Abstract
Recombinant adenovirus serotype 5 (Ad5) vectors have been studied extensively in preclinical gene therapy models and in a range of clinical trials. However, innate immune responses to adenovirus vectors limit effectiveness of Ad5 based therapies. Moreover, extensive pre-existing Ad5 immunity in human populations will likely limit the clinical utility of adenovirus vectors, unless methods to circumvent neutralizing antibodies that bind virus and block target cell transduction can be developed; Furthermore, memory T cell and humoral responses to Ad5 are associated with increased toxicity, raising safety concerns for therapeutic adenovirus vectors in immunized hosts. Most preclinical studies have been performed in naïve animals; although pre-existing immunity is among the greatest hurdles for adenovirus therapies, it is also one of the most neglected experimentally. Here we summarize findings using adenovirus vectors in naïve animals, in Ad-immunized animals and in clinical trials, and review strategies proposed to overcome innate immune responses and pre-existing immunity.
Keywords: ADENOVIRUS, INNATE IMMUNITY, NEUTRALIZING ANTIBODY, GENE THERAPY, VACCINATION
Adenovirus vectors have many promising traits for gene therapy; they efficiently transduce many proliferating and quiescent cell types, can “package” large amounts of foreign DNA, can be grown to high titers and are not germ line transmitted. However, adenovirus-mediated transgene expression is generally transient; ranging from weeks to a few months. Temporal limitation of transgene expression results mainly from immune responses that develop against both vector and transgene products.
Adenovirus vectors often elicit strong innate inflammatory responses within hours after administration [Muruve et al., 1999]. Following the initial innate immune response, neutralizing antibodies to viral capsid proteins (fiber, hexon, penton) develop in naïve animals within 1 week. Neutralizing antibodies reduce the infectious titer of a virus and the transduction of target cells by adenovirus gene therapy vectors. [Neutralizing titers are determined in vitro by serially diluting immune sera and determining the highest serum dilution that inhibits transgene expression or plaque formation by 50%. The neutralizing titer is the reciprocal of this dilution value. Sprangers et al. [2003Q3] discuss the advantages and disadvantages of several in vitro neutralization assays; Pichla-Gollon et al. [2009] report that in vitro antibody neutralization assays may, in fact, underestimate Ad5 inhibition by circulating anti-Ad5 antibodies in vivo.]
Anti-adenovirus antibodies inhibit virus transduction after vector re-administration. Moreover, CD8+ T cell immune responses against both vector capsid components and transgene products eliminate target cells expressing these proteins. Thus, even in the absence of pre-existing immunity, adenovirus vectors are not suitable for correction of chronic disorders requiring repeated vector administration, and might be adequate only for therapeutic strategies that require short-term expression, unless the immune response resulting from an initial vector challenge can be eliminated or circumvented.
Because immune responses prevent long-term expression or repeated adenoviral vector challenge, most clinical trials have been based on protocols that target cancer. For such an application, transient transgene expression is designed to eliminate target cells or to initiate anti-tumor responses. Adenoviruses are also utilized to promote vaccine development; in this application investigators attempt to exploit as therapeutic advantage—rather than circumvent—adenovirus immunogenicity. However, pre-existing immunity is likely to limit adenovirus utility both as a gene therapy vector and as a vaccine vector. Forty to ninety seven per cent of humans have neutralizing antibodies to Ad5, the most frequently used adenovirus vector in gene therapy and vaccine protocols [Chirmule et al., 1999; Vogels et al., 2003].
We review here Ad5 cell interactions, innate and adaptive immune responses to adenovirus vectors and studies in which adenovirus vectors are used in immunized animals and in clinical trials. We conclude by discussing strategies to circumvent humoral and cellular capsid responses, and consider approaches to improve adenovirus gene therapy in seropositive individuals.
ROUTES OF ADENOVIRUS CELL ENTRY
The most widely studied adenovirus cell entry pathway, commonly studied with cultured fibroblasts, epithelial and endothelial cells, begins when the adenovirus fiber protein “knob” engages cells through an interaction with the cellular cocksackie and adenovirus receptor (CAR). Virus internalization is subsequently facilitated by interaction of the viral penton base protein RGD motif with cellular integrins [Wickham et al., 1993].
Following intravenous injection into naïve mice, nearly all Ad5-directed transgene expression occurs in the liver [Stratford-Perricaudet et al., 1990]. Elevated hepatic transgene expression was initially attributed to high hepatic CAR levels [Tomko et al., 1997] and to the relative accessibility of liver cells to circulating adenovirus, compared to other tissues, because of differences in vascular endothelial barriers [Zinn et al., 1998]. Moreover, genetic ablation of viral CAR and integrin binding was reported to reduce liver transduction following systemic virus administration [Einfeld et al., 2001]. Several studies, however, suggest CAR is not present on epithelial cell surfaces in vivo, but is located in tight junctions where it is inaccessible to virus [Cohen et al., 2001]. Moreover, additional studies report that ablation of adenovirus CAR and integrin binding does not reduce liver transduction by intravenously administered virus [Nicklin et al., 2005], suggesting other receptors/entry pathways are responsible for hepatic adenovirus transduction in vivo.
Shayakhmetov et al. [2005] suggested circulating proteins (e.g., Factor IX and complement C4 binding protein) act as “molecular bridges,” binding to fiber knobs and mediating virus entry into cells through receptors such as heparan sulfate proteoglycan (HSPG) or low density lipoprotein receptor-related protein. However, systemic adenovirus administration to FIX knockout and wild-type mice results in equivalent hepatic transgene expression. In contrast, in the absence of all vitamin K-dependent serum coagulation factors (including Factor IX), hepatic adenovirus transduction is diminished [Parker et al., 2006]. Moreover, Factor X alone restored hepatic transduction [Parker et al., 2006]. Several labs subsequently demonstrated human and murine Factor X bind, with high affinity, to the adenovirus hexon protein, and suggested HSPGs mediate adenovirus hepatocyte entry [Kalyuzhniy et al., 2008; Waddington et al., 2008] (Fig. 1, left panel). Adenovirus vectors unable to bind to CAR were similarly unable to transduce liver in the absence of vitamin-K dependent coagulation factors [Waddington et al., 2007]. These results suggest CAR plays at best a minor role in hepatic adenovirus transduction in vivo in the experimental animal models described here.
Fig. 1.
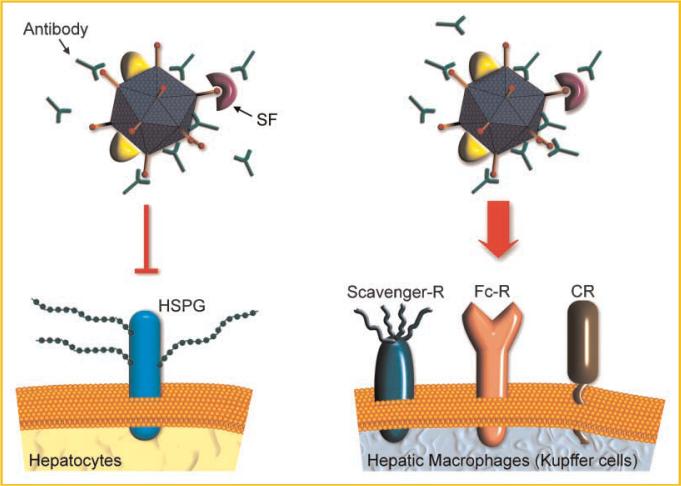
Adenovirus transduction of hepatocytes and Kupffer cells following intravenous administration in naïve mice. After intravenous injection into naïve mice, adenovirus vectors very efficiently transduce liver hepatocytes. This transduction is now thought to be dependent on serum factors such as FX that bridge the virus to hepatic heparan sulfate proteoglycans (HSPGs). Other serum factors (SF), including FIX and complement components, also bind to capsid proteins. However, their role in hepatocyte virus transduction is less clear at this time. Transduction into hepatocytes is followed by robust transgene expression. Kupffer cells (liver macrophages) take up virus mainly through scavenger receptors, complement receptors (CR) and Fc-receptors for immunoglobulin (Fc-R). However, transgene expression is substantially less in Kupffer cells than that observed in transduced hepatocytes.
Kupffer cells (liver macrophages) are responsible for rapid hepatic uptake of low adenovirus doses (up to 3 × 1010 particles); higher virus doses overcome this biological filter effect and initiate hepatocyte transduction [Tao et al., 2001]. Kupffer cells take up virus mainly through scavenger receptors, complement receptors (CR) and immunoglobulin Fc-receptors (Fc-R) [Xu et al., 2008] (Fig. 1, right panel). Although Kupffer cells are transduced, transgene expression is inefficient. Di Paolo et al. [2009] showed, by analyzing viral DNA, that FX-ablated virus or virus injected into Kupffer cell-depleted animals is still sequestered in the liver. Only when a virus that was depleted of its capsid RGD peptide was administered to Kupffer cell depleted mice in the presence of Warfarin (i.e., in the absence of FX), was adenovirus sequestration by the liver reduced by ~80%. The results of Di Paolo et al. [2009] suggest that adenovirus liver sequestration is mediated by multiple redundant mechanisms. We should point out that the extent of adenovirus sequestration by the liver following intravenous challenge varies with species.
