Abstract
It is firmly established that the hippocampus, a brain region implicated in spatial learning, episodic memory, and consolidation, contains a high concentration of CB1 receptors. Moreover, systemic and intrahippocampal administration of cannabinoid agonists have been shown to impair hippocampal-dependent memory tasks. However, the degree to which CB1 receptors in the hippocampus play a specific functional role in the memory disruptive effects of marijuana or its primary psychoactive constituent Δ9-tetrahydrocannabinol (Δ9-THC) is unknown. The present study was designed to determine whether hippocampal CB1 receptors play a functional role in the memory disruptive effects of systemically administered cannabinoids, using the radial arm maze, a well characterized rodent model of working memory. Male Sprague-Dawley rats were implanted with bilateral cannulae aimed at the CA1 region of the dorsal hippocampus. The CB1 receptor antagonist, rimonabant, was delivered into the hippocampus prior to a systemic injection of either Δ9-THC or the potent cannabinoid analog, CP-55,940. Strikingly, intrahippocampal administration of rimonabant completely attenuated the memory disruptive effects of both cannabinoids in the radial arm maze task, but did not affect other pharmacological properties of cannabinoids, as assessed in the tetrad assay (i.e., hypomotility, analgesia, catalepsy, and hypothermia). Infusions of rimonabant just dorsal or ventral to the hippocampus did not prevent Δ9-THC-induced memory impairment, indicating that its effects on mnemonic function were regionally selective. These findings provide compelling evidence in support of the view that hippocampal CB1 receptors play a necessary role in the memory disruptive effects of marijuana.
Keywords: Cannabinoids, Learning & Memory, Psychopharmacology, Animal models, marijuana, CB1 receptor, CP55, 940, delta-9-tetrahydrocannabinol (THC), radial arm maze, hippocampus
Introduction
It has long been known that cannabis, the most widely used illicit substance (Johnston 2007), as well as naturally occurring and synthetic cannabinoids, impair learning and memory in humans and laboratory animals (Ranganathan and D'Souza 2006; Riedel and Davies 2005). Electrophysiological evidence suggests that the hippocampus plays a predominant role in the memory disruptive effects of marijuana. Δ9-tetrahydrocannabinoid (Δ9-THC), the primary psychoactive constituent of marijuana, and other cannabinoids activate cannabinoid-1 (CB1) receptors, which are widely distributed throughout the CNS, and are particularly abundant in the hippocampus (Matsuda, et al 1993). These compounds disrupt synaptic long-term plasticity in the hippocampus by reducing presynaptic neurotransmitter release (Misner and Sullivan 1999). Moreover, in vivo administration of Δ9-THC has been found to disrupt synaptic plasticity for up to three days (Mato, et al 2004).
In laboratory rodents, administration of Δ9-THC disrupts hippocampal-dependent learned behavior in operant and spatial maze models of memory (Brodkin and Moerschbaecher 1997; Ferrari, et al 1999; Heyser, et al 1993; Lichtman, et al 1995; Mallet and Beninger 1998; Nakamura, et al 1991; Varvel, et al 2001). Behavioral studies have provided compelling support for the involvement of the hippocampus in cannabinoid-induced memory impairment. Hampson et al. (2000) reported that systemic administration of Δ9-THC or the synthetic cannabinoid receptor agonist, WIN 55,212-2, elicited deficits in a delayed non-match-to-sample operant task that were related to depressed hippocampal cell firing (Hampson and Deadwyler 2000). Several other groups have demonstrated that intrahippocampal administration of Δ9-THC, WIN55,212-2, or CP-55,940, a potent, bicyclic cannabinoid analogue impaired spatial memory in rat radial arm maze, delayed alternation t-maze, or water maze tasks (Egashira, et al 2002; Lichtman, et al 1995; Suenaga, et al 2008; Yim, et al 2008).
While direct administration of cannabinoids into the hippocampus reliably impairs spatial memory (Egashira, et al 2002; Lichtman, et al 1995; Mishima, et al 2001; Wegener, et al 2008), it is unclear whether hippocampal CB1 receptors play a critical role in the memory disruptive effects of systemically administered cannabinoids. Thus, the primary objective of the present study was to determine whether intrahippocampal administration of the selective CB1 receptor antagonist, rimonabant, would prevent the memory disruptive effects of systemically administered Δ9-THC or CP-55,940 in the radial arm maze, a well established hippocampus-dependent spatial memory task (Olton 1987) that is sensitive to the memory disruptive effects of cannabinoids (Lichtman, et al 1995; Lichtman and Martin 1996; Nakamura, et al 1991). In an initial experiment, we established the dose of rimonabant that would block the memory disruptive effects of CP-55,940, when both drugs were infused bilaterally into the hippocampus. Subsequent studies evaluated whether intrahippocampal administration of the active rimonabant dose would block the memory disruptive effects of systemically administered cannabinoids. To control for the possibility that rimonabant elicited its effects because of diffusion to distal areas, we also evaluated whether rimonabant infused outside the borders of the hippocampus would block memory deficits caused by systemic cannabinoid administration.
In addition to interfering with mnemonic processes, systemically administered cannabinoid receptor agonists produce a wide range of sensorimotor, physiological, and subjective effects (Jarbe and McMillan 1980; Little, et al 1988). Accordingly, the second goal of the present study was to determine whether intrahippocampal administration of rimonabant would block non-mnemonic pharmacological effects of cannabinoids using the tetrad assay (Smith, et al 1994), which assesses rodents for locomotor activity, antinociception, catalepsy, and hypothermia.
