Epidemiologic studies indicate that green tea consumption decreases cancer risk (1–3). These data are supported by the results of numerous preclinical studies, which have shown that green and black tea are potent inhibitors of carcinogenesis in various rodent models (4–6), including models for cancers of the skin, lung, esophagus, stomach, liver, duodenum, small intestine, pancreas, colon-rectum, and mammary gland (1, 4, 6–11).
Different tea preparations contain varying amounts of polyphenols, and epigallocatechin 3-gallate (EGCG) is the most abundant, best-studied, and possibly most potent (against cancer) polyphenol found in tea (4–6, 12–15). Besides EGCG, which may account for 50% to 80% of the total antioxidant polyphenols called catechins in tea (6, 12–16), other tea cate(−)chins include (−)-epigallocatechin, (−)-epicatechin gallate, and (−)-epicatechin. The achievable tissue concentrations of these polyphenols are in the low micromolar range, and therefore, anticarcinogenic effects observed with much higher concentrations in vitro may not be relevant to the in vivo anticarcinogenic process (4, 5, 17).
Green tea, EGCG, or other dietary components clearly have both direct and indirect effects. Numerous proteins that can directly bind with EGCG include the plasma proteins fibronectin, fibrinogen, and histidine-rich glycoprotein (18), which may act as carrier proteins for EGCG. EGCG also binds with Fas (19), which might trigger the Fas-mediated apoptosis cascade. Laminin and the 67 kDa laminin receptor (20, 21) also interact with EGCG, and this binding seems to regulate the biological functions of the 67 kDa laminin receptor that have possible implications for prion-related diseases. Other directly bound protein targets include the intermediate filament protein, vimentin (22), ζ chain–associated 70 kDa protein (ZAP-70) kinase (23), Fyn (24), insulin-like growth factor-1 receptor (25), and the molecular chaperone glucose-regulated protein 78 (26; Fig. 1). All of these directly bound proteins play important roles in carcinogenesis. Zap-70 plays a critical role in Tcell receptor–mediated signal transduction and in the immune response of leukemia cells, and Fyn plays a major role in malignant cell transformation. Insulin-like growth factor-1 receptor plays a functional role in cell transformation and cancer formation, and glucose-regulated protein 78 is associated with the multidrug resistance phenotype of many types of cancer cells. The many targets of polyphenols that have been discovered and continue to be discovered are very likely dependent on the concentration of the tea polyphenol used and the specific cell, tissue, or organ—for example, proteins that bind EGCG in the lung, breast, colon, or skin might be very different from one another, and EGCG very likely targets multiple proteins in each tissue.
Fig. 1.
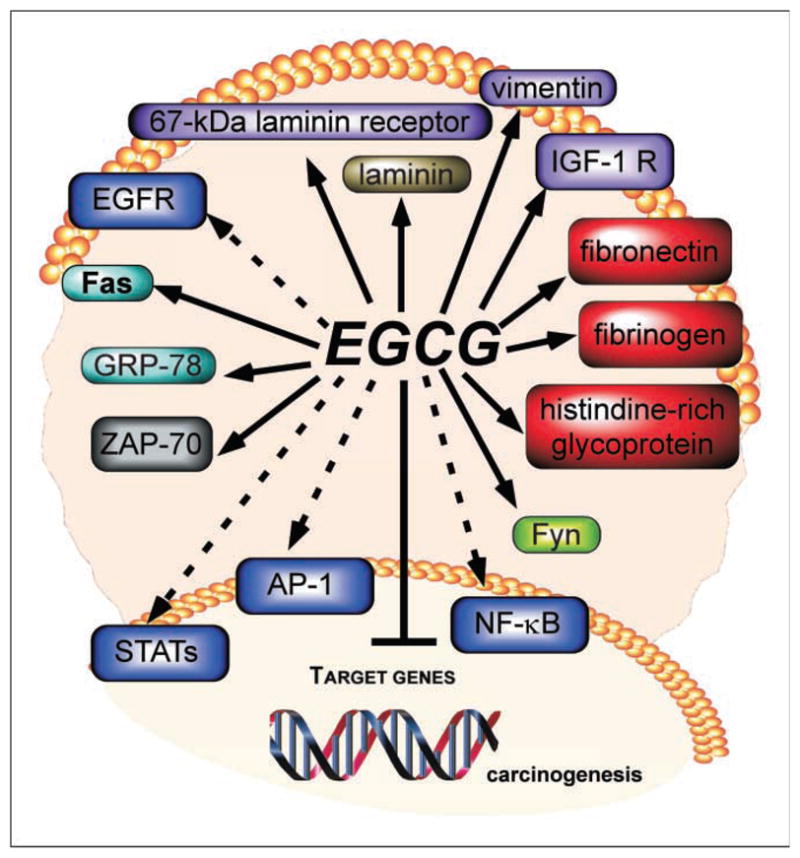