Adenovirus also interact with blood cells [Lyons et al., 2006; Stone et al., 2007b]. Systemically administered adenovirus can cause thrombocytopenia in patients. Adenovirus is associated with platelets in murine liver sinusoids [Stone et al., 2007b]. Platelet depletion reduced adenovirus accumulation in murine liver following intravenous injection, suggesting platelet binding contributes to murine liver transduction [Stone et al., 2007b]. However, platelet removal does not reduce adenovirus uptake by murine Kupffer cells [Xu et al., 2008]. In blood samples from clinical trials with patients who received intratumoral adenovirus injections, 99% of adenovirus DNA in the circulation was associated with human blood cells. When incubated with human erythrocytes ex vivo, Ad5 was sequestered; incubation of erythrocyte-bound virus with epithelial cells reduced transgene expression [Lyons et al., 2006]. In contrast, incubation of Ad5 with murine blood did not reduce the ability of the virus to transduce cells in culture. Seiradake et al. [2009] demonstrated that Ads of different serotypes, all of which agglutinate human red blood cells, interact with these cells via different cellular receptors. CAR present on human red blood cells has recently been reported to be responsible for Ad5 sequestration [Carlisle et al., 2009]. The studies described in this paragraph, which suggest that alternative adenovirus vectors interact differently with murine and human blood cells, clearly require additional investigation and suggest caution in extrapolating to clinical contexts results of investigations using systemic adenovirus administration in mice. It will be of great interest to examine binding to human and murine blood cells of various types by adenovirus with mutations in proposed receptor recognition sites.
INNATE AND ADAPTIVE IMMUNE RESPONSES TO ADENOVIRUS
Adenovirus induces both innate and adaptive immune responses. The innate immune system is comprised of (1) cells, including macrophages, dendritic cells, neutrophils, mast cells and natural killer (NK) cells, (2) serum protein immune mediators, including chemokines, cytokines and complement and (3) “hardwired” intracellular and extracellular innate receptors, termed pattern recognition receptors (PRR), that recognize pathogen-associated molecular patterns (PAMPS) such as bacterial cell-wall components. The innate immune system is activated rapidly in response to incoming pathogens. These features—in particular, the rapidity of the response—distinguish the innate immune response from the adaptive immune response, which requires B and T cell maturation and function, and needs approximately 1 week to become effective.
In animal models, adenovirus administered intravenously to naïve individuals is sequestered in multiple organs, including spleen, heart, lung and liver. However, transgene expression occurs mainly in hepatocytes [Koizumi et al., 2007]; transgene expression in other cells—including those of the immune system—is usually lower than that observed in hepatocytes, despite effective cell entry. Because of preferential hepatic transgene expression, adenovirus uptake in other organs is often underestimated.
Acute inflammatory responses to adenovirus in livers of naïve recipients begin with rapid induction of pro-inflammatory cytokine and chemokine expression. This “cytokine storm” is followed by leukocyte infiltration, resulting in necrosis and tissue damage [Muruve et al., 1999; Schnell et al., 2001]. Adenovirus-induced acute toxicity is dose-dependent and independent of viral gene expression, indicating the viral capsid elicits the innate immune response [Muruve et al., 1999]. The innate immune response can lead to tissue damage that culminates in elimination of virus-transduced cells and consequent reduction in the level and duration of transgene expression [Guidotti and Chisari, 2001]. In addition, interferons can be induced that block transcription in infected cells that remain viable [Guidotti and Chisari, 2001; Sadler and Williams, 2008].
Adenovirus bound to serum proteins (e.g., complement factors, coagulation factors, non-specific immunoglobulins) in naïve individuals engage receptors such as complement receptors or Fc-receptors on immune cells which, in turn, contain PRRs. Activation of innate receptors such as TLR9 and NALP3 in cells of the immune system can induce inflammatory responses, leading to type I interferon (IFN) induction in murine dendritic cells and to IL-1β-processing in murine and human macrophages [Muruve et al., 2008; Zhu et al., 2007].
Innate pathways of viral clearance are very effective, even in naïve recipients. Recall that Kupffer cells clear adenovirus more effectively at low doses than do hepatocytes [Tao et al., 2001]. CD11c + spleen cells take up adenovirus vector and secrete IL-6, but—like Kupffer cells—express viral transgenes poorly. Similarly, alveolar macrophages internalize adenovirus administered to the lung, and produce IL-6 and TNF-α [Zsengeller et al., 2000]. These findings suggest cells of the immune system play key roles in initial virus uptake and in cytokine/chemokine expression observed immediately following intravenous virus administration, even in naïve individuals. They also demonstrate the differences among cell types in virus uptake versus vector transgene expression.
The innate immune response subsequently initiates the adaptive response and modulates its progression. Adenovirus-transduced macrophages and dendritic cells function as antigen presenting cells and elicit adaptive responses, including cytotoxic T-cell responses to both viral proteins and vector-encoded transgene products expressed by transduced cells [Yang et al., 1995]. Macrophages transport adenovirus to lymph nodes, where B cells produce antibodies to capsid proteins [Junt et al., 2007]. Memory CD8+ T cells, primed for life, recognize antigen-expressing tissue cells, leading to their rapid elimination. Following vector re-challenge, circulating neutralizing antibodies prevent virus transduction and memory T cells reduce transgene expression and kill transduced cells.
Because of the potential value of adenovirus vectors for gene therapy and vaccine development, and the roadblocks presented by Ad5-induced immunity, the mechanisms by which immune responses to adenovirus vectors are generated have been the topic of extensive effort. However, most studies of innate and adaptive responses to adenovirus vector transduction have been performed in naïve mice, not previously exposed to virus. Antibody binding to adenovirus in immune individuals is likely to inhibit vector transduction of target epithelial cells such as hepatocytes and to elicit increased uptake by Fc-receptor bearing immune cells such as Kupffer cells, resulting in rapid vector clearance and exacerbated inflammatory response (Fig. 2).
Fig. 2.
Adenovirus transduction of hepatocytes and Kupffer cells following intravenous administration in mice with circulating anti-adenovirus antibodies. In an immunized mouse, hepatocyte transduction following intravenous injection is blocked by antibodies that bind to the adenovirus capsid. In contrast, antibody coating of virus leads to increased uptake by the Kupffer cells, through Fc receptors on their surface. Because Kupffer cells do not efficiently express viral transgenes, virus administration to immunized animals results in overall reduced transgene expression.
ADENOVIRUS VECTORS IN ANIMAL MODELS AND CLINICAL TRIALS: EFFECT OF PRE-EXISTING IMMUNITY
Ad5 binds efficiently to dendritic cells (DCs), in an FcR-dependent fashion, in the presence of anti-Ad5 antibodies. In addition to increasing Ad binding, Ad5 antibodies also increase viral uptake into DCs. However, despite anti-Ad5 antibody increased binding and uptake by DCs, Ad-immunized mice injected with an adenovirus vector develop a reduced transgene-specific CD8+ T-cell immune response compared to naïve mice, suggesting antibody-enhanced vector DC uptake does not result in enhanced transduction (i.e., transgene expression) in vivo [Mercier et al., 2004]. Perreau et al. [2008] compared the interaction of adenovirus vectors with human DCs in the presence or absence of antibodies and found that adenovirus-immune complexes (IC) stimulated increased DC activation and maturation in an Fc-receptor dependent fashion. Ad-IC also increased the ability of DCs to prime Ad-specific CD8 T cells in vitro. Anti-adenovirus antibodies also increase FcR-dependent uptake of virus by macrophages, but target virus to phagolysosomes. Moreover, anti-adenovirus stimulated increased uptake into macrophages amplified innate intracellular pathways, leading to elevated expression of genes associated with the innate immune response in vitro and in vivo in mice [Zaiss et al., 2009]. Vlachaki et al. [2002] studied the effects of pre-immunization on intratumorally administered adenovirus transduction in a subcutaneous mouse mammary tumor model. Mice were immunized with adenovirus lacking a transgene. Two weeks later immunized and naïve mice received an intratumoral Ad-luciferase injection. Pre-immunization resulted in reduced tumor luciferase expression levels and reduced duration of transgene expression. Moreover, viral toxicity was greater in immunized animals; increased histologic hepatic damage, increased circulating hepatic enzyme levels and increased mortality relative to naïve mice occurred. These results suggest that preimmunity can exacerbate toxicity, rather than protecting the host, following viral administration.
In contrast, mice pre-immunized by intramuscular adenovirus injection showed less hepatic toxicity following rechallenge, reflected both by liver histopathology and by serum transaminase analysis [Varnavski et al., 2005]. However, pre-immunization did not prevent systemic toxicity, reflected by increased serum pro-inflammatory cytokines. Consistent with the previous study [Vlachaki et al., 2002], pre-immunized animals were at higher mortality risk following vector challenge; 17/81 pre-immunized mice died with 24 h following adenovirus administration (11 within the first hour), while no deaths occurred in this time period among 46 naïve mice.
When immunodeficient mice were supplemented with rabbit serum IgG fractions containing anti-adenovirus antibodies and subsequently challenged with adenovirus, they exhibited less toxicity than control mice. Moreover, mortality was reduced [Chen et al., 2000]. The action of rabbit anti-adenovirus antibodies in mice is restricted to virus neutralization; the Fc-portion of rabbit IgG is incompatible with murine immune cells. This result suggests the Fc portion of anti-adenovirus antibodies in immunized mice is responsible for the toxicity and mortality observed following virus administration.
Adenovirus toxicity has also been studied in immunized rhesus monkeys [Varnavski et al., 2002]. Immunity was induced by intramuscular adenovirus immunization, followed after 6 months with intravenous vector rechallenge. Virus pre-exposure diminished subsequent β-galactosidase transgene expression, following a second virus challenge, in the liver and in other tissues, but had little effect on viral DNA levels present in most tissues. Like the murine studies, the data suggest virus is taken up in the presence of antibodies, but transgene expression is suppressed. Cell fractionation, histologic and genomic DNA analysis suggested anti-adenovirus antibody re-directed vector uptake into innate immune cells. Pre-immunity did not eliminate toxicity; like the murine studies [Vlachaki et al., 2002; Varnavski et al., 2005], elevated serum pro-inflammatory cytokine levels were present in monkeys receiving the second virus challenge. A reduction of erythroid progenitor cells in the bone marrow [Varnavski et al., 2002] of pre-immunized—but not naïve—animals was also observed.