Methods
Subjects
All experiments were performed on Sprague Dawley (Harlan, IN) male rats that were individually housed in a temperature-controlled (20–22°C) environment with a 12-h light/dark cycle. Subjects were maintained on a food-restricted diet in order to sustain body weights of approximately 85% of free-feeding weight. Water was available ad libitum. All animal protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee and were in concordance with the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Drugs
Rimonabant (National Institute on Drug Abuse, Rockville, MD), Δ9-THC (National Institute on Drug Abuse, Rockville, MD), and CP-55,940 (Pfizer, Groton, CT) were dissolved in a 1:1 mixture of absolute ethanol and alkamuls-620 (Rhone-Poulenc, Princeton, NJ), and diluted with saline in a final ratio of 1:1:18 (ethanol/alkamuls/saline). The vehicle consisted of the 1:1:18 (ethanol/alkamuls/saline) solution. All systemic injections were given through the i.p. route of administration in a volume of 1 ml/kg. All intracerebral injections were given bilaterally in an injection volume of 0.5 μl per side.
Cannulae Implantation
After initial training in the radial arm maze rats were implanted with bilateral cannulae directed to the CA1 region of the rostral hippocampus. The CA1 region was selected based on previous findings demonstrating that intracerebral injections of cannabinoid agonists directed at this area disrupt memory performance in the radial arm maze (Egashira, et al 2002; Lichtman, et al 1995). Surgery was conducted under isoflurane anesthesia using a standard stereotaxic apparatus. The rat's fur on the head was shaved and cleaned with alcohol and Betadine, and ophthalmic gel was applied to each rat's eyes. An incision was made at the midline of the head with a scalpel blade to expose the skull. The coordinates for the intracerebral infusion sites from bregma (mm) were: 1) dorsal hippocampus: A/P: -3.3, L: +/-1.5, D/V: -3.0; 2) dorsal to the original target site: A/P: -3.3, L: +/-1.5, D/V: -2.0; and 3) ventral to the original target site: A/P: -3.3, L: +/-1.5, D/V: -4.0 (Paxinos and Watson 2007). Subjects were given a two week recovery period after cannulae implantation before commencing the experiments. Each intracerebral infusion was administered in a volume of 0.5 μl over a 1 min period and the injector needle was left in each respective cannula for an additional 1 min. At the conclusion of each experiment, all rats were euthanized with pentobarbital overdose. The brains were removed from the skull, postfixed in -30°C isopentane (2-methylbutane), and frozen at -80°C. Coronal sections (40-μm) were then cut using a freezing microtome and Nissl stained with thionin. A dissecting microscope (Swift Instruments International, Tokyo, Japan) was used to visualize the location of the intracerebral injection sites, which were then verified according to a rat brain atlas (Paxinos and Watson 2007).
Radial Arm Maze
The apparatus and training procedure were identical to that previously described (Lichtman, et al 1995). Each of the eight arms was baited with a 45-mg Noyes pellet placed 5 cm from the end and guillotine doors were used to increase the likelihood that the rats would use a spatial search strategy. At the start of each session, the subject was placed in the center platform with all doors down. Five s later, all of the doors were raised and the subject was allowed to enter a maze arm. The subject was considered to have entered an arm once all four of its paws crossed the threshold into a maze arm. The other seven guillotine doors were then gently lowered. After the subject returned to the center platform the remaining door was lowered and a 5-s ITI was imposed. All eight doors were then raised for the next trial. The session ended when all eight arms had been visited or 10 min had elapsed, whichever came first. An observer scored the number of correct responses, as well as re-entry errors and errors of omission committed by each rat. In addition, the duration of time required to obtain all the available food pellets was recorded for each session.
Rats were trained in the eight arm radial maze tasks until they visited each arm and committed no more than one re-entry error on three consecutive sessions. Once these criteria were achieved, the subjects underwent stereotaxic surgery, as described above. Two weeks after cannulae implantation, the rats were re-trained to these same criteria (i.e. 0 or 1 re-entry errors on three consecutive days) before drug testing in the radial arm maze. The initial training period required between 15 and 20 sessions and the post-surgical training required an additional 8 to 10 days. In each experiment, rimonabant or vehicle was administered 10 min before CP-55,940, Δ9-THC, or vehicle. Rats were then tested in the radial arm maze 20 min later. These time points were based on previous experiments from our laboratory (Lichtman and Martin 1996). All drug conditions were tested in a counterbalanced order, with 5-7 days between tests. Additionally, the rats received a minimum of two days of radial arm maze training between test days.
Tetrad behavioral assessment
Dependent measures of interest that are typically sensitive to the systemic effects of cannabinoids include locomotor activity, antinociception, catalepsy, and hypothermia (Little, et al 1988). To assess locomotor behavior, rats were placed in clean plastic cages (28 × 16 cm) inside sound-attenuating chambers and distance traveled was recorded for 5 min and analyzed by the ANY-maze (Stoelting, Wood Dale, IL) video tracking system. Antinociception was assessed in the tail-flick test as previously described previously (D'Amour and Smith 1941). To minimize tissue damage, a maximum cut-off latency of 10 s was used. Catalepsy was determined using the bar test (Pertwee and Wickens 1991), in which the front paws of each subject were placed on a rod (0.75 cm diameter) that was elevated 4.5 cm from the bench top. The duration of time that the rat remained motionless (with the exception of respiratory movements) with their front paws on the bar for 10 s was scored. Rectal temperature was determined using a telethermometer (Physitemp Instruments, Inc., Clifton, New Jersey) by inserting a thermocouple probe 4.5 cm into the rectum. The rats were assessed for locomotor activity, nociception, catalepsy, and temperature at 20, 25, 40 and 60 min, respectively, after the i.p. injection as previously described (Lichtman, et al 1995; Little, et al 1988). Pre-injection measures for rectal temperature and tail flick were obtained. The subjects were randomly assigned to one of the following three treatment conditions: 1) intrahippocampal vehicle and i.p. vehicle; 2) intrahippocampal vehicle and i.p. CP-55,940 (0.15 mg/kg); and 3) intrahippocampal rimonabant (0.6 μg total) and i.p. CP-55,940 (0.15 mg/kg).
