Abstract
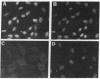
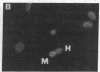
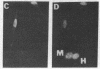
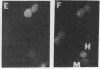
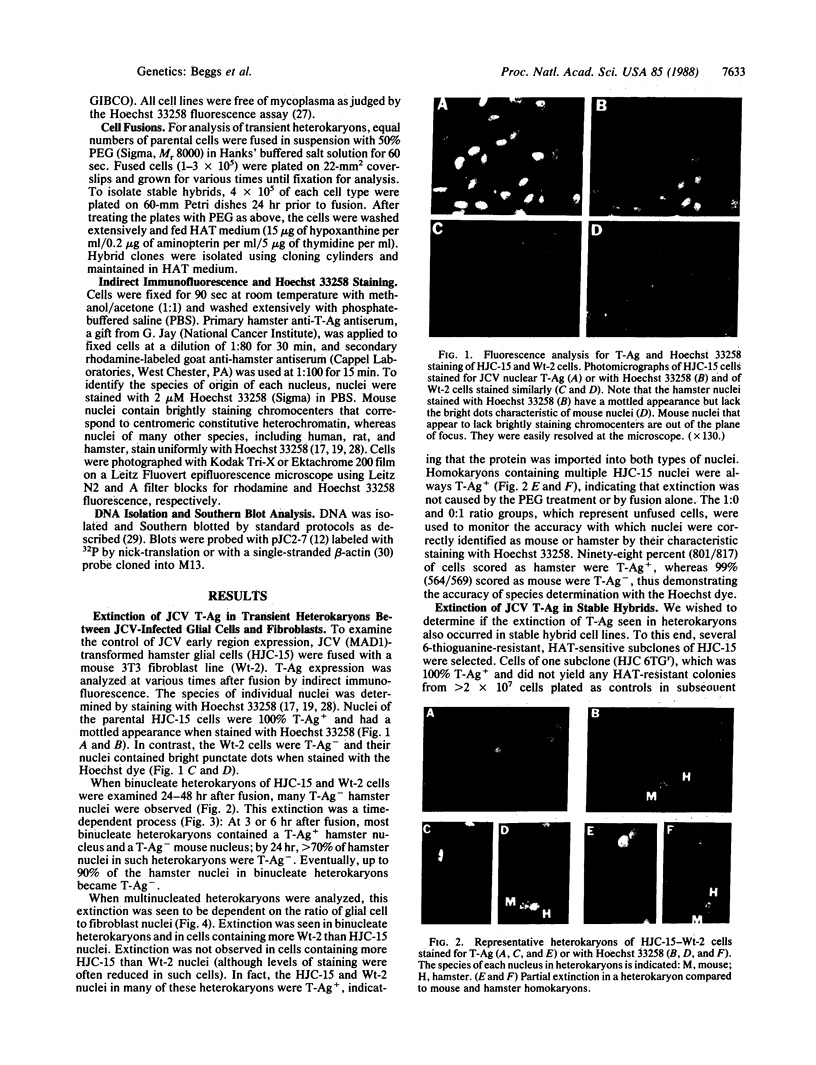
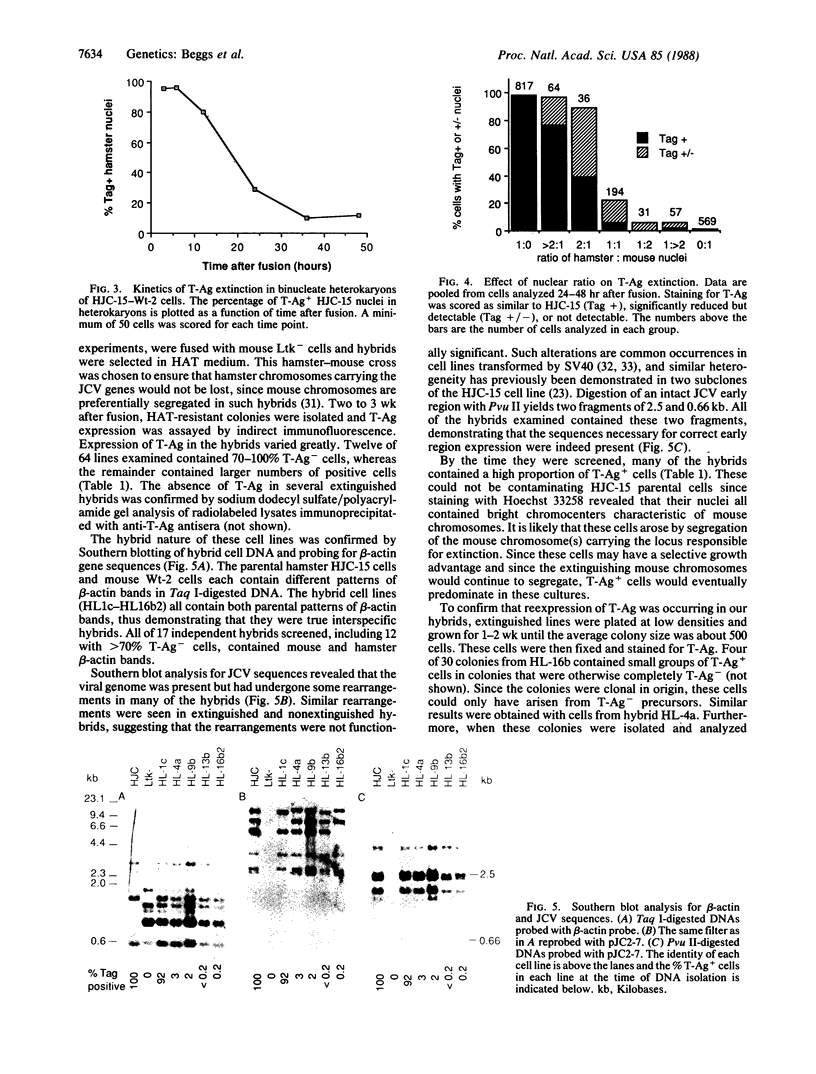
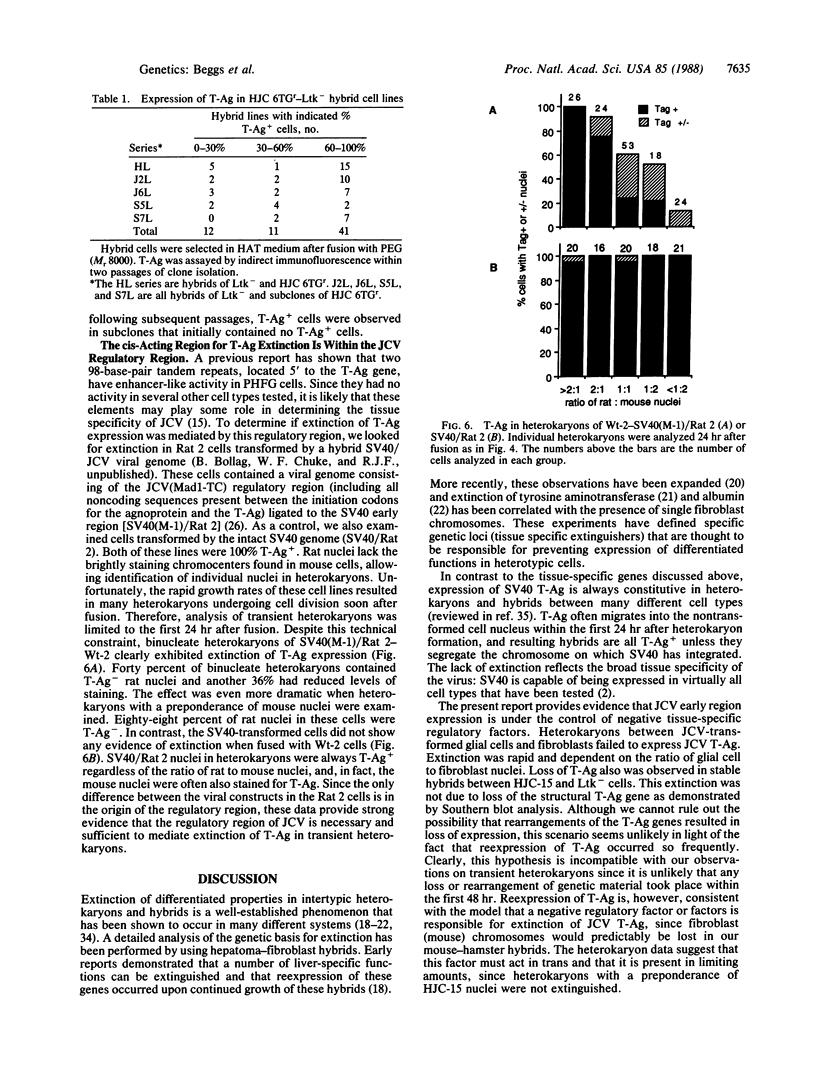
JC virus (JCV) is a ubiquitous human papovavirus that shares sequence and structural homology with simian virus 40 (SV40). In contrast to SV40, expression of JCV is restricted to a small number of cell types, including human fetal glial cells, uroepithelial cells, amnion cells, and some endothelial cells. To study the control of JCV early region expression, we made heterokaryons and stable hybrids between JCV-transformed hamster glial cells and mouse fibroblasts. Binucleate heterokaryons exhibited extinction of large tumor antigen expression in the hamster nuclei as assayed by indirect immunofluorescence. This extinction was both time and dose dependent: extinction reached maximal levels at 24-36 hr after fusion and was dependent on the ratio of glial cell to fibroblast nuclei in multinucleated heterokaryons. Extinction also was observed in stable hybrids between the glial cells and mouse Ltk- cells. Southern blot analysis showed that the extinguished hybrids contained viral sequences. Reexpression of large tumor antigen was observed in several subclones, suggesting that extinction was correlated with the loss of murine fibroblast chromosomes from these hybrids. The cis-acting region that mediates extinction resides within the viral regulatory region, which contains two 98-base-pair repeats that have enhancer activity. These data demonstrate that cellular factors that negatively regulate viral gene expression contribute to the restricted cell-type specificity of this virus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron M. H., Maniatis T. Rapid reprogramming of globin gene expression in transient heterokaryons. Cell. 1986 Aug 15;46(4):591–602. doi: 10.1016/0092-8674(86)90885-8. [DOI] [PubMed] [Google Scholar]
