenee WK. The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res. 1998;58:5002–5008. [PubMed] [Google Scholar]
- 36.Vlashi E, Kim K, Dealla Donna L, Donna LD, McDonald JT, Eghbali M, Sayre JW, Stefani E, McBride W, Pajonk F. In-vivo imaging, tracking, and targeting of cancer stem cells. J Natl Cancer Inst. 2009;101:350–359. doi: 10.1093/jnci/djn509. [DOI] [PMC free article] [PubMed] [Google Scholar]