Statistical analysis
A two-way analysis of variance (ANOVA) was used to analyze errors (i.e. entries into non-baited arms) and time to complete the task (s/arm) in the radial arm maze task. The first factor was the cannabinoid receptor antagonist rimonabant and the second factor was the cannabinoid receptor agonist (Δ9-THC or CP-55,950). The tail-flick data were expressed as percent maximal possible effect (%MPE), where %MPE = [(test - control) / (10 - control)] × 100. The rectal temperature data were expressed as post-injection temperature – pre-injection temperature. One-way ANOVAs were used to analyze dependent measures in the tetrad assay. The Tukey-Kramer post hoc test was used to analyze differences between treatment conditions. Differences were considered significant at the p < 0.05 level.
Results
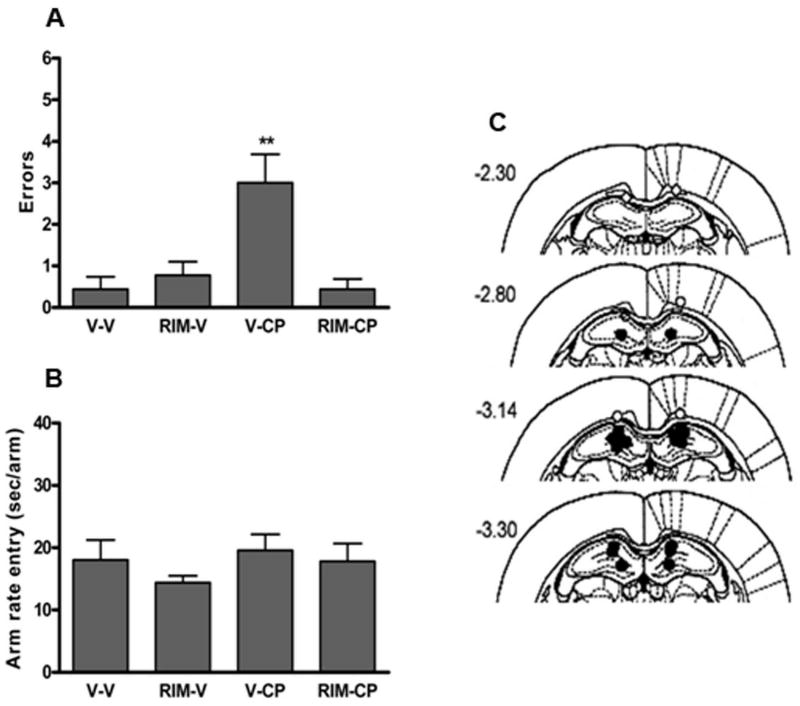
In a preliminary experiment, we sought to determine an effective intrahippocampal dose of rimonabant that antagonizes the memory disruptive effects of the potent cannabinoid analog CP-55,940 (10 μg/rat) given in the same injection site. CP-55,950 produced a significant increase in the number of re-entry errors (Figure 1A), but did not affect the rate of entry into each arm (Figure 1B). A dose of 0.06 μg rimonabant completely blocked the memory disruptive effects of CP-55,940, as indicated by a significant interaction between rimonabant and CP-55,940 treatment, F (1,32) = 13.59, p < 0.01. Post hoc comparisons showed that microinjections of vehicle + CP-55,940 into the hippocampus elicited significantly more errors than each of the other three treatment conditions. Virtually no re-entry errors were committed by rats in the other three treatment conditions. Neither drug given alone nor in combination affected rate of arm entry, as indicated by no significant interaction between the two drugs (p = 0.51), as well as no significant main effect for either rimonabant treatment (p = 0.79) or CP-55,940 treatment (p = 0.25). The data include rats whose cannulae were correctly aimed at the hippocampus (see Figure 1C for cannulae placements). Thus, 0.06 μg was selected as the dose of rimonabant for intracerebral injections in subsequent experiments.
Figure 1.
Establishing an effective dose of rimonabant for intrahippocampal administration. A. Intrahippocampal rimonabant (Rim; 0.06 μg/rat) blocked the memory disruptive effects of intrahippocampal CP-55,940 (CP; 10 μg/rat) in the eight arm radial maze task. B. Intrahippocampal injection of CP-55,940 and rimonabant given separately or in combination did not affect maze running speed. C. Location of intracerebral infusion sites. Drugs were tested in a counterbalanced order. ** p < 0.01 for each group vs. vehicle-vehicle (v-v) treated rats. Results are shown as mean ± SE. n = 9 rats/group.