- Beckmann A. M., Shah K. V., Padgett B. L. Propagation and primary isolation of papovavirus JC in epithelial cells derived from human urine. Infect Immun. 1982 Nov;38(2):774–777. doi: 10.1128/iai.38.2.774-777.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich C., Yoshioka T., Tanaka K., Jay G., Scangos G. Functional expression of a heterologous major histocompatibility complex class I gene in transgenic mice. Mol Cell Biol. 1987 Nov;7(11):4003–4009. doi: 10.1128/mcb.7.11.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H. M., Pavlath G. K., Hardeman E. C., Chiu C. P., Silberstein L., Webster S. G., Miller S. C., Webster C. Plasticity of the differentiated state. Science. 1985 Nov 15;230(4727):758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Chin A. C., Fournier R. E. A genetic analysis of extinction: trans-regulation of 16 liver-specific genes in hepatoma-fibroblast hybrid cells. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1614–1618. doi: 10.1073/pnas.84.6.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy B., Scangos G. Expression of transferred thymidine kinase genes is controlled by methylation. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6299–6303. doi: 10.1073/pnas.79.20.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuke W. F., Walker D. L., Peitzman L. B., Frisque R. J. Construction and characterization of hybrid polyomavirus genomes. J Virol. 1986 Dec;60(3):960–971. doi: 10.1128/jvi.60.3.960-971.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J., Bream G. L., Cannella M. T. Human polyomavirus JC virus genome. J Virol. 1984 Aug;51(2):458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J., Martin J. D., Padgett B. L., Walker D. L. Infectivity of the DNA from four isolates of JC virus. J Virol. 1979 Nov;32(2):476–482. doi: 10.1128/jvi.32.2.476-482.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J., Rifkin D. B., Walker D. L. Transformation of primary hamster brain cells with JC virus and its DNA. J Virol. 1980 Jul;35(1):265–269. doi: 10.1128/jvi.35.1.265-269.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B. W., Berg D. T., Walls J. D. Negative regulation of the human polyomavirus BK enhancer involves cell-specific interaction with a nuclear repressor. Mol Cell Biol. 1988 Aug;8(8):3448–3457. doi: 10.1128/mcb.8.8.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower M. J., Lucas J. J. Construction of viable mouse-human hybrid cells by nuclear transplantation. J Cell Physiol. 1980 Oct;105(1):93–103. doi: 10.1002/jcp.1041050112. [DOI] [PubMed] [Google Scholar]