EGCG interacts with and binds numerous proteins to prevent carcinogenesis. EGCG has been reported to directly bind with the plasma proteins fibronectin, fibrinogen and histidine-rich glycoprotein, Fas, laminin and the 67 kDa laminin receptor, vimentin ZAP-70, Fyn, insulin-line growth factor-I receptor (IGF-IR), and glucose-regulated protein 78 (GRP-78; solid arrows). EGCG also indirectly targets a number of other oncogenic proteins including EGFR and the activator protein 1 (AP-1), signal transducers and activators of transcription (STAT), and nuclear factor κB (NF-κB) transcription factors. The net result is the inhibition of carcinogenesis in a variety of tissues.
EGCG and other polyphenols also exert strong indirect effects on a number of important regulatory proteins and transcription factors, adding further complexity to these agents’ multitargeted anticancer effects. In particular, EGCG inhibited tumor promoter–induced activator protein-1 (15, 27), signal transducers and activators of transcription (28), phosphatidylinositol 3-kinase/Akt (29), and nuclear factor-κB (30) activation. Phorbol ester tumor promoters such as 12-O-tetradecanoylphorbol-13-acetate are known to stimulate activator protein-1 activity through the epidermal growth factor receptor (EGFR; ref. 31), and theaflavins and EGCG were reported to down-regulate the EGFR (32) or its phosphorylation (33). However, the direct target(s) of EGCG, theaflavins, and other polyphenols in suppressing EGFR, activator protein-1, signal transducers and activators of transcription, nuclear factor-κB, or other transcription factor activations have not yet been identified.
Polyphenon E (Poly E) is a well-defined pharmaceutical-grade mixture that contains at least five different catechins, including epicatechin, gallocatechin gallate, epigallocatechin, epicatechingallate, and most abundantly, EGCG (~65%; refs. 34, 35). Poly E is the form of green tea used in clinical cancer trials funded through the National Cancer Institute to investigate the benefits of tea catechins in humans. Poly E has effectively inhibited lung cancer in a number of mouse model studies. Female A/J mice with benzo(a)pyrene-induced precancerous lesions formed over a period of 21 weeks received Poly E (1% in the diet) for an additional 25 weeks. Poly E treatment reduced the average tumor load per animal but did not significantly inhibit average tumor multiplicity (36). It is notable that Poly E reduced the largest carcinomas (compared with these tumors in untreated mice; ref. 36). Another study found that Poly E in the diet significantly reduced pulmonary adenoma multiplicity and tumor load in a dose-dependent fashion in A/J mice (37). Poly E in drinking fluid significantly reduced the incidence (by 52%) and multiplicity (by 63%) of 4-(methyl-nitrosamino)-1-(3-pyridyl)-1-butanone–induced lung tumor progression from adenoma to adenocarcinomas in female A/J mice (38). The number of visible lung tumors was also reduced (38). These studies indicate that administration of Poly E in the diet or drinking fluid is effective in suppressing lung cancer in animal models.