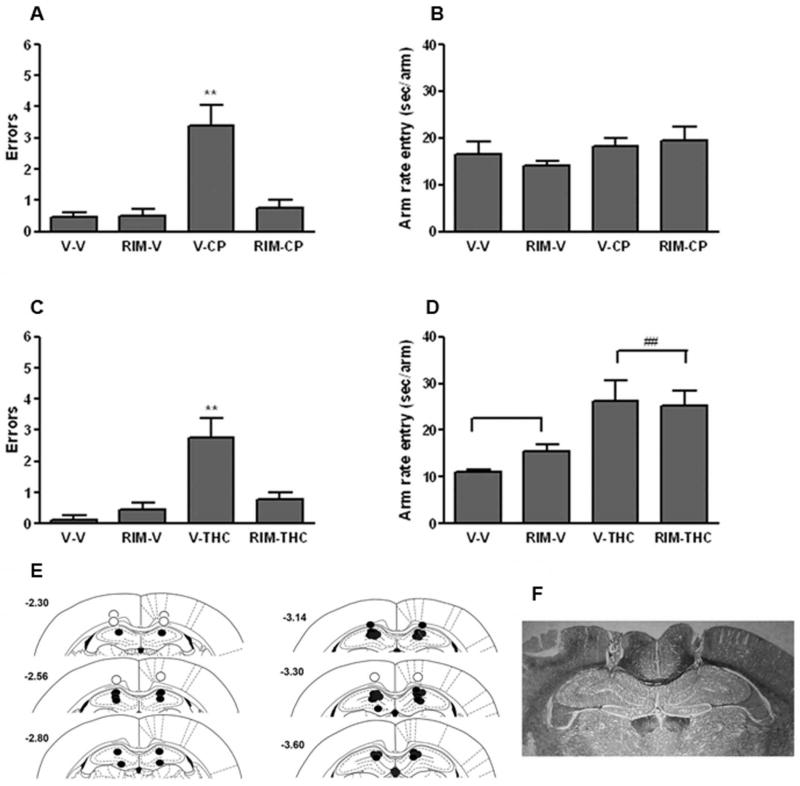
We next evaluated whether intrahippocampal administration of rimonabant (0.06 μg) would prevent radial arm maze performance deficits caused by either CP-55,940 (0.05 mg/kg) or Δ9-THC (5.6 mg/kg). Both cannabinoid receptor agonists significantly impaired radial arm maze choice accuracy in rats given intrahippocampal infusions of vehicle (see Figure 2A and 2C), as previously reported (Lichtman, et al 1995). Intrahippocampal rimonabant administration completely blocked the memory deficits elicited by systemically administered CP-55,940. A two-way ANOVA revealed a significant interaction between rimonabant and CP-55,940, F (1,54) = 15.24, p < 0.001. Treatment with vehicle + CP-55,940 resulted in significantly more errors than each of the other three drug combinations, indicating that rimonabant blocked the memory disruptive effects of this cannabinoid receptor agonist. In contrast, there were no main effects of rimonabant treatment (p = 0.79) and CP-55,940 (p = 0.25), as well as no interaction between rimonabant and CP-55,940 (p = 0.51) for the maze completion data (Figure 2B).
Figure 2.
Hippocampal CB1 receptors mediate the memory disruptive effects of systemically administered cannabinoid receptor agonists in the radial arm maze task. A. Intracerebral administration of rimonabant (Rim; 0.06 μg/rat) into the dorsal hippocampal blocked re-entry errors caused by the potent cannabinoid CP-55,940 (CP; 0.05 mg/kg; i.p.). B. CP-55,940 and rimonabant given separately or in combination did not affect maze running speed. C. Intracerebral administration of rimonabant (0.06 μg/rat) into the dorsal hippocampal blocked re-entry errors caused by Δ9-THC (THC; 5.6 mg/kg; i.p.). D. Δ9-THC led to a significant decrease in the rate of entry into each arm, which was not affected by rimonabant. E. Location of intracerebral infusion sites. Closed and open circles respectively reflect injections sites properly placed within the hippocampus and outside the hippocampus. E. Photomicrograph of cannulae placement in dorsal hippocampus from a representative rat. ** p < 0.01 versus each other group. ## p < 0.01 for Δ9-THC vs. vehicle treatment. Results are shown as mean ± SE. n=7-17 rats/group.
Likewise, Δ9-THC elicited a significant increase in re-entry errors that was blocked by rimonabant, as indicated by a significant interaction between these two drugs, F (1,30) = 15.81, p < 0.01 (Figure 2C). However, systemic administration of Δ9-THC (5.6 mg/kg) produced an increase in maze completion time that was not blocked by intracerebral administration of rimonabant (Figure 2D). A two-way ANOVA revealed no interaction (p = 0.23) or main effect of rimonabant (p = 0.43), but there was a significant main effect of Δ9-THC treatment, F (1,16) = 16.60, p < 0.001. The cannulae placement sites are depicted in Figure 2E and a photomicrograph of the cannulae tracks from a representative rat is shown in Figure 2F.
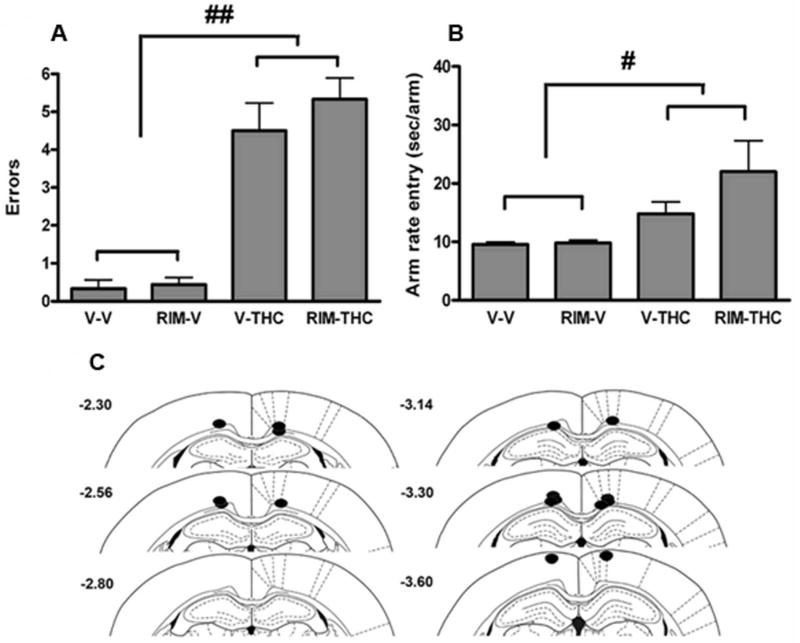
Because of the possibility that intracerebral rimonabant may prevent the disruptive effects of cannabinoids because of diffusion to sites distal to the injection site, we next evaluated whether its infusion just dorsal (Figure 3) or ventral (Figure 4) to the borders of the hippocampus would also block Δ9-THC-induced memory impairment. As shown in Figure 3A, i.p. administration of Δ9-THC led to a significant increase in the number of re-entry errors (main effect of Δ9-THC treatment, F (1,14) = 53.98, p < 0.0001). However, microinjection of rimonabant dorsal to the hippocampus failed to block these memory disruptive effects, as indicated by no significant interaction between rimonabant and Δ9-THC (p = 0.24) and no significant main effect of rimonabant (p = 0.24). Systemically administered Δ9-THC decreased entry rate into each arm (main effect of Δ9-THC: F (1,14) = 7.39, p < 0.05; Figure 3B). Rimonabant infused into the region dorsal to the hippocampus did not block this effect, as indicated by a lack of interaction between the two drug (p = 0.17) and no main effect of rimonabant (p = 0.15). All cannulae were placed dorsal to the hippocampus in the prefrontal cortex or corpus callosum (Figure 3C).