Although neither intramuscular nor oral administration of an Ad5 vaccine vector designed to protect mice from Ebola challenge were able to prevent Ebola-induced lethality in mice previously immunized with Ad5, intranasal administration of the Ad5 vaccine vector protected against subsequent Ebola challenge [Croyle et al., 2008]. These data and additional observations suggest that alternative routes of delivery for adenoviral therapeutic and vaccine vectors should continue to be examined as potential methods for evading pre-existing Ad immunity.
Unlike the non-replicating adenovirus vectors discussed above, oncolytic adenoviruses are engineered to be unable to replicate in normal human cells, but to be capable of replicating within and lyse human tumor cells. However, adenovirus does not replicate in mice, thus limiting the utility of murine models. Tsai et al. [2004] studied the impact of humoral immunity on oncolytic adenovirus therapy in a mouse tumor xenograft model. Nude mice were reconstituted with human sera containing anti-adenovirus antibodies, to establish immunity equivalent to humans. The sera did not decrease intravenously administered oncolytic adenovirus anti-tumor efficacy. While oncolytic adenovirus vectors may overcome neutralizing antibodies, since only a small number of replicating virus that reach the tumors might be sufficient to have a therapeutic effect, questions remain regarding this experiment. The Fc-portion of human IgG may be incompatible with murine cells; Fc-dependent effector functions might not occur in this model.
In contrast to mice, Syrian hamsters—like humans—are permissive for Ad infection. Dhar et al. [2009] recently examined the anti-tumor efficacy of a replicating oncolytic adenovirus (INGN 007) in previously immunized, immunocompetent Syrian hamsters. Preimmunity to Ad5 did not significantly change the anti-tumor effects of the virus when the virus was injected intratumorally into subcutaneous renal tumor grafts. Preimmunity also prevented virus spill-over to other organs.
In the one example we are aware of where baseline anti-adenovirus antibody titers were examined in a clinical trial (an oncolytic adenovirus injected intratumorally into prostate cancers), “there was no evident correlation between baseline antibody titer and subsequent PSA response” [DeWeese et al., 2001]. The three studies cited here on the potential for evasion of anti-Ad immunity by oncolytic adenovirus vectors are provocative. It will be of great interest to extend studies of the efficacy of replicating oncolytic adenovirus tumor therapy in previously immunized, immunocompetent animal models and in additional clinical trials, particularly following systemic virus administration.
Of note, the only currently approved clinical adenovirus therapy application employs an oncolytic adenovirus. In clinical trials, patients treated with the virus develop fevers after injections and show increased anti-viral antibody titers. No objective tumor response was demonstrated with virus alone. Because tumor stabilization was observed in patients treated with high viral doses, in combination with chemotherapy, this virus was approved in China for head and neck cancer treatment by direct intratumoral injection [Garber, 2006].
Exploitation of adenovirus immunogenicity has been used in attempts to develop vaccination vectors for tumors and for infectious diseases. Robust T cell responses are observed when HIV antigens are expressed from adenovirus vectors, resulting in protection against HIV infection in naïve primate models. Similar vectors induce anti-HIV CD8+ T cell responses in humans [Vanniasinkam and Ertl, 2005], suggesting this approach might limit disease progression However, results in primate trials were less encouraging; monkeys injected with an empty adenovirus vector prior to injections with Ad5-HIV gag had attenuated anti-gag T cell responses, compared to naïve animals [Casimiro et al., 2003].
The high prevalence of human anti-Ad5 antibodies, especially in sub-Saharan Africa, suggests adenovirus vector administration will likely lead both to antibody neutralization and to a rapid, exacerbated cellular immune response in many individuals. The resulting vector elimination is anticipated to be an impediment to the development of a T cell response to the vector-driven antigen, since enhanced viral clearance would result in greatly reduced transgene expression.
The practical scope of this problem is illustrated by the HIV-STEP trial, the first clinical trial of vaccine-induced cellular HIV immunity in humans. Three thousand healthy volunteers were immunized with a combination of adenovirus vectors expressing gag, pol, and nef genes. The trial was intended to test the vaccine's ability to reduce infection and/or viral load. The vaccine showed promising results in Phase I and II studies. However, although vaccination was successful in inducing HIV-specific CD8+ T cells, it failed to protect Ad5 seronegative individuals from HIV infection [Buchbinder et al., 2008]. Moreover, in participants with high initial anti-Ad5 antibody titers, the vaccine appears to have increased HIV infection [McElrath et al., 2008]. Two subsequent phase I trials using Ad5 vectors expressing only the gag gene suggest that systemic adverse events (22 categories) were more frequent in subjects with low (<200) baseline anti-Ad5 antibody titers versus subjects with higher Ad5 (>200) titers. HIV gag peptide immune responses (ELISPOT analyses) were also more frequent in individuals with low baseline anti-Ad5 antibody titers, varying inversely with baseline anti-Ad5 neutralizing antibody titer [Harro et al., 2009]. The results of the STEP trial and the subsequent study by Harro et al. [2009] demonstrate the practical consequences of the evidence that Ad5-mediated vaccine efficacy is likely to be impaired in humans that have even moderate anti-Ad5 antibody titers. These studies emphasize the need to develop strategies to overcome pre-existing immunity, if adenovirus vectors are to become valuable general tools in gene therapy and vaccination applications.
In a very interesting exception to the rule, Tuve et al. [2009] found that direct intratumoral injection of “non-replicating, transgene-devoid” Ad5 into transplanted subcutaneous mammary tumors in a syngeneic, immunocompetent mouse model elicited an immune response, mediated primarily by CD4+ and CD8+ T cells that inhibited tumor growth and increased survival time. Remarkably, pre-existing anti-Ad immunity enhanced the efficacy of intratumoral Ad5 therapy, increasing survival time. While the effect of this approach on tumors at distant sites is not yet known, the observation leads to a number of surprising and interesting potential avenues of exploitation.
STRATEGIES TO OVERCOME PRE-EXISTING IMMUNITY
HELPER-DEPENDENT VECTORS: ESCAPE FROM IMMUNE DETECTION?
Cells transduced with non-replicating first generation adenovirus vectors express viral genes, leading to display of capsid protein epitopes on transduced cell membranes. Continued capsid expression in transduced cells likely contributes to continued inflammation, enhanced immune-mediated virus clearance and memory T cell killing of transduced cells in an immunized host. Helper-dependent (HD) adenovirus vectors (HD-Ads, also called “third generation” or “gutless” adenoviruses) have nearly the entire viral genome deleted; no viral genes are transcribed after gene transfer [Parks et al., 1996]. HD-Ads can accommodate up to 35 kb of foreign DNA. Although generally reported to elicit reduced immunogenicity and prolonged transgene expression, several groups report that HD-Ad vectors induce innate immune responses in a pattern similar to that induced by first-generation adenovirus vectors. Like mice injected with first generation vectors, mice injected intravenously with HD-Ad vectors express innate immune response-associated genes, including inflammatory cytokines and chemokines in the liver within the first 24 h post injection [Muruve et al., 2004; McCaffrey et al., 2008]. In baboons, intravenous HD-Ad caused acute, dose-dependent toxicity in naïve animals [Brunetti-Pierri et al., 2004]. These HD-Ad studies support a growing body of evidence that the innate immune response to adenovirus is independent of viral gene expression [Kafri et al., 1998; Muruve et al., 1999; Zsengeller et al., 2000]. HD-Ad vectors can also provoke transgene-specific immunity [Muruve et al., 2004; McCaffrey et al., 2008].
First generation, conventional adenovirus vectors induce a second liver inflammation phase that includes additional inflammatory gene expression and hepatic lymphocyte infiltration at 7 days post-transduction [Muruve et al., 2004]. This second peak of inflammation likely originates from cytotoxic T lymphocytes (CTLs) that destroy transduced cells, which continuously express viral genes and, therefore, display capsid peptides on their surface. In contrast, HD-Ad do not induce expression of inflammatory genes beyond 24 h post-injection [Muruve et al., 2004]. Moreover, lymphocyte infiltration at 7 days does not occur. Since HD-Ad vectors lack viral genes, capsid proteins are provided only by the incoming virus and are only transiently presented by MHC I molecules in transduced cells. Once epitopes from the HD-Ad capsid are metabolized, cells containing vector DNA are no longer recognized by capsid-specific T cells; consequently transgene expression persists. These HD-Ad properties are likely reasons for improved transgene expression in naïve animals following transduction with helper-dependent vectors versus transduction with conventional adenovirus vectors.
Whether HD-Ad vectors confer much advantage over-first generation vectors in immunized hosts remains to be determined. In immunized hosts, HD-Ads will still be neutralized by anti-adenovirus antibodies. In addition, transient capsid epitope display on HD-Ad transduced cells in immunized hosts is likely to be sufficient for re-activation of memory T cells and consequent killing of vector-transduced cells [Molinier-Frenkel et al., 2000]. Thus, hepatic HD-Ad transgene expression in pre-immunized mice or baboons was only achieved with an HD-Ad vector of a different serotype [Morral et al., 1999; Parks et al., 1999], suggesting that HD-Ad vectors do not escape pre-existing immunity. In contrast, when an HD-Ad was injected intramuscularly into immunized mice, stable transgene expression could be achieved. Stable transduction was, however, not obtained when the HD-Ad was injected intravenously [Maione et al., 2001] into pre-immunized mice. These data suggest HD-Ad organ/tissue injection might circumvent systemic immunity in certain circumstances. In cell culture, [HD-Ad][neutralizing anti-Ad antibody] complexes are less potent in activating dendritic cells than are first-generation [Ad][neutralizing anti-Ad antibody] complexes [Perreau et al., 2008].