In this issue of the journal, Ming You’s group (Fu et al.) report a study of aerosolized Poly E (39) that builds on an earlier study by the same group. In the previous study, aerosolized Poly E or EGCG alone was administered to A/J mice beginning 2 weeks after carcinogen treatment and continued daily for 20 weeks. Poly E decreased tumor load by ~59%, whereas EGCG alone at the same or a higher dose failed to inhibit lung carcinogenesis (40). The new study reported in this issue of the journal tested Poly E versus Poly E stripped of its EGCG content, or Poly E-light, in A/J mice (39). Poly E decreased benzo (a)pyrene-induced tumor multiplicity by 53%, but the same dose of Poly E-light failed to inhibit lung carcinogenesis. Ineffectiveness for EGCG alone was shown in the first study and for Poly E minus its most abundant component, EGCG, in the second study, indicating that aerosolized Poly E may require all its components but certainly requires EGCG in order to be effective in treating or preventing pulmonary adenoma formation and growth in A/J mice.
These results are supported by studies of Poly E versus EGCG in other tissues. ApcMin/+ mice fed Poly E (0.12% in diet) or EGCG (0.08% in drinking water) exhibited significantly decreased tumor multiplicity, suggesting that Poly E was more effective than was EGCG alone (41). It is notable that more EGCG was found in the small intestine of animals receiving Poly E than EGCG (41), suggesting that EGCG may be metabolized more efficiently when given in a combination of catechins rather than alone. The effects of Poly E or EGCG might also be tissue-specific. For example, Poly E caused a dose-dependent decrease in palpable 4-hydroxybutyl(butyl)-nitrosamine–induced urinary bladder tumors but had little effect on the prevention of methylnitrosourea-induced mammary cancers (42). Relatively high levels of various polyphenols, but not EGCG, were found in the urine, and the levels of these polyphenols were ~50 to 1,000 times lower in serum. Therefore, the bioavailability of these tea polyphenols to different organ sites might contribute to the differential preventive efficacy of Poly E against urinary bladder and mammary cancers (42).
These results suggest what many have begun to suspect—isolating a single compound from complex foods may not be effective cancer prevention even at high, relatively toxic doses, whereas combinations of lower, less-toxic doses of each compound might be effective (43–47). Clearly, EGCG or other polyphenol chemicals may require interreactivity or dependency on other components in the whole food source. This idea is supported by the general clinical findings that individual dietary components have not been very successful in preventing cancer. Results from the Alpha-Tocopherol, Beta-Carotene (ATBC) Cancer Prevention Study in Finnish men smokers at risk of lung cancer indicated that the incidence of lung cancer was not affected by α-tocopherol and unexpectedly increased in men receiving β-carotene compared with those who did not (48). Subsequent findings across subgroups of participants in the Alpha-Tocopherol, Beta-Carotene study supported these findings but also indicated that the adverse β-carotene effect might have been associated with heavier smoking and higher alcohol intake (49). The findings of the Beta-Carotene and Retinol Efficacy Trial in smokers, former smokers, and workers exposed to asbestos (50), similar with the Alpha-Tocopherol, Beta-Carotene Study, was that β-carotene (combined with retinol) had a higher risk of lung cancer compared with placebo (50). Folic acid did not prevent colorectal adenomas in patients with a recent history of colorectal adenomas and no previous invasive large intestine carcinoma (51). Furthermore, the Selenium and Vitamin E Cancer Prevention Trial showed that neither selenium nor vitamin E prevented prostate cancer (52). On the other hand, recent results of a phase III trial of low doses of combined sulindac (a nonsteroidal anti-inflammatory drug) and difluoromethylornithine prevented colon polyp recurrence by 70% overall and by 92% for the highest-risk, advanced adenomas (43).