Figure 3.
Rimonabant (Rim) infused dorsal to the hippocampus does not reduce Δ9-THC-induced memory impairment. Systemic administration of Δ9-THC (5.6 mg/kg) produced significant increases in re-entry errors (A) and arm entry rates (B) that were not blocked by rimonabant (0.06 μg/rat) microinjected in sites dorsal to the hippocampus. C. Location of intracerebral infusion sites. Closed circles depict intracerebral infusion sites from cannulae implanted dorsal to the hippocampus. Results are shown as mean ± SE. n = 6-13 rats/group. # p < 0.05, ## p < 0.01 for Δ9-THC vs. vehicle treatment.
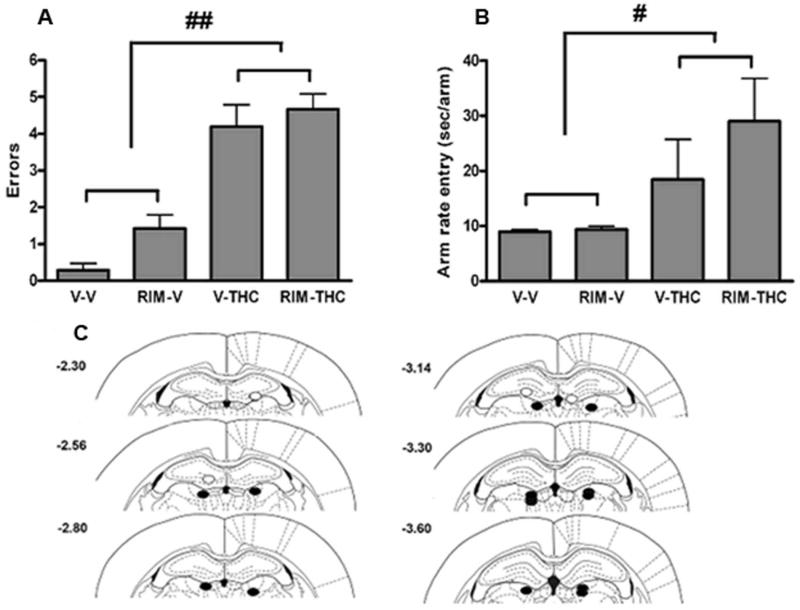
Figure 4.
Rimonabant (Rim) infused ventral to the hippocampus does not reduce Δ9-THC-induced memory impairment. Systemic administration of Δ9-THC (5.6 mg/kg) produced significant increases in re-entry errors (A) and arm entry rates (B) that were not blocked by rimonabant (0.06 μg/rat) given ventral to the border of the hippocampus. C. Location of intracerebral infusion sites. Closed circles depict intracerebral infusion sites from cannulae implanted ventral to the hippocampus. Results are shown as mean ± SE. n = 8 rats/group. # p < 0.05, ## p < 0.01 for Δ9-THC vs. vehicle treatment.
A similar pattern of results was found when rimonabant was infused ventral to the hippocampus. Systemic administration of Δ9-THC impaired choice accuracy (main effect of Δ9-THC, F (1,11) = 162.88, p < 0.0001; Figure 4A) and slowed running speed (main effect of Δ9-THC, F (1,11) = 6.27, p < 0.05; Figure 4B). Microinfusion of rimonabant below the hippocampus did not modify the disruptive effects Δ9-THC on either choice accuracy (main effect of rimonabant; p = 0.09; interaction between rimonabant and Δ9-THC: p = 0.45) or radial arm entry rate (main effect of rimonabant; p = 0.06; interaction between rimonabant and Δ9-THC: p = 0.08). Cannulae placements are shown in Figure 4C.
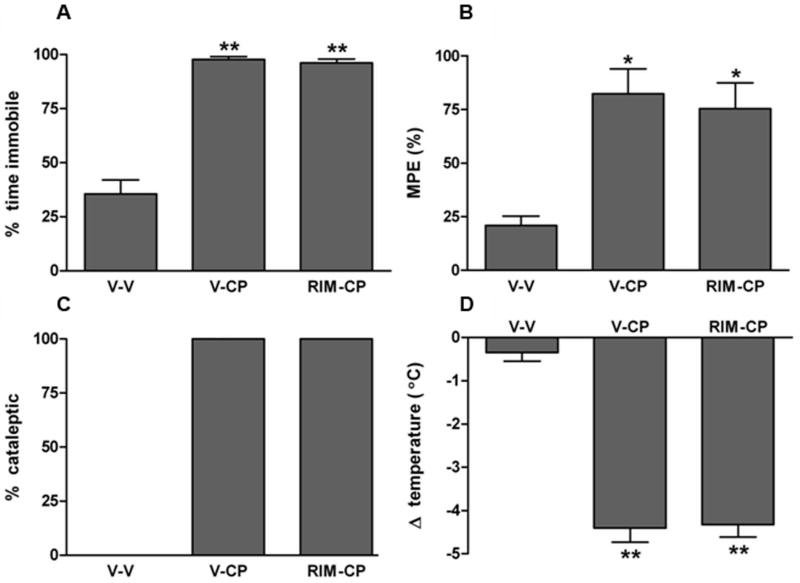
In the final experiment, we assessed whether intrahippocampal rimonabant administration would attenuate non-mnemonic effects produced by cannabinoids, as assessed in the tetrad assay. As previously reported (Compton, et al 1992), CP-55,940 (0.15 mg/kg, i.p.) produced locomotor suppressive (F (2,16) = 121, p < 0.001; Figure 5A), analgesic (F (2,16) = 6.1, p < 0.05; Figure 5B), cataleptic (Figure 5C), and hypothermic (F (2,16) = 42, p < 0.001; Figure 5D) effects. Intrahippocampal rimonabant (0.06 μg) administration failed to attenuate any of these effects, as indicated by post hoc analyses.