- Hiscott J. B., Murphy D., Defendi V. Instability of integrated viral DNA in mouse cells transformed by simian virus 40. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1736–1740. doi: 10.1073/pnas.78.3.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley P. M., Rentier-Delrue F., Heilman C. A., Law M. F., Chowdhury K., Israel M. A., Takemoto K. K. Cloned human polyomavirus JC DNA can transform human amnion cells. J Virol. 1980 Dec;36(3):878–882. doi: 10.1128/jvi.36.3.878-882.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney S., Natarajan V., Strike D., Khoury G., Salzman N. P. JC virus enhancer-promoter active in human brain cells. Science. 1984 Dec 14;226(4680):1337–1339. doi: 10.1126/science.6095453. [DOI] [PubMed] [Google Scholar]
- Killary A. M., Fournier R. E. A genetic analysis of extinction: trans-dominant loci regulate expression of liver-specific traits in hepatoma hybrid cells. Cell. 1984 Sep;38(2):523–534. doi: 10.1016/0092-8674(84)90507-5. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Coleman J. R. Extinction of muscle-specific properties in somatic cell heterokaryons. Dev Biol. 1984 Feb;101(2):463–476. doi: 10.1016/0012-1606(84)90160-x. [DOI] [PubMed] [Google Scholar]
- London W. T., Houff S. A., Madden D. L., Fuccillo D. A., Gravell M., Wallen W. C., Palmer A. E., Sever J. L., Padgett B. L., Walker D. L. Brain tumors in owl monkeys inoculated with a human polyomavirus (JC virus). Science. 1978 Sep 29;201(4362):1246–1249. doi: 10.1126/science.211583. [DOI] [PubMed] [Google Scholar]
- Martin J. D., King D. M., Slauch J. M., Frisque R. J. Differences in regulatory sequences of naturally occurring JC virus variants. J Virol. 1985 Jan;53(1):306–311. doi: 10.1128/jvi.53.1.306-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. D., Padgett B. L., Walker D. L. Characterization of tissue culture-induced heterogeneity in DNAs of independent isolates of JC virus. J Gen Virol. 1983 Oct;64(Pt 10):2271–2280. doi: 10.1099/0022-1317-64-10-2271. [DOI] [PubMed] [Google Scholar]
- Migeon B. R. Hybridization of somatic cells derived from mouse and syrian hamster: evolution of karyotype and enzyme studies. Biochem Genet. 1968 Mar;1(4):305–322. doi: 10.1007/BF00491487. [DOI] [PubMed] [Google Scholar]