A cautionary note; helper virus contamination in HD-Ad preparations can vary substantially, and is often not well-described. Consequently, comparisons of both innate and adaptive responses among various HD-Ad reports may be compromised. Additional investigation is needed to determine effects of pre-existing adenovirus immunity on therapeutic applications of HD-Ad vectors.
GENE THERAPY IN THE BRAIN: IMMUNE-PRIVILEGED SITES AND SYSTEMIC IMMUNITY?
Adenovirus vectors injected into the brain can cause a dose-dependent local inflammatory response [Byrnes et al., 1995]. However, adenovirus-induced brain inflammation has a threshold of ~1 × 108 infectious units in the rat [Lowenstein et al., 2007]. Once this threshold is crossed, interferon (IFN) and chemokine expression, microglia activation, and macrophage/lymphocyte recruitment occurs, resulting in chronic inflammation and toxicity. At vector doses below threshold, however, long-term transgene expression continues for up to 1 year [Lowenstein et al., 2007]. Thus, although adenovirus vectors elicit only temporary expression in peripheral organs, long-term expression in brain is possible in naïve animals—as long as non-inflammatory viral doses are administered.
To test whether adenovirus-mediated transgene expression in brain is affected by subsequent systemic immunization, rats initially injected in the brain with a sub-threshold adenovirus vector dose were injected subcutaneously, 2 months later, with a vector encoding a different transgene. Brain inflammation and loss of brain transgene expression followed, accompanied by lymphocyte and macrophage infiltration into brain parenchyma [Byrnes et al., 1995, 1996; Barcia et al., 2006]. Immunological synapses were detected in the brain during clearing of transduced astrocytes, confirming that virus-transduced brain cells were cleared by activated cytotoxic lymphocytes [Barcia et al., 2006; Lowenstein et al., 2007].
As few as 1,000 vector particles injected into the mouse brain were subsequently recognized and cleared by the adaptive immune system following peripheral immunization. The inflammatory immune response in the brain was independent of either the promoter or the transgene used. Reversing the challenge led to a similar result; when mice were pre-immunized intraperitoneally with an adenovirus vector to establish anti-Ad immunity and then injected 30 days later in the brain with a vector encoding a different transgene, transgene expression was eliminated [Barcia et al., 2007; Lowenstein et al., 2007].
In contrast to conventional adenovirus vectors, HD-Ad can efficiently transduce brain and mediate stable transgene expression for up to 1 year, even in the presence of pre-existing anti-Ad immunity [Thomas et al., 2000; Barcia et al., 2007]. HD-Ad efficacy in promoting long-term transgene expression, even in mice with pre-existing anti-Ad immunity, suggests that brain-directed gene therapy using HD-Ad vectors may be practical even in seropositive patients. Pre-existing systemic immunity against the transgene does abrogate even HD-Ad transgene expression in the brain and is accompanied by brain inflammation [Xiong et al., 2008]. However, pre-existing immunity to therapeutic gene products is not likely to be nearly as prevalent as pre-existing immunity to adenovirus capsid proteins. Indeed, this same group has demonstrated a potent therapeutic response to HD-Ad directed Herpes Simplex Virus thymidine kinase/ganciclovir therapy and long term survival of rats carrying a syngeneic intracranial glioma, “even in the presence of systemic antiadenovirus immunity” [King et al., 2008].
In summary, using a combination of brain as an immune-privileged site for gene transfer and HD-Ad vectors, pre-existing immunity may be overcome; long-term transgene expression can be achieved in brain, providing systemic immunity against the transgene is not present. However, this observation has not been generalized to a variety of transgenes. We expect clinical trials for HD-Ad therapy of brain tumors to soon be initiated.
CAN PRE-EXISTING IMMUNITY BE AVOIDED BY CHANGING ADENOVIRUS SEROTYPES?
More than 50 adenovirus serotypes infect humans. Frequencies of serotype-specific antibodies vary widely in human populations. Ad5 antibodies are most common [Vogels et al., 2003]; vectors derived from other adenovirus serotypes may be able to escape Ad5 immunity. Several laboratories demonstrated (i) Ad35 immunity is less prevalent than Ad5 immunity in humans and (ii) Ad35 vectors are able to evade Ad5 immunity in mice and in human cells [Vogels et al., 2003; Barouch et al., 2004; Brouwer et al., 2007], leading to transgene expression (or to a transgene immune response in vaccine applications) in the presence of Ad5 antibodies. Other relatively rare human serotypes that appear promising in evading Ad5 immunity include Ad2 [Morral et al., 1999], Ad49 [Lemckert et al., 2006], Ad11 [Holterman et al., 2004] and Ad50, Ad26, and Ad48 [Abbink et al., 2007].
Lore et al. [2007] compared Ad35 and Ad5 as vaccine vectors. Cultured human dendritic cells were more susceptible to Ad35 transduction than to Ad5 transduction, likely due to the presence of CD46 on dendritic cells; CD46 is the primary Ad35 receptor. Ad35 was also able to more effectively stimulate dendritic cell antigen presentation to T cells, suggesting that Ad35 might be superior to Ad5 for vaccine development. However, these results suggest caution when employing Ad35 for gene therapy applications other than vaccination, since Ad35 vectors may elicit potent transgene-specific immune responses.
CD46, the receptor for many of these Ad serotypes, is expressed in humans but not mice. Stone et al. [2007a] analyzed immune responses and biodistribution for several adenovirus serotypes (serotypes 3, 4, 5, 11, 35, and 41). Immunogenicity was tested by injecting the vectors intravenously into naïve CD46 transgenic mice. Several serotypes induced unexpectedly high toxicity, with death rates of 25% for Ad11 (1 of 4), 75% for Ad3 (3 of 4) and 100% for Ad4 (4 of 4) at the highest dose (1 × 1011 particles/mouse). In contrast, at this virus titer death is rare for mice that receive Ad5. Despite differences in biodistribution, receptor usage and mortality, all adenovirus serotypes induced substantial IL-6, MCP-1, and IFN-γ release [Stone et al., 2007a], suggesting that use of high intravenous doses of any adenovirus for human gene therapy will need to be approached with caution.
Ni et al. [2006] report that when naïve C57-CD46 transgenic mice were injected with Ad5/35-GFP, a virus in which the Ad5 fiber was replaced with the fiber from Ad35, cytokine levels (IL5, TNFα, IFNα, IL12, MCP1) reflecting the innate immune response were all significantly lower than when these mice were injected with Ad5-GFP. Ad5/35 can also target metastatic and intraperitoneal tumors that express hCD46, following intravenous and intraperitoneal injection. To date, we are unaware of any studies of immune responses to Ad vectors in pre-immunized CD46 transgenic mice.
Most humans possess circulating antibodies against a range of adenovirus serotypes [Vogels et al., 2003]. In addition, T cells active against one human adenovirus serotype may cross-react with related serotypes [Smith et al., 1998]. One possibility for more effective adenovirus vectors for gene therapy and vaccine use, therefore, may be to develop vectors from different species, to escape pre-existing Ad5 immunity. Farina et al. [2001] developed vaccine vectors based on chimpanzee adenovirus serotype C68, to circumvent pre-existing Ad5 neutralizing antibodies. In mice, immunization with one Ad5 vector abrogated transgene immunogenicity elicited by a second Ad5 vector. In contrast, transgene immunity was not eliminated when an AdC68 vector was used for transgene expression [Fitzgerald et al., 2003]. However, transgene immunity was slightly reduced in Ad5 pre-immunized animals when using the chimp vector, because of Ad5-specific memory CD8+ T cells that cross-react with chimp vector epitopes [Fitzgerald et al., 2003].
Adenovirus vectors for gene therapy and vaccination have also been developed from ovine [Hofmann et al., 1999], bovine [Reddy et al., 1999], and porcine [Tuboly and Nagy, 2001] adenoviruses and canine adenovirus serotype 2 (CAV-2) [Perreau and Kremer, 2006]. CAV-2 vectors elicited transgene expression for 1 year in rats, without any obvious toxicity. Ad5 immunized mice exhibited strong transgene efficacy following challenge with a CAV-2 vector. Moreover, CAV-2 was not neutralized by 98% of human sera that contained a wide range of antibodies to human adenovirus serotypes. In addition, CAV-2 did not transduce or induce maturation of human dendritic cells [Perreau and Kremer, 2006]. The development of an HD-CAV-2 vector may further expand its potential as a clinical gene therapy vector; CAV-2 might be a safer, more effective vector, when compared to Ad5, for long-term transgene expression. However, inability of CAV-2 vectors to elicit human dendritic cell maturation might reduce applicability of these vectors for vaccination.
CAPSID-MODIFIED VECTORS AND ANTIVIRAL IMMUNITY
Genetic modification of adenovirus capsid proteins
Most adenovirus capsid modifications have been made to alter virus tropism. However, immune responses by tropism-modified adenovirus vectors have been compared with immune responses to the parental vectors. Schoggins and Falck-Pedersen [2006] and Schoggins et al. [2005] mutated both the Ad5 fiber protein and penton base capsid protein, on the assumption that hepatocyte viral entry is mediated by knob-CAR and penton–integrin interactions. These vectors generated a greatly reduced innate inflammatory response. However, they still provoked strong neutralizing capsid immunity and anti-transgene antibodies. Indeed, three of their CAR/penton “untargeted” adenovirus mutants induced anti-transgene immunity greater than that induced by the unmodified Ad5 vector. These vectors may, therefore, have substantial value for vaccine production. However, since the bulk of the antibody response to adenovirus vectors is directed toward the hexon protein [Sumida et al., 2005], they are also likely to be poor gene therapy vectors for patients with pre-existing immunity.