The available clinical evidence suggests that Poly E is more bioavailable than is EGCG alone, which may explain the differences in efficacy between the two agents in different models. In general, the oral bioavailability of green tea catechins is low, which means that systemic catechin levels in humans are several-fold less than the effective concentrations determined in in vitro systems (53). Phase I pharmacokinetic studies have tested increasing single oral doses of EGCG or Poly E (decaffeinated) to assess their systemic availability. Following Poly E administration, EGCG was present mostly in the free form, whereas epicatechin and epigallocatechin were present at low/undetectable levels as glucuronide and sulfate conjugates in plasma or urine (53). Following EGCG administration in another study, none of these compounds were detectable (indicating the purity of the EGCG used), and the systemic availability of EGCG was increased at higher doses (54). In addition, oral administration of EGCG or Poly E under a fasting condition increased their bioavailability (53). A study of the safety and pharmacokinetics of 4 weeks of daily oral EGCG or Poly E (decaffeinated; ref. 55) found that healthy individuals can take green tea polyphenol products in amounts equivalent to the EGCG content of 8 to 16 cups of green tea and that a high daily bolus dose (800 mg EGCG or Poly E once daily) increased the systemic availability of free EGCG by >60% (55).
Cell culture studies suggest that EGCG alone is just as effective as is Poly E in inhibiting cancer cell growth. For example, as little as 1 μg/mL of EGCG or Poly E (containing ~65% EGCG) for 96 hours inhibited the growth of Caco2, HCT116, HT29, SW480, and SW837 colon cancer cells but had no effect on the FHC normal human fetal colon cell line (33). Poly E and EGCG alone had similar potencies to suppressed EGFR and HER2 phosphorylation and downstream target activation with similar potency. Of note, as little as 1 μg/mL of epicatechin combined with EGCG (10 μg/mL) had synergistic inhibitory effects on cell growth (33), suggesting an interdependency for optimal activity. Accumulating evidence from animal models strongly suggests that EGCG and perhaps other catechins are not as effective in vivo on their own as they are combined.
Clinical studies support the concept that combinations (such as Poly E) of chemopreventive compounds and agents may be superior to such agents used singly. Compared with purified chemicals such as erlotinib (tarceva; an EGFR inhibitor), celecoxib (a cyclooxygenase-2 inhibitor), or difluoromethylornithine (an ornithine decarboxylase inhibitor), compounds such as EGCG found in complex foods seem to be much less potent or toxic. Therefore, combinations of natural or synthetic agents for cancer prevention might be more effective and have fewer side effects because they likely will require lower doses of natural compounds such as EGCG or of molecular-targeted agents such as inhibitors of EGFR, cyclooxygenase-2, or ornithine decarboxylase. Indeed, a report by Amin et al. (56), in this issue of the journal, provides evidence that a combination of EGCG and the EGFR inhibitor erlotinib synergistically inhibited the growth of squamous cell carcinoma of the head and neck by suppressing nuclear factor-κB activation. Results of this type support the hypothesis that isolating single compounds such as selenium, vitamin E, and β-carotene may cause them to lose their potential anticancer and other beneficial effects, possibly even causing them to exhibit undesired cancer promotion effects, as in the case of β-carotene. Likewise, EGCG or other polyphenol chemicals may require their complex, natural combination forms to be active anticancer agents because they depend on interactions with other whole-food components for efficacy, illustrating the age-old principle—united they stand, divided they fall.