Figure 5.
Intracerebral administration of rimonabant (Rim; 0.06 μg/rat) into the dorsal hippocampal does not block the non-mnemonic effects of systemically administered CP-55,940 (CP; 0.15 mg/kg, i.p.), as assessed in the tetrad assay that includes hypomotility (A), antinociception (B), catalepsy (C), and hypothermia (D). * p < 0.05, ** p < 0.01 for each group vs. vehicle-vehicle (V-V) treatment. Results are shown as mean ± SE. n=4-9 rats/group.
Discussion
The results from the present study are unique in that they are the first to demonstrate that microinjection of a CB1 receptor antagonist into the hippocampus blocked spatial memory deficits caused by systemic administration of Δ9-THC, the primary active constituent of marijuana, as well as CP55-940, a potent cannabinoid analogue. Moreover, the effects of intrahippocampal infusion of rimonabant on radial arm choice accuracy were behaviorally selective. Intrahippocampal rimonabant administration did not attenuate non-mnemonic effects of cannabinoids, including behaviors assessed in the tetrad test and decreased radial arm running speeds in the radial arm maze. Finally, the effects of rimonabant were regionally selective, as its administration to sites just dorsal or ventral to the borders of the hippocampus did not antagonize the memory disruptive effects of systemically administered cannabinoids. These finding support the contention that hippocampal CB1 receptors are necessary for the memory disruptive effects of marijuana.
Given the importance of the hippocampus in spatial memory (Ferbinteanu and McDonald 2001; Ferbinteanu, et al 2003) and its high density of CB1 receptors (Herkenham, et al 1991; Matsuda, et al 1993), it is not surprising that this brain region plays an integral role in the disruptive effects of marijuana on memory. Consistent with this hypothesis, systemic administration of Δ9-THC or WIN55,212-2 reliably impairs performance in delayed-match-to-sample (DMTS) and delayed-non-match-to-sample (DNTS) tasks, accompanied with decreases in hippocampal cell firing during the sample phases of the task (Hampson and Deadwyler 1999; 2000; Heyser, et al 1993). In addition, WIN 55212-2 reduced encoding in the hippocampus that was required to perform long-delay trials in a DNTS task (Deadwyler, et al 2007). Other supporting evidence came from studies examining the effects of intracerebral administration of cannabinoids on learning and memory. In particular, intrahippocampal infusions of CP-55,940, Δ9-THC, or WIN 55,212-2 were found to disrupt performance in radial arm maze, t-maze delayed alternation, passive avoidance, and place recognition memory tasks (Egashira, et al 2002; Lichtman, et al 1995; Mishima, et al 2001; Suenaga and Ichitani 2008; Suenaga, et al 2008; Wegener, et al 2008) . Moreover, studies have demonstrated that infusions of Δ9-THC into the hippocampus, as compared to other brain regions, impair memory performance in the radial arm maze task (Egashira, et al 2002). Similarly, administration of WIN 55,212-2 into the dorsal hippocampus, but not into the ventral hippocampus, nucleus accumbens, ventral tegmental area, or medial prefrontal cortex, selectively impaired retrieval memory in the radial arm maze without effecting prepulse inhibition (PPI) or locomotor activity (Wegener, et al 2008). In addition, post-training intrahippocampal administration of WIN 55,212-2 disrupted long term spatial memory, but not acquisition or short term memory, in a rat reference memory task in the water maze (Yim, et al 2008). Systemic administration of the CB1 receptor, AM281, blocked the memory disruptive effects of intrahippocampally administered WIN 55,212-2 in the t-maze delayed alternation and place recognition tasks (Suenaga and Ichitani 2008; Suenaga, et al 2008).These findings, taken together, suggest that the hippocampus is an important target for systemically administered cannabinoids.
The results of the present study indicate that CB1 receptors in the hippocampus play a necessary role in Δ9-THC-induced memory impairment; however, it is unclear which specific hippocampal neurons mediated these memory impairing effects. CB1 receptors are predominantly localized on the terminals of a subset of GABAergic basket cell interneurons (Marsicano and Lutz 1999); however, they have also been demonstrated to inhibit glutamatergic transmission in cultured hippocampal cells (Shen, et al 1996). Overall, the evidence favors a predominant role for GABAergic pathways in the memory disruptive effects of cannabinoids. Specifically, activation of hippocampal CB1 receptors decreases GABA release (Hajos, et al 2000; Hoffman and Lupica 2000; Hoffman, et al 2003; Katona, et al 1999). CB1 receptors located on GABAergic axon terminals are activated by lower concentrations of cannabinoid receptor agonists than CB1 receptors located on glutamatergic terminals (Hoffman, et al 2007; Ohno-Shosaku, et al 2002) and CB1 receptor expression is significantly lower on glutamatergic terminals than GABA axon terminals in the hippocampus (Katona, et al 2006; Kawamura, et al 2006). Moreover, chronic exposure to Δ9-THC in vitro results in tolerance to the inhibitory effects of the cannabinoid agonist WIN, 212-2 but does not affect glutamate release in the hippocampus (Hoffman, et al 2007). Of importance, both Δ9-THC and CP-55,940 decreased the power of theta, gamma, and ripple oscillations in the hippocampus of rats that correlated with memory impairment in the delayed alternation memory paradigm, a hippocampus-dependent task (Robbe, et al 2006). Finally, the GABAA antagonist, bicucculine, blocked Δ9-THC-induced memory deficits in a mouse Morris water maze task (Varvel, et al 2004b). Taken together, these findings are consistent with the notion that CB1 receptors located on inhibitory axon terminals may be the primary target of Δ9-THC in the hippocampus.