Iacobelli-Martinez and Nemerow [2007] analyzed IFNα production from human peripheral blood mononuclear cells (PBMC) transduced with Ad5 vector or Ad5 derivatives expressing fiber proteins from Ads 37, 16, or 35. Ad5 vectors expressing the Ad35 fiber (which binds to CD46) induced more type I interferons in PBMC than did wild type Ad5 (which binds to CAR). Adenovirus can transduce HeLa cells both by CAR-mediated and CD46-mediated pathways. Despite equivalent transduction rates by both viruses, CD46-mediated viral entry “showed a preferential induction of TLR9-mediated events,” including increased cytokine induction and activation of NF-κB, relative to CAR-mediated transduction. Thus the viral entry route plays a significant role in subsequent TLR9-mediated innate immune responses; alterations in intracellular trafficking due to capsid modifications can change the innate immune response to adenovirus and the resulting toxicity. These data suggest that CD46 mediated binding of adenovirus vectors to mononuclear cells and dendritic cells plays a substantial role in the innate immune response. Since mice do not express CD46, the validity of mouse models for examination of innate and acquired adenovirus immunity becomes an issue in translation to the clinic.
Although antibodies directed against adenovirus fiber proteins and penton base protein do exist, the bulk of neutralizing anti-adenovirus antibodies are directed against the hexon protein [Sumida et al., 2005]. Several investigators constructed adenovirus vectors in which the hexon gene of one serotype replaced the hexon gene or hexon sequences of another serotype. Gall et al. [1998] replaced the Ad5 hexon gene with either the entire hexon or with specific hexon sequences (loop 1 and 2) from Ad2 and tested whether the vector could escape pre-existing Ad5 immunity. The hexon-modified viruses were neutralized to some degree by Ad5 antiserum in vitro, probably due to the epitope similarity between the two vectors. Pre-existing anti-Ad5 immunity also suppressed gene expression by the Ad5 hexon-modified vector in vivo [Gall et al., 1998]. In contrast, when Ad5 viruses in which the endogenous hexon gene was replaced with hexon genes from Ad3, Ad6, or Ad12 were administered to Ad5 immunized mice, there was no significant loss in transgene expression [Roy et al., 1998; Wu et al., 2002; Youil et al., 2002]. However, a large percentage of humans have neutralizing anti-Ad3, anti-Ad6, and/or anti-Ad12 antibodies [Vogels et al., 2003]. This proof-of-principle experiment will need to be explored more thoroughly, with additional hexon modifications, to determine its practical feasibility for evading naturally occurring neutralizing antibodies in human applications.
Roberts et al. [2006] engineered Ad5 vectors to obtain capsids that can evade pre-existing immunity. Ad5 neutralizing antibodies are mainly directed against the seven hypervariable regions (HVRs) of the hexon subunit. Ad48 neutralizing antibodies are rare in the human population. By exchanging all seven Ad5 HVRs for Ad48 HVRs, they constructed a virus that could evade Ad5 neutralizing antibody and produce CD8+ T cell responses to adenovirus-driven SIV gag protein in Ad5 preimmunized mice. Ad5 vector did not induce measurable anti-transgene immunity in Ad5 pre-immunized mice or rhesus monkeys. In contrast, gag immunogenicity induced by Ad5/HVR48 virus was comparable in both Ad5 pre-immunized and naïve mice and monkeys, suggesting the modified vector evades Ad5 neutralizing antibodies. This study suggests that antibodies to fiber and penton base proteins, generally present in low titers in Ad5 immunized hosts, do not play significant roles in virus neutralization in this challenge protocol for Ad5/HVR8 immunization.
Chemical modification of adenovirus capsid proteins
Another approach to reduce innate and adaptive adenovirus immunity in naïve individuals has been to “mask” viral epitopes by attaching chemical polymers such as polyethylene glycol (PEG) to adenovirus capsid proteins. PEGylated virus, administered intravenously to naïve mice, resulted in significantly reduced IL-6 serum levels 6 h after injection when compared to the response to unmodified virus, despite equivalent liver transduction [Croyle et al., 2005; Mok et al., 2005]. PEGylated virus association with liver Kupffer cells was reduced, as was IL-6 secretion from PEGylated virus-transduced RAW 264.7 macrophages in culture [Mok et al., 2005]. Unlike unmodified virus, PEGylated virus did not reduce platelet counts leading to thrombocytopenia in mice, suggesting the polymer might interfere with virus association with platelets in murine blood [Croyle et al., 2005]. PEGylation also reduced cytotoxic T cell activation and neutralizing antibody production when modified virus was administered intravenously to naïve animals [Croyle et al., 2002].
Capsid PEGylation has also been proposed as a way to escape adenovirus antibody neutralization in individuals immune to adenovirus. The pioneering work of Chillon et al. [1998] demonstrated adenovirus PEGylation enhanced transduction of cells otherwise difficult to transduce; for example, mouse lung. PEG-coated virus could also escape antibody neutralization in cell culture. However, in vivo, PEGylated virus could escape antibody neutralization only when administered intranasally. PEGylated virus failed to induce transgene expression (measured as virus transgene encoded α1-antitrypsin) when administered intravenously to immunized mice. Similarly, in another study, PEGylated virus was nearly equally effective in eliciting transgene expression in lungs of adenovirus-immunized mice and naïve mice following intranasal administration [O'Riordan et al., 1999]. In contrast, delivery of PEGylated virus to lungs of pre-immunized mice resulted in 100-fold lower transgene expression when compared to the same PEGylated virus administered to lungs of naïve mice [Croyle et al., 2001].
In naïve mice, intravenously injected PEGylated virus increased transgene expression in the liver fivefold relative to native virus; moreover, hepatic transgene expression was prolonged in mice receiving PEGylated adenovirus. Although rechallenge with PEGylated vector of preimmunized mice expressing circulating neutralizing antibody resulted in hepatic transgene expression, this expression was substantially less (~1%) than that observed in naïve mice receiving the same vector [Croyle et al., 2002]. In contrast, liver transgene expression in immunized mice following injection of PEGylated helper-dependent adenovirus was only one log lower than hepatic expression in naïve mice [Croyle et al., 2005].
Wortmann et al. [2008] challenged (intramuscularly) naïve mice that had been supplemented with sera from Ad5 immunized mice either with a control vector expressing the small surface antigen of hepatitis B antigen (AdHBsAg) or with PEGylated AdHBsAg, and measured the cellular immune response to HBsAg 4 weeks later. Passively Ad5-immunized mice receiving AdHBsAg had a 15% response compared to naïve mice receiving this same challenge. Ad5-immunized mice receiving PEGylatd AdHBsAG had a 67% response compared to naïve mice, suggesting PEGylation can mask intramuscularly injected virus from neutralizing antibody. Kreppel and Kochanek [2008] recently reviewed polymer modification effects on Ad transgene expression, innate immune response and evasion of pre-existing immunity. Studies of immune escape by PEGylated adenovirus in mice are growing in number; whether the protection reported will be sufficient to use adenovirus vectors in individuals with substantial anti-adenovirus titers, either for vaccine development or for gene transfer therapeutic applications, however, remains to be seen.
CONCLUSIONS
Both innate immune responses to adenovirus challenge and pre-existing adenovirus immunity represent fundamental problems, with great implications for both safety and efficacy of adenovirus vector gene therapy applications. The limitations posed by pre-existing immunity to gene therapy are not unique to adenovirus vector systems. Although adeno-associated virus (AAV) vectors elicit greatly reduced innate immune responses compared to adenovirus vectors, pre-existing adaptive immunity in humans has also limited the success of AAV2-gene therapy clinical trials [Mingozzi and High, 2007]. While the problem(s) have been recognized for some time, progress has lagged on these major obstacles for clinical applications of an otherwise robust reagent.
Recall that the majority of the human population is adenovirus seropositive [Chirmule et al., 1999]. Therefore, in the majority of potential patients for adenoviral therapy, contact between virus and blood will result in adenovirus-antibody complex formation. These complexes are likely to induce inflammatory reactions in patients equivalent to those observed in experimental animals. Indeed, limited experience has demonstrated that adenovirus vectors can cause severe inflammatory reactions in patients; reactions which can result in systemic sepsis-like syndromes and even death.
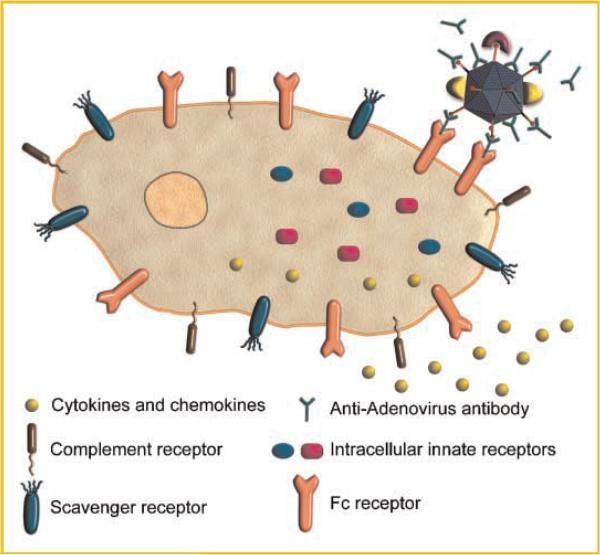
Studies in immunized animals and clinical trials data suggest the presence of anti-Ad5 antibodies results—not surprisingly—in overall reduced target cell transduction and transgene expression. Adenovirus immunity also results in decreased duration of transgene expression, most likely due to memory T cell responses that rapidly clear cells expressing viral capsid proteins. In addition to reducing efficacy of gene therapy applications, neutralizing anti-adenovirus antibodies can also abrogate induction of immunity to expressed transgenes in adenoviral vector vaccine applications. Pre-existing immunity also can lead to increased toxicity and even mortality in mouse and non-human primate models in response to adenovirus vector challenge [Varnavski et al., 2002, 2005; Vlachaki et al., 2002]. These increased immune responses may be due to increased vector uptake by innate immune cells; innate immune cells can take up antibody-opsonized virus via Fc-receptor-mediated pathways. Enhanced uptake of antibody-opsonized vector by immune cells might also result in increased exposure of intracellular innate receptors such as NALP3 or TLR9 to the virus, resulting in inflammatory gene expression [Muruve et al., 2008; Zaiss et al., 2009] (Fig. 3).