Acknowledgments
Grant support: The Hormel Foundation and NIH grants R37 CA081064, CA120388, and CA111536.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Dreosti IE, Wargovich MJ, Yang CS. Inhibition of carcinogenesis by tea: the evidence from experimental studies. Crit Rev Food Sci Nutr. 1997;37:761–70. doi: 10.1080/10408399709527801. [DOI] [PubMed] [Google Scholar]
- 2.Katiyar SK, Mukhtar H. Tea in chemoprevention of cancer: epidemiologic and experimental studies. Int J Oncol. 1996;8:221–38. doi: 10.3892/ijo.8.2.221. [DOI] [PubMed] [Google Scholar]
- 3.Yoshizawa S, Horiuchi T, Fukiji H, Yosida T, Oduad T, Sugimura T. Antitumor promoting activity of (−)-epigallocatechin gallate, the main constituent of “tannin” in green tea. Photother Res. 1987;1:44–7. [Google Scholar]
- 4.Yang CS. Inhibition of carcinogenesis by tea. Nature. 1997;389:134–5. doi: 10.1038/38154. [DOI] [PubMed] [Google Scholar]
- 5.Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- 6.Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993;85:1038–49. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- 7.Gensler HL, Timmermann BN, Valcic S, et al. Prevention of photocarcinogenesis by topical administration of pure epigallocatechin gallate isolated from green tea. Nutr Cancer. 1996;26:325–35. doi: 10.1080/01635589609514488. [DOI] [PubMed] [Google Scholar]
- 8.Huang MT, Ho CT, Wang ZY, et al. Inhibitory effect of topical application of a green tea polyphenol fraction on tumor initiation and promotion in mouse skin. Carcinogenesis. 1992;13:947–54. doi: 10.1093/carcin/13.6.947. [DOI] [PubMed] [Google Scholar]
- 9.Wang ZY, Khan WA, Bickers DR, Mukhtar H. Protection against polycyclic aromatic hydrocarbon-induced skin tumor initiation in mice by green tea polyphenols. Carcinogenesis. 1989;10:411–5. doi: 10.1093/carcin/10.2.411. [DOI] [PubMed] [Google Scholar]
- 10.Chung FL, Wang M, Rivenson A, et al. Inhibition of lung carcinogenesis by black tea in Fischer rats treated with a tobacco-specific carcinogen: caffeine as an important constituent. Cancer Res. 1998;58:4096–101. [PubMed] [Google Scholar]
- 11.Huang MT, Xie JG, Wang ZY, et al. Effects of tea, decaffeinated tea, and caffeine on UVB light-induced complete carcinogenesis in SKH-1 mice: demonstration of caffeine as a biologically important constituent of tea. Cancer Res. 1997;57:2623–9. [PubMed] [Google Scholar]
- 12.Bode AM, Dong Z. Signal transduction pathways: targets for chemoprevention of skin cancer. Lancet Oncol. 2000;1:181–8. doi: 10.1016/s1470-2045(00)00029-2. [DOI] [PubMed] [Google Scholar]
- 13.Bode AM, Dong Z. Signal transduction pathways: targets for green and black tea polyphenols. J Biochem Mol Biol. 2003;36:66–77. [PubMed] [Google Scholar]
- 14.Bode AM, Dong Z. Molecular and cellular targets. Mol Carcinog. 2006;45:422–30. doi: 10.1002/mc.20222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong Z, Ma W, Huang C, Yang CS. Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols, (−)-epigallocatechin gallate, and theaflavins. Cancer Res. 1997;57:4414–9. [PubMed] [Google Scholar]
- 16.Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21:334–50. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- 17.Jankun J, Selman SH, Swiercz R, Skrzypczak-Jankun E. Why drinking green tea could prevent cancer. Nature. 1997;387:561. doi: 10.1038/42381. [DOI] [PubMed] [Google Scholar]
- 18.Sazuka M, Isemura M, Isemura S. Interaction between the carboxyl-terminal heparin-binding domain of fibronectin and (−)-epigallocatechin gallate. Biosci Biotechnol Biochem. 1998;62:1031–2. doi: 10.1271/bbb.62.1031. [DOI] [PubMed] [Google Scholar]
- 19.Hayakawa S, Saeki K, Sazuka M, et al. Apoptosis induction by epigallocatechin gallate involves its binding to Fas. Biochem Biophys Res Commun. 2001;285:1102–6. doi: 10.1006/bbrc.2001.5293. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki Y, Isemura M. Inhibitory effect of epigallocatechin gallate on adhesion of murine melanoma cells to laminin. Cancer Lett. 2001;173:15–20. doi: 10.1016/s0304-3835(01)00685-1. [DOI] [PubMed] [Google Scholar]