The observations that global CB1 receptor knockout mice (Ledent, et al 1999; Varvel and Lichtman 2002; Zimmer, et al 1999) or animals treated with CB1 receptor antagonists (Compton, et al 1996; Hampson and Deadwyler 1999; Lichtman and Martin 1996; Mallet and Beninger 1998; Rinaldi-Carmona, et al 1994) are resistant to the effects of Δ9-THC in the tetrad assay or on spatial memory indicates that this receptor is predominantly responsible for the CNS effects of marijuana. Research using conditional knockout mouse lines has revealed that CB1 receptors expressed on discrete neuronal subpopulations control the various effects Δ9-THC (Monory, et al 2007). As discussed above, there appears to be a strong GABAergic component to the memory disruptive effects Δ9-THC. However, GABA does not appear to play an appreciable role in the non-mnemonic effects of cannabinoids. Specifically, Δ9-THC produced full tetrad effects in mutant mice lacking CB1 receptors on GABAergic neurons (Monory, et al 2007). Likewise, bicucculine did not block the effects of this drug in the tetrad assay (Varvel, et al 2004a). In contrast, mice baring a deletion of the CB1 receptor in principal neurons were resistant to the antinociceptive, cataleptic, and hypothermic effects of Δ9-THC, though the locomotor depressive effects were only partially reduced (Monory, et al 2007). In addition, Δ9-THC-induced hypomotility and hypothermia were reduced in mice lacking CB1 receptors on glutamatergic neurons. It will be of great interest to evaluate the effects of Δ9-THC in these different lines of conditional CB1 (-/-) mice in learning and memory paradigms.
Although the present findings implicate an important role for the hippocampus in the memory disruptive effects of the chief psychoactive component of marijuana and other cannabinoids, the involvement of CB1 receptors in other brain regions on learning and memory cannot be excluded. For instance, cannabinoids are known to disrupt synaptic plasticity in several brain regions (Iversen 2003). In particular, Δ9-THC infused into the prefrontal cortex impaired memory in a radial arm maze procedure that incorporated a 1 h delay (Silva de Melo, et al 2005), but not in the standard radial arm maze task (Egashira, et al 2002). Thus, the demands of the task are likely to determine the neural substrates underlying marijuana-induced memory impairment.
Collectively, the results of the present study provide compelling evidence that Δ9-THC impairs memory function through a direct action of CB1 receptors in the hippocampus. Specifically, intrahippocampal administration of the CB1 receptor antagonist, rimonabant, completely blocked the disruptive effects of systemically administered Δ9-THC, the primary constituent responsible for marijuana's CNS effects, or the potent cannabinoid receptor agonist CP-55,940 in the radial arm maze task. Rimonabant's effects were regionally selective, as its infusion just outside the borders of the hippocampus failed to block Δ9-THC-induced memory impairment. While pharmacological antagonism of CB1 receptor signaling in the hippocampus blocked cannabinoid-induced memory impairment, it failed to attenuate other common cannabinoid pharmacological effects, including analgesia, motor alterations, and hypothermia. Likewise, intrahippocampal administration of CP-55,940 impaired spatial memory in the radial arm maze, without eliciting these other pharmacological effects (Lichtman, et al 1995). In conclusion, these findings support the hypothesis that CB1 receptors in the hippocampus are necessary for the memory disruptive effects of marijuana, but are not essential for the other common CNS actions of this drug.
Acknowledgments
The authors thank Dr. Carl Lupica for his insightful comments and suggestions on an earlier version of this manuscript. This research was supported by the National Institute on Drug Abuse (R01DA 015683, R01DA003672, and T23DA07027).
Footnotes
Disclosure/Conflict of Interest: None of the authors report any conflicts of interest with the work presented in this manuscript. This research has been supported solely by the National Institutes of Health (NIH). AHL declares that over the past three years that he has received compensation from Pfizer, Ironwood Pharmaceuticals, and Allergan. In addition, AHL has received funding from Pfizer and Ironwood for contracts unrelated to the research presented in this paper. AJP declares full-time employment at Abbott, since completing his postdoctoral fellowship and contributions to this paper. LEW declares no financial support or compensation has been received from any individual or corporate entity over the past three years.