Fig. 3.
Cytokine and chemokine production in mice with circulating anti-adenovirus antibodies. Opsonization of adenovirus by neutralizing antibodies promotes increased virus particle binding to, and uptake by, Fc receptors present on macrophages and other cells of the immune system. As a result of increased virus uptake by immune cells, adenovirus vectors are more rapidly cleared from the circulation than they are in naïve animals, and transgene expression is reduced in target tissues. The increased Fc-receptor mediated uptake of vector by immune cells leads to enhanced engagement of innate receptors such as TLR9 or NALP3, increased cytokine and chemokine release and increased host toxicity.
Considering the potential increased toxicity and reduced vector efficacy in immunized hosts, it is clear that identifying means to evade antibody neutralization is a major necessity if we are to develop systemic adenovirus therapies. Current strategies to overcome Ad5 vector antibody include, but are not limited to, use of alternative adenovirus serotypes, modification or chimerism of capsid hexon proteins and coating virus with PEG and similar polymers.
Targeting adenovirus to specific organs, tissues or cell types, reducing interactions with undesired target organs, tissues or cells, and modulating immune responses to adenovirus vectors and/or transgenes are major objectives for adenovirus gene therapy in the future. To understand how capsid modifications affect both targeting and immune responses, we require more detailed knowledge about underlying mechanisms that determine cell uptake, transduction, productive infection and immune induction. Additional studies using these vectors with cells ex vivo, in animal models and in phase I clinical trials should provide valuable information about antibody escape, host safety and vector biological efficacy. In light of recently emerging data on adenovirus interactions with serum proteins and blood cells in vivo, however, current capsid modifications aimed at tropism changes need to be re-evaluated. New approaches to reducing initial innate immune responses and to evading pre-existing adenovirus immunity are critically needed, if we are to develop reliable, robust adenovirus therapeutic agents.
ACKNOWLEDGMENTS
This study was supported by the grant National Cancer Institute (P50 CA86306 and R01 CA084572 to H.R.H. and P.I.).
REFERENCES
- Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, O'Brien KL, Carville A, Mansfield KG, Goudsmit J, Havenga MJ, Barouch DH. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcia C, Thomas CE, Curtin JF, King GD, Wawrowsky K, Candolfi M, Xiong WD, Liu C, Kroeger K, Boyer O, Kupiec-Weglinski J, Klatzmann D, Castro MG, Lowenstein PR. In vivo mature immunological synapses forming SMACs mediate clearance of virally infected astrocytes from the brain. J Exp Med. 2006;203:2095–2107. doi: 10.1084/jem.20060420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcia C, Jimenez-Dalmaroni M, Kroeger KM, Puntel M, Rapaport AJ, Larocque D, King GD, Johnson SA, Liu C, Xiong W, Candolfi M, Mondkar S, Ng P, Palmer D, Castro MG, Lowenstein PR. One-year expression from high-capacity adenoviral vectors in the brains of animals with pre-existing anti-adenoviral immunity: Clinical implications. Mol Ther. 2007;15:2154–2163. doi: 10.1038/sj.mt.6300305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, Truitt DM, Sumida SM, Kishko MG, Arthur JC, Korioth-Schmitz B, Newberg MH, Gorgone DA, Lifton MA, Panicali DL, Nabel GJ, Letvin NL, Goudsmit J. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- Brouwer E, Havenga MJ, Ophorst O, de Leeuw B, Gijsbers L, Gillissen G, Hoeben RC, ter Horst M, Nanda D, Dirven C, Avezaat CJ, Goudsmit J, Sillevis Smitt P. Human adenovirus type 35 vector for gene therapy of brain cancer: Improved transduction and bypass of pre-existing anti-vector immunity in cancer patients. Cancer Gene Ther. 2007;14:211–219. doi: 10.1038/sj.cgt.7701010. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M, Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study). A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes AP, Rusby JE, Wood MJ, Charlton HM. Adenovirus gene transfer causes inflammation in the brain. Neuroscience. 1995;66:1015–1024. doi: 10.1016/0306-4522(95)00068-t. [DOI] [PubMed] [Google Scholar]
- Byrnes AP, MacLaren RE, Charlton HM. Immunological instability of persistent adenovirus vectors in the brain: Peripheral exposure to vector leads to renewed inflammation, reduced gene expression, and demyelination. J Neurosci. 1996;16:3045–3055. doi: 10.1523/JNEUROSCI.16-09-03045.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle RC, Di Y, Cerny AM, Sonnen AF, Sim RB, Green NK, Subr V, Ulbrich K, Gilbert RJ, Fisher KD, Finberg RW, Seymour LW. Human erythrocytes bind and inactivate type 5 adenovirus by presenting Coxsackie virus-adenovirus receptor and complement receptor 1. Blood. 2009;113:1909–1918. doi: 10.1182/blood-2008-09-178459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, Tang A, Chen M, Huang L, Harris V, Freed DC, Wilson KA, Dubey S, Zhu DM, Nawrocki D, Mach H, Troutman R, Isopi L, Williams D, Hurni W, Xu Z, Smith JG, Wang S, Liu X, Guan L, Long R, Trigona W, Heidecker GJ, Perry HC, Persaud N, Toner TJ, Su Q, Liang X, Youil R, Chastain M, Bett AJ, Volkin DB, Emini EA, Shiver JW. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003;77:6305–6313. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yu DC, Charlton D, Henderson DR. Pre-existent adenovirus antibody inhibits systemic toxicity and antitumor activity of CN706 in the nude mouse LNCaP xenograft model: Implications and proposals for human therapy. Hum Gene Ther. 2000;11:1553–1567. doi: 10.1089/10430340050083289. [DOI] [PubMed] [Google Scholar]
- Chillon M, Lee JH, Fasbender A, Welsh MJ. Adenovirus complexed with polyethylene glycol and cationic lipid is shielded from neutralizing antibodies in vitro. Gene Ther. 1998;5:995–1002. doi: 10.1038/sj.gt.3300665. [DOI] [PubMed] [Google Scholar]
- Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci USA. 2001;98:15191–15196. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croyle MA, Chirmule N, Zhang Y, Wilson JM. “Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J Virol. 2001;75:4792–4801. doi: 10.1128/JVI.75.10.4792-4801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croyle MA, Chirmule N, Zhang Y, Wilson JM. PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum Gene Ther. 2002;13:1887–1900. doi: 10.1089/104303402760372972. [DOI] [PubMed] [Google Scholar]
- Croyle MA, Le HT, Linse KD, Cerullo V, Toietta G, Beaudet A, Pastore L. PEGylated helper-dependent adenoviral vectors: Highly efficient vectors with an enhanced safety profile. Gene Ther. 2005;12:579–587. doi: 10.1038/sj.gt.3302441. [DOI] [PubMed] [Google Scholar]
- Croyle MA, Patel A, Tran KN, Gray M, Zhang Y, Strong JE, Feldmann H, Kobinger GP. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PLoS One. 2008;3:e3548. doi: 10.1371/journal.pone.0003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWeese TL, van der Poel H, Li S, Mikhak B, Drew R, Goemann M, Hamper U, DeJong R, Detorie N, Rodriguez R, Haulk T, DeMarzo AM, Piantadosi S, Yu DC, Chen Y, Henderson DR, Carducci MA, Nelson WG, Simons JW. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–7472. [PubMed] [Google Scholar]
- Dhar D, Spencer JF, Toth K, Wold WS. Effect of preexisting immunity on oncolytic adenovirus vector INGN 007 antitumor efficacy in immunocompetent and immunosuppressed Syrian hamsters. J Virol. 2009;83:2130–2139. doi: 10.1128/JVI.02127-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo NC, van Rooijen N, Shayakhmetov DM. Redundant and synergistic mechanisms control the sequestration of blood-born adenovirus in the liver. Mol Ther. 2009;17:675–684. doi: 10.1038/mt.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einfeld DA, Schroeder R, Roelvink PW, Lizonova A, King CR, Kovesdi I, Wickham TJ. Reducing the native tropism of adenovirus vectors requires removal of both CAR and integrin interactions. J Virol. 2001;75:11284–11291. doi: 10.1128/JVI.75.23.11284-11291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina SF, Gao GP, Xiang ZQ, Rux JJ, Burnett RM, Alvira MR, Marsh J, Ertl HC, Wilson JM. Replication-defective vector based on a chimpanzee adenovirus. J Virol. 2001;75:11603–11613. doi: 10.1128/JVI.75.23.11603-11613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JC, Gao GP, Reyes-Sandoval A, Pavlakis GN, Xiang ZQ, Wlazlo AP, Giles-Davis W, Wilson JM, Ertl HC. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J Immunol. 2003;170:1416–1422. doi: 10.4049/jimmunol.170.3.1416. [DOI] [PubMed] [Google Scholar]