- 21.Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol. 2004;11:380–1. doi: 10.1038/nsmb743. [DOI] [PubMed] [Google Scholar]
- 22.Ermakova S, Choi BY, Choi HS, Kang BS, Bode AM, Dong Z. The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J Biol Chem. 2005;280:16882–90. doi: 10.1074/jbc.M414185200. [DOI] [PubMed] [Google Scholar]
- 23.Shim JH, Choi HS, Pugliese A, et al. (−)-Epigallocatechin gallate regulates CD3-mediated T cell receptor signaling in leukemia through the inhibition of ZAP-70 kinase. J Biol Chem. 2008;283:28370–9. doi: 10.1074/jbc.M802200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Z, Tang F, Ermakova S, et al. Fyn is a novel target of (−)-epigallocatechin gallate in the inhibition of JB6 Cl41 cell transformation. Mol Carcinog. 2008;47:172–83. doi: 10.1002/mc.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, He Z, Ermakova S, et al. Direct inhibition of insulin-like growth factor-I receptor kinase activity by (−)-epigallocatechin-3-gallate regulates cell transformation. Cancer Epidemiol Biomarkers Prev. 2007;16:598–605. doi: 10.1158/1055-9965.EPI-06-0892. [DOI] [PubMed] [Google Scholar]
- 26.Ermakova SP, Kang BS, Choi BY, et al. (−)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006;66:9260–9. doi: 10.1158/0008-5472.CAN-06-1586. [DOI] [PubMed] [Google Scholar]
- 27.Chen NY, Ma WY, Yang CS, Dong Z. Inhibition of arsenite-induced apoptosis and AP-1 activity by epigallocatechin-3-gallate and theaflavins. J Environ Pathol Toxicol Oncol. 2000;19:287–95. [PubMed] [Google Scholar]
- 28.Zykova TA, Zhang Y, Bode AM, Dong Z. The signal transduction networks required for phosphorylation of STAT1 at Ser727 in mouse epidermal JB6 cells in the UVB response and inhibitory mechanisms of tea polyphenols. Carcinogenesis. 2005;26:331–42. doi: 10.1093/carcin/bgh334. [DOI] [PubMed] [Google Scholar]
- 29.Nomura M, Kaji A, He Z, et al. Inhibitory mechanisms of tea polyphenols on the ultraviolet B-activated phosphatidylinositol 3-kinase-dependent pathway. J Biol Chem. 2001;276:46624–31. doi: 10.1074/jbc.M107897200. [DOI] [PubMed] [Google Scholar]
- 30.Nomura M, Ma W, Chen N, Bode AM, Dong Z. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced NF-κB activation by tea polyphenols, (−)-epigallocatechin gallate and theaflavins. Carcinogenesis. 2000;21:1885–90. doi: 10.1093/carcin/21.10.1885. [DOI] [PubMed] [Google Scholar]
- 31.Chen N, Ma WY, She QB, et al. Transactivation of the epidermal growth factor receptor is involved in 12-O-tetradecanoylphorbol-13-acetate-induced signal transduction. J Biol Chem. 2001;276:46722–8. doi: 10.1074/jbc.M107156200. [DOI] [PubMed] [Google Scholar]
- 32.Mizuno H, Cho YY, Zhu F, et al. Theaflavin-3, 3′-digallate induces epidermal growth factor receptor downregulation. Mol Carcinog. 2006;45:204–12. doi: 10.1002/mc.20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res. 2005;11:2735–46. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- 34.Chang PY, Mirsalis J, Riccio ES, et al. Genotoxicity and toxicity of the potential cancer-preventive agent polyphenon E. Environ Mol Mutagen. 2003;41:43–54. doi: 10.1002/em.10129. [DOI] [PubMed] [Google Scholar]
- 35.Clark J, You M. Chemoprevention of lung cancer by tea. Mol Nutr Food Res. 2006;50:144–51. doi: 10.1002/mnfr.200500135. [DOI] [PubMed] [Google Scholar]
- 36.Anderson MW, Goodin C, Zhang Y, et al. Effect of dietary green tea extract and aerosolized difluoromethylornithine during lung tumor progression in A/J strain mice. Carcinogenesis. 2008;29:1594–600. doi: 10.1093/carcin/bgn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan Y, Wang Y, Tan Q, et al. Efficacy of polyphenon E, red ginseng, and rapamycin on benzo(a)pyrene-induced lung tumorigenesis in A/J mice. Neoplasia. 2006;8:52–8. doi: 10.1593/neo.05652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu G, Liao J, Yang G, Reuhl KR, Hao X, Yang CS. Inhibition of adenoma progression to adenocarcinoma in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis model in A/J mice by tea polyphenols and caffeine. Cancer Res. 2006;66:11494–501. doi: 10.1158/0008-5472.CAN-06-1497. [DOI] [PubMed] [Google Scholar]