- Mével-Ninio M., Weiss M. C. Immunofluorescence analysis of the time-course of extinction, reexpression, and activation of albumin production in rat hepatoma-mouse fibroblast heterokaryons and hybrids. J Cell Biol. 1981 Aug;90(2):339–350. doi: 10.1083/jcb.90.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norum R. A., Migeon B. R. Non-random loss of human markers from man-mouse somatic cell hybrids. Nature. 1974 Sep 6;251(5470):72–74. doi: 10.1038/251072a0. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Rogers C. M., Walker D. L. JC virus, a human polyomavirus associated with progressive multifocal leukoencephalopathy: additional biological characteristics and antigenic relationships. Infect Immun. 1977 Feb;15(2):656–662. doi: 10.1128/iai.15.2.656-662.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. New human papovaviruses. Prog Med Virol. 1976;22:1–35. [PubMed] [Google Scholar]
- Petit C., Levilliers J., Ott M. O., Weiss M. C. Tissue-specific expression of the rat albumin gene: genetic control of its extinction in microcell hybrids. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2561–2565. doi: 10.1073/pnas.83.8.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Anisowicz A., Howell N. Genomic rearrangements in a mouse cell line containing integrated SV40 DNA. Cell. 1981 Jan;23(1):41–50. doi: 10.1016/0092-8674(81)90268-3. [DOI] [PubMed] [Google Scholar]
- Small J. A., Scangos G. A., Cork L., Jay G., Khoury G. The early region of human papovavirus JC induces dysmyelination in transgenic mice. Cell. 1986 Jul 4;46(1):13–18. doi: 10.1016/0092-8674(86)90855-x. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Howley P. M., Miyamura T. JC human papovavirus replication in human amnion cells. J Virol. 1979 Apr;30(1):384–389. doi: 10.1128/jvi.30.1.384-389.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. L., Padgett B. L., ZuRhein G. M., Albert A. E., Marsh R. F. Human papovavirus (JC): induction of brain tumors in hamsters. Science. 1973 Aug 17;181(4100):674–676. doi: 10.1126/science.181.4100.674. [DOI] [PubMed] [Google Scholar]
- Wold W. S., Green M., Mackey J. K., Martin J. D., Padgett B. L., Walker D. L. Integration pattern of human JC virus sequences in two clones of a cell line established from a JC virus-induced hamster brain tumor. J Virol. 1980 Mar;33(3):1225–1228. doi: 10.1128/jvi.33.3.1225-1228.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]