- Gall JG, Crystal RG, Falck-Pedersen E. Construction and characterization of hexon-chimeric adenoviruses: Specification of adenovirus serotype. J Virol. 1998;72:10260–10264. doi: 10.1128/jvi.72.12.10260-10264.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber K. China approves world's first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- Harro CD, Robertson MN, Lally MA, O'Neill LD, Edupuganti S, Goepfert PA, Mulligan MJ, Priddy FH, Dubey SA, Kierstead LS, Sun X, Casimiro DR, DiNubile MJ, Shiver JW, Leavitt RY, Mehrotra DV. Safety and immunogenicity of adenovirus-vectored near-consensus HIV type 1 clade B gag vaccines in healthy adults. AIDS Res Hum Retroviruses. 2009;25:103–114. doi: 10.1089/aid.2008.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann C, Loser P, Cichon G, Arnold W, Both GW, Strauss M. Ovine adenovirus vectors overcome preexisting humoral immunity against human adenoviruses in vivo. J Virol. 1999;73:6930–6936. doi: 10.1128/jvi.73.8.6930-6936.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holterman L, Vogels R, van der Vlugt R, Sieuwerts M, Grimbergen J, Kaspers J, Geelen E, van der Helm E, Lemckert A, Gillissen G, Verhaagh S, Custers J, Zuijdgeest D, Berkhout B, Bakker M, Quax P, Goudsmit J, Havenga M. Novel replication-incompetent vector derived from adenovirus type 11 (Ad11) for vaccination and gene therapy: Low seroprevalence and non-cross-reactivity with Ad5. J Virol. 2004;78:13207–13215. doi: 10.1128/JVI.78.23.13207-13215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobelli-Martinez M, Nemerow GR. Preferential activation of Toll-like receptor nine by CD46-utilizing adenoviruses. J Virol. 2007;81:1305–1312. doi: 10.1128/JVI.01926-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, van Rooijen N, Mempel TR, Whelan SP, von Andrian UH. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- Kafri T, Morgan D, Krahl T, Sarvetnick N, Sherman L, Verma I. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: Implications for gene therapy. Proc Natl Acad Sci USA. 1998;95:11377–11382. doi: 10.1073/pnas.95.19.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL, Shayakhmetov DM. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci USA. 2008;105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GD, Muhammad AK, Xiong W, Kroeger KM, Puntel M, Larocque D, Palmer D, Ng P, Lowenstein PR, Castro MG. High-capacity adenovirus vector-mediated anti-glioma gene therapy in the presence of systemic antiadenovirus immunity. J Virol. 2008;82:4680–4684. doi: 10.1128/JVI.00232-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi N, Yamaguchi T, Kawabata K, Sakurai F, Sasaki T, Watanabe Y, Hayakawa T, Mizuguchi H. Fiber-modified adenovirus vectors decrease liver toxicity through reduced IL-6 production. J Immunol. 2007;178:1767–1773. doi: 10.4049/jimmunol.178.3.1767. [DOI] [PubMed] [Google Scholar]
- Kreppel F, Kochanek S. Modification of adenovirus gene transfer vectors with synthetic polymers: A scientific review and technical guide. Mol Ther. 2008;16:16–29. doi: 10.1038/sj.mt.6300321. [DOI] [PubMed] [Google Scholar]
- Lemckert AA, Grimbergen J, Smits S, Hartkoorn E, Holterman L, Berkhout B, Barouch DH, Vogels R, Quax P, Goudsmit J, Havenga MJ. Generation of a novel replication-incompetent adenoviral vector derived from human adenovirus type 49: Manufacture on PER.C6 cells, tropism and immunogenicity. J Gen Virol. 2006;87:2891–2899. doi: 10.1099/vir.0.82079-0. [DOI] [PubMed] [Google Scholar]
- Lore K, Adams WC, Havenga MJ, Precopio ML, Holterman L, Goudsmit J, Koup RA. Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. J Immunol. 2007;179:1721–1729. doi: 10.4049/jimmunol.179.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein PR, Kroeger K, Castro MG. Immunology of neurological gene therapy: How T cells modulate viral vector-mediated therapeutic transgene expression through immunological synapses. Neurotherapeutics. 2007;4:715–724. doi: 10.1016/j.nurt.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons M, Onion D, Green NK, Aslan K, Rajaratnam R, Bazan-Peregrino M, Phipps S, Hale S, Mautner V, Seymour LW, Fisher KD. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol Ther. 2006;14:118–128. doi: 10.1016/j.ymthe.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Maione D, Della Rocca C, Giannetti P, D'Arrigo R, Liberatoscioli L, Franlin LL, Sandig V, Ciliberto G, La Monica N, Savino R. An improved helper-dependent adenoviral vector allows persistent gene expression after intramuscular delivery and overcomes preexisting immunity to adenovirus. Proc Natl Acad Sci USA. 2001;98:5986–5991. doi: 10.1073/pnas.101122498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey AP, Fawcett P, Nakai H, McCaffrey RL, Ehrhardt A, Pham TT, Pandey K, Xu H, Feuss S, Storm TA, Kay MA. The host response to adenovirus, helper-dependent adenovirus, and adeno-associated virus in mouse liver. Mol Ther. 2008;16:931–941. doi: 10.1038/mt.2008.37. [DOI] [PubMed] [Google Scholar]
- McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, Buchbinder SP, Robertson MN, Mehrotra DV, Self SG, Corey L, Shiver JW, Casimiro DR. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: A case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier S, Rouard H, Delfau-Larue MH, Eloit M. Specific antibodies modulate the interactions of adenovirus type 5 with dendritic cells. Virology. 2004;322:308–317. doi: 10.1016/j.virol.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7:316–324. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- Mok H, Palmer DJ, Ng P, Barry MA. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol Ther. 2005;11:66–79. doi: 10.1016/j.ymthe.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Molinier-Frenkel V, Gahery-Segard H, Mehtali M, Le Boulaire C, Ribault S, Boulanger P, Tursz T, Guillet JG, Farace F. Immune response to recombinant adenovirus in humans: Capsid components from viral input are targets for vector-specific cytotoxic T lymphocytes. J Virol. 2000;74:7678–7682. doi: 10.1128/jvi.74.16.7678-7682.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morral N, O'Neal W, Rice K, Leland M, Kaplan J, Piedra PA, Zhou H, Parks RJ, Velji R, Aguilar-Cordova E, Wadsworth S, Graham FL, Kochanek S, Carey KD, Beaudet AL. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci USA. 1999;96:12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruve DA, Barnes MJ, Stillman IE, Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- Muruve DA, Cotter MJ, Zaiss AK, White LR, Liu Q, Chan T, Clark SA, Ross PJ, Meulenbroek RA, Maelandsmo GM, Parks RJ. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J Virol. 2004;78:5966–5972. doi: 10.1128/JVI.78.11.5966-5972.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- Ni S, Gaggar A, Di Paolo N, Li ZY, Liu Y, Strauss R, Sova P, Morihara J, Feng Q, Kiviat N, Toure P, Sow PS, Lieber A. Evaluation of adenovirus vectors containing serotype 35 fibers for tumor targeting. Cancer Gene Ther. 2006;13:1072–1081. doi: 10.1038/sj.cgt.7700981. [DOI] [PubMed] [Google Scholar]
- Nicklin SA, Wu E, Nemerow GR, Baker AH. The influence of adenovirus fiber structure and function on vector development for gene therapy. Mol Ther. 2005;12:384–393. doi: 10.1016/j.ymthe.2005.05.008. [DOI] [PubMed] [Google Scholar]
- O'Riordan CR, Lachapelle A, Delgado C, Parkes V, Wadsworth SC, Smith AE, Francis GE. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum Gene Ther. 1999;10:1349–1358. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]
- Parker AL, Waddington SN, Nicol CG, Shayakhmetov DM, Buckley SM, Denby L, Kemball-Cook G, Ni S, Lieber A, McVey JH, Nicklin SA, Baker AH. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108:2554–2561. doi: 10.1182/blood-2006-04-008532. [DOI] [PubMed] [Google Scholar]
- Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL. A helper-dependent adenovirus vector system: Removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks R, Evelegh C, Graham F. Use of helper-dependent adenoviral vectors of alternative serotypes permits repeat vector administration. Gene Ther. 1999;6:1565–1573. doi: 10.1038/sj.gt.3300995. [DOI] [PubMed] [Google Scholar]
- Perreau M, Kremer EJ. The conundrum between immunological memory to adenovirus and their use as vectors in clinical gene therapy. Mol Biotechnol. 2006;34:247–256. doi: 10.1385/MB:34:2:247. [DOI] [PubMed] [Google Scholar]