- 39.Fu H, He J, Mei F, et al. Anti-lung cancer effect of epigallocatechin-3-gallate is dependent on its presence in a complex mixture (polyphenon E) Cancer Prev Res (Phila Pa) 2009;2:XXX. doi: 10.1158/1940-6207.CAPR-08-0185. [DOI] [PubMed] [Google Scholar]
- 40.Yan Y, Cook J, McQuillan J, et al. Chemopreventive effect of aerosolized polyphenon E on lung tumorigenesis in A/J mice. Neoplasia. 2007;9:401–5. doi: 10.1593/neo.07160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hao X, Sun Y, Yang CS, et al. Inhibition of intestinal tumorigenesis in Apc(min/+) mice by green tea polyphenols (polyphenon E) and individual catechins. Nutr Cancer. 2007;59:62–9. doi: 10.1080/01635580701365050. [DOI] [PubMed] [Google Scholar]
- 42.Lubet RA, Yang CS, Lee MJ, et al. Preventive effects of polyphenon E on urinary bladder and mammary cancers in rats and correlations with serum and urine levels of tea polyphenols. Mol Cancer Ther. 2007;6:2022–8. doi: 10.1158/1535-7163.MCT-07-0058. [DOI] [PubMed] [Google Scholar]
- 43.Meyskens FL, Jr, McLaren CE, Pelot D, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila Pa) 2008;1:32–8. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torrance CJ, Jackson PE, Montgomery E, et al. Combinatorial chemoprevention of intestinal neoplasia. Nat Med. 2000;6:1024–8. doi: 10.1038/79534. [DOI] [PubMed] [Google Scholar]
- 45.Brown PH, Subbaramaiah K, Salmon AP, et al. Combination chemoprevention of HER2/neu-induced breast cancer using a cyclooxygenase-2 inhibitor and a retinoid X receptor-selective retinoid. Cancer Prev Res (Phila Pa) 2008;1:208–14. doi: 10.1158/1940-6207.CAPR-08-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerner EW, Meyskens FL., Jr Combination chemoprevention for colon cancer targeting polyamine synthesis and inflammation. Clin Cancer Res. 2009;15:758–61. doi: 10.1158/1078-0432.CCR-08-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao H, Yang CS. Combination regimen with statins and NSAIDs: a promising strategy for cancer chemoprevention. Int J Cancer. 2008;123:983–90. doi: 10.1002/ijc.23718. [DOI] [PubMed] [Google Scholar]
- 48.The effect of vitamin E and β-carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–35. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 49.Albanes D, Heinonen OP, Taylor PR, et al. α-Tocopherol and β-carotene supplements and lung cancer incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88:1560–70. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 50.Omenn GS, Goodman G, Thornquist M, et al. The Beta-Carotene and Retinol Efficacy Trial (CARET) for chemoprevention of lung cancer in high risk populations: smokers and asbestos-exposed workers. Cancer Res. 1994;54:2038–43s. [PubMed] [Google Scholar]
- 51.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–9. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 52.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Jama. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chow HH, Hakim IA, Vining DR, et al. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clin Cancer Res. 2005;11:4627–33. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- 54.Chow HH, Cai Y, Alberts DS, et al. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001;10:53–8. [PubMed] [Google Scholar]
- 55.Chow HH, Cai Y, Hakim IA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–9. [PubMed] [Google Scholar]
- 56.Amin AR, Khuri FR, Chen Z, Shin DM. Synergistic growth inhibition of squamous cell carcinoma of the head and neck by erlotinib and EGCG: the role of p53-dependent inhibition of nuclear factor-κB. Cancer Prev Res. 2009 doi: 10.1158/1940-6207.CAPR-09-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]