References
- Brodkin J, Moerschbaecher JM. SR141716A antagonizes the disruptive effects of cannabinoid ligands on learning in rats. J Pharmacol Exp Ther. 1997;282:1526–1532. [PubMed] [Google Scholar]
- Compton D, Aceto M, Lowe J, Martin B. In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): Inhibition of Δ9-tetrahdrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther. 1996;277:586–594. [PubMed] [Google Scholar]
- Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacological profile of a series of bicyclic cannabinoid analogs: Classification as cannabimimetic agents. J Pharmacol Exp Ther. 1992;260:201–209. [PubMed] [Google Scholar]
- D'Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharm Exp Ther. 1941;72:74–79. [Google Scholar]
- Deadwyler SA, Goonawardena AV, Hampson RE. Short-term memory is modulated by the spontaneous release of endocannabinoids: evidence from hippocampal population codes. Behav Pharmacol. 2007;18:571–580. doi: 10.1097/FBP.0b013e3282ee2adb. [DOI] [PubMed] [Google Scholar]
- Egashira N, Mishima K, Iwasaki K, Fujiwara M. Intracerebral microinjections of delta 9-tetrahydrocannabinol: search for the impairment of spatial memory in the eight-arm radial maze in rats. Brain Res. 2002;952:239–245. doi: 10.1016/s0006-8993(02)03247-x. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, McDonald RJ. Dorsal/ventral hippocampus, fornix, and conditioned place preference. Hippocampus. 2001;11:187–200. doi: 10.1002/hipo.1036. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, Ray C, McDonald RJ. Both dorsal and ventral hippocampus contribute to spatial learning in Long-Evans rats. Neurosci Lett. 2003;345:131–135. doi: 10.1016/s0304-3940(03)00473-7. [DOI] [PubMed] [Google Scholar]
- Ferrari F, Ottani A, Vivoli R, Giuliani D. Learning impairment produced in rats by the cannabinoid agonist HU 210 in a water-maze task. Pharmacol Biochem Behav. 1999;64:555–561. doi: 10.1016/s0091-3057(99)00106-9. [DOI] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem S, MacKie K, Ledent C, Mody I, Freund T. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–1349. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids, hippocampal function and memory. Life Sci. 1999;65:715–723. doi: 10.1016/s0024-3205(99)00294-5. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. Journal of Neuroscience. 2000;20:8932–8942. doi: 10.1523/JNEUROSCI.20-23-08932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Hampson RE, Deadwyler SA. Effects of Δ9-tetrahydrocannabinol on delayed match to sample performance in rats: Alterations in short-term memory associated with changes in task specific firing of hippocampal cells. J Pharmacol Exp Ther. 1993;264:294–307. [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Caulder T, Lupica CR. Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J Neurosci. 2003;23:4815–4820. doi: 10.1523/JNEUROSCI.23-12-04815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Yang R, Lichtman AH, Lupica CR. Opposing actions of chronic { Delta } 9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation. Learn Mem. 2007;14:63–74. doi: 10.1101/lm.439007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126:1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, McMillan DE. delta 9-THC as a discriminative stimulus in rats and pigeons: generalization to THC metabolites and SP-111. Psychopharmacology (Berl) 1980;71:281–289. doi: 10.1007/BF00433063. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Volume II: College students and adults ages 19-45 (NIH Publication No 07-6206) National Institute on Drug Abuse, National Institute on Drug Abuse; 2007. Monitoring the Future national survey results on drug use, 1975-2006. [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Dimen KR, Martin BR. Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacol. 1995;119:282–290. doi: 10.1007/BF02246292. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Delta 9-tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacology (Berl) 1996;126:125–131. doi: 10.1007/BF02246347. [DOI] [PubMed] [Google Scholar]
- Little PJ, Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther. 1988;247:1046–1051. [PubMed] [Google Scholar]
- Mallet PE, Beninger RJ. The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by delta-9-tetrahydrocannabinol or anandamide. Psychopharmacol. 1998;140:11–19. doi: 10.1007/s002130050733. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Mato S, Chevaleyre V, Robbe D, Pazos A, Castillo PE, Manzoni OJ. A single in-vivo exposure to delta 9THC blocks endocannabinoid-mediated synaptic plasticity. Nat Neurosci. 2004;7:585–586. doi: 10.1038/nn1251. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Mishima K, Egashira N, Hirosawa N, Fujii M, Matsumoto Y, Iwasaki K, Fujiwara M. Characteristics of learning and memory impairment induced by delta9-tetrahydrocannabinol in rats. Jpn J Pharmacol. 2001;87:297–308. doi: 10.1254/jjp.87.297. [DOI] [PubMed] [Google Scholar]
- Misner DL, Sullivan JM. Mechanism of cannabinoid effects on long-term potential and depression in hippocampal CA1 neurons. J Neurosci. 1999;19:6795–6805. doi: 10.1523/JNEUROSCI.19-16-06795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schutz G, Wotjak CT, Lutz B, Marsicano G. Genetic dissection of behavioural and autonomic effects of Delta(9)-tetrahydrocannabinol in mice. PLoS Biol. 2007;5:e269. doi: 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura EM, da Silva EA, Concilio GV, Wilkinson DA, Masur J. Reversible effects of acute and long-term administration of Δ9-tetrahydrocannabinol (THC) on memory in the rat. Drug Alc Depend. 1991;28:167–175. doi: 10.1016/0376-8716(91)90072-7. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci. 2002;22:3864–3872. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olton DS. The radial arm maze as a tool in behavioral pharmacology. Physiol Behav. 1987;40:793–797. doi: 10.1016/0031-9384(87)90286-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th. Elsevier, Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Wickens AP. Enhancement by chlordiazepoxide of catalepsy induced in rats by intravenous or intrapallidal injections of enantiomeric cannabinoids. Neuropharmacol. 1991;30:237–244. doi: 10.1016/0028-3908(91)90150-a. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, D'Souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl) 2006;188:425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Riedel G, Davies SN. Cannabinoid function in learning, memory and plasticity. Handbook Exp Pharmacol. 2005:445–477. doi: 10.1007/3-540-26573-2_15. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Néliat G, Caput D, Ferrara P, Soubrié P, Brelière JC, Le Fur G. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat Neurosci. 2006;9:1526–1533. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- Shen M, Piser TM, Seybold VS, Thayer SA. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci. 1996;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva de Melo LC, Cruz AP, Rios Valentim SJ, Jr, Marinho AR, Mendonca JB, Nakamura-Palacios EM. Delta(9)-THC administered into the medial prefrontal cortex disrupts the spatial working memory. Psychopharmacology (Berl) 2005;183:54–64. doi: 10.1007/s00213-005-0141-1. [DOI] [PubMed] [Google Scholar]
- Smith PB, Compton DR, Welch SP, Razdan RK, Mechoulam R, Martin BR. The pharmacological activity of anandamide, a putative endogenous cannabinoid, in mice. J Pharmacol Exp Ther. 1994;270:219–227. [PubMed] [Google Scholar]
- Suenaga T, Ichitani Y. Effects of hippocampal administration of a