- Perreau M, Pantaleo G, Kremer EJ. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. J Exp Med. 2008;205:2717–2725. doi: 10.1084/jem.20081786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichla-Gollon SL, Lin SW, Hensley SE, Lasaro MO, Herkenhoff-Haut L, Drinker M, Tatsis N, Gao GP, Wilson JM, Ertl HC, Bergelson JM. Effect of preexisting immunity on an adenovirus vaccine vector: In vitro neutralization assays fail to predict inhibition by antiviral antibody in vivo. J Virol. 2009;83:5567–5573. doi: 10.1128/JVI.00405-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PS, Idamakanti N, Chen Y, Whale T, Babiuk LA, Mehtali M, Tikoo SK. Replication-defective bovine adenovirus type 3 as an expression vector. J Virol. 1999;73:9137–9144. doi: 10.1128/jvi.73.11.9137-9144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DM, Nanda A, Havenga MJ, Abbink P, Lynch DM, Ewald BA, Liu J, Thorner AR, Swanson PE, Gorgone DA, Lifton MA, Lemckert AA, Holterman L, Chen B, Dilraj A, Carville A, Mansfield KG, Goudsmit J, Barouch DH. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- Roy S, Shirley PS, McClelland A, Kaleko M. Circumvention of immunity to the adenovirus major coat protein hexon. J Virol. 1998;72:6875–6879. doi: 10.1128/jvi.72.8.6875-6879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell MA, Zhang Y, Tazelaar J, Gao GP, Yu QC, Qian R, Chen SJ, Varnavski AN, LeClair C, Raper SE, Wilson JM. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol Ther. 2001;3:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- Schoggins JW, Falck-Pedersen E. Fiber and penton base capsid modifications yield diminished adenovirus type 5 transduction and proinflammatory gene expression with retention of antigen-specific humoral immunity. J Virol. 2006;80:10634–10644. doi: 10.1128/JVI.01359-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins JW, Nociari M, Philpott N, Falck-Pedersen E. Influence of fiber detargeting on adenovirus-mediated innate and adaptive immune activation. J Virol. 2005;79:11627–11637. doi: 10.1128/JVI.79.18.11627-11637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiradake E, Henaff D, Wodrich H, Billet O, Perreau M, Hippert C, Mennechet F, Schoehn G, Lortat-Jacob H, Dreja H, Ibanes S, Kalatzis V, Wang JP, Finberg RW, Cusack S, Kremer EJ. The cell adhesion molecule “CAR” and sialic acid on human erythrocytes influence adenovirus in vivo biodistribution. PLoS Pathog. 2009;5:e1000277. doi: 10.1371/journal.ppat.1000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Woodruff LS, Rooney C, Kitchingman GR. Extensive cross-reactivity of adenovirus-specific cytotoxic T cells. Hum Gene Ther. 1998;9:1419–1427. doi: 10.1089/hum.1998.9.10-1419. [DOI] [PubMed] [Google Scholar]
- Stone D, Liu Y, Li ZY, Tuve S, Strauss R, Lieber A. Comparison of adenoviruses from species B, C, E, and F after intravenous delivery. Mol Ther. 2007a;15:2146–2153. doi: 10.1038/sj.mt.6300319. [DOI] [PubMed] [Google Scholar]
- Stone D, Liu Y, Shayakhmetov D, Li ZY, Ni S, Lieber A. Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver. J Virol. 2007b;81:4866–4871. doi: 10.1128/JVI.02819-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford-Perricaudet LD, Levrero M, Chasse JF, Perricaudet M, Briand P. Evaluation of the transfer and expression in mice of an enzyme-encoding gene using a human adenovirus vector. Hum Gene Ther. 1990;1:241–256. doi: 10.1089/hum.1990.1.3-241. [DOI] [PubMed] [Google Scholar]
- Sumida SM, Truitt DM, Lemckert AA, Vogels R, Custers JH, Addo MM, Lockman S, Peter T, Peyerl FW, Kishko MG, Jackson SS, Gorgone DA, Lifton MA, Essex M, Walker BD, Goudsmit J, Havenga MJ, Barouch DH. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J Immunol. 2005;174:7179–7185. doi: 10.4049/jimmunol.174.11.7179. [DOI] [PubMed] [Google Scholar]
- Tao N, Gao GP, Parr M, Johnston J, Baradet T, Wilson JM, Barsoum J, Fawell SE. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol Ther. 2001;3:28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: Toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci USA. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomko RP, Xu R, Philipson L. HCAR and MCAR: The human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai V, Johnson DE, Rahman A, Wen SF, LaFace D, Philopena J, Nery J, Zepeda M, Maneval DC, Demers GW, Ralston R. Impact of human neutralizing antibodies on antitumor efficacy of an oncolytic adenovirus in a murine model. Clin Cancer Res. 2004;10:7199–7206. doi: 10.1158/1078-0432.CCR-04-0765. [DOI] [PubMed] [Google Scholar]
- Tuboly T, Nagy E. Construction and characterization of recombinant porcine adenovirus serotype 5 expressing the transmissible gastroenteritis virus spike gene. J Gen Virol. 2001;82:183–190. doi: 10.1099/0022-1317-82-1-183. [DOI] [PubMed] [Google Scholar]
- Tuve S, Liu Y, Tragoolpua K, Jacobs DJ, Yumul RC, Li Z-Y, Strauss R, Hellstrom K-E, Disis ML, Roffler S, Lieber A. In situ adenovirus vaccination engages T effector cells against cancer. Vaccine. 2009;27:4225–4239. doi: 10.1016/j.vaccine.2009.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanniasinkam T, Ertl HC. Adenoviral gene delivery for HIV-1 vaccination. Curr Gene Ther. 2005;5:203–212. doi: 10.2174/1566523053544236. [DOI] [PubMed] [Google Scholar]
- Varnavski AN, Zhang Y, Schnell M, Tazelaar J, Louboutin JP, Yu QC, Bagg A, Gao GP, Wilson JM. Preexisting immunity to adenovirus in rhesus monkeys fails to prevent vector-induced toxicity. J Virol. 2002;76:5711–5719. doi: 10.1128/JVI.76.11.5711-5719.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnavski AN, Calcedo R, Bove M, Gao G, Wilson JM. Evaluation of toxicity from high-dose systemic administration of recombinant adenovirus vector in vector-naive and pre-immunized mice. Gene Ther. 2005;12:427–436. doi: 10.1038/sj.gt.3302347. [DOI] [PubMed] [Google Scholar]
- Vlachaki MT, Hernandez-Garcia A, Ittmann M, Chhikara M, Aguilar LK, Zhu X, Teh BS, Butler EB, Woo S, Thompson TC, Barrera-Saldana H, Aguilar-Cordova E. Impact of preimmunization on adenoviral vector expression and toxicity in a subcutaneous mouse cancer model. Mol Ther. 2002;6:342–348. doi: 10.1006/mthe.2002.0669. [DOI] [PubMed] [Google Scholar]
- Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Bethune MP, Kostense S, Penders G, Helmus N, Koudstaal W, Cecchini M, Wetterwald A, Sprangers M, Lemckert A, Ophorst O, Koel B, van Meerendonk M, Quax P, Panitti L, Grimbergen J, Bout A, Goudsmit J, Havenga M. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: Efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol. 2003;77:8263–8271. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington SN, Parker AL, Havenga M, Nicklin SA, Buckley SM, McVey JH, Baker AH. Targeting of adenovirus serotype 5 (Ad5) and 5/47 pseudotyped vectors in vivo: Fundamental involvement of coagulation factors and redundancy of CAR binding by Ad5. J Virol. 2007;81:9568–9571. doi: 10.1128/JVI.00663-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SM, Greig JA, Denby L, Custers J, Morita T, Francischetti IM, Monteiro RQ, Barouch DH, van Rooijen N, Napoli C, Havenga MJ, Nicklin SA, Baker AH. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Wortmann A, Vohringer S, Engler T, Corjon S, Schirmbeck R, Reimann J, Kochanek S, Kreppel F. Fully detargeted polyethylene glycol-coated adenovirus vectors are potent genetic vaccines and escape from pre-existing anti-adenovirus antibodies. Mol Ther. 2008;16:154–162. doi: 10.1038/sj.mt.6300306. [DOI] [PubMed] [Google Scholar]
- Wu H, Dmitriev I, Kashentseva E, Seki T, Wang M, Curiel DT. Construction and characterization of adenovirus serotype 5 packaged by serotype 3 hexon. J Virol. 2002;76:12775–12782. doi: 10.1128/JVI.76.24.12775-12782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Candolfi M, Kroeger KM, Puntel M, Mondkar S, Larocque D, Liu C, Curtin JF, Palmer D, Ng P, Lowenstein PR, Castro MG. Immunization against the transgene but not the TetON switch reduces expression from gutless adenoviral vectors in the brain. Mol Ther. 2008;16:343–351. doi: 10.1038/sj.mt.6300375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Tian J, Smith JS, Byrnes AP. Clearance of adenovirus by Kupffer cells is mediated by scavenger receptors, natural antibodies, and complement. J Virol. 2008;82:11705–11713. doi: 10.1128/JVI.01320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youil R, Toner TJ, Su Q, Chen M, Tang A, Bett AJ, Casimiro D. Hexon gene switch strategy for the generation of chimeric recombinant adenovirus. Hum Gene Ther. 2002;13:311–320. doi: 10.1089/10430340252769824. [DOI] [PubMed] [Google Scholar]
- Zaiss AK, Vilaysane A, Cotter MJ, Clark SA, Meijndert HC, Colarusso P, Yates RM, Petrilli V, Tschopp J, Muruve DA. Antiviral antibodies target adenovirus to phagolysosomes and amplify the innate immune response. J Immunol. 2009;182:7058–7068. doi: 10.4049/jimmunol.0804269. [DOI] [PubMed] [Google Scholar]
- Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn KR, Douglas JT, Smyth CA, Liu HG, Wu Q, Krasnykh VN, Mountz JD, Curiel DT, Mountz JM. Imaging and tissue biodistribution of 99mTc-labeled adenovirus knob (serotype 5). Gene Ther. 1998;5:798–808. doi: 10.1038/sj.gt.3300659. [DOI] [PubMed] [Google Scholar]
- Zsengeller Z, Otake K, Hossain SA, Berclaz PY, Trapnell BC. Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection. J Virol. 2000;74:9655–9667. doi: 10.1128/jvi.74.20.9655-9667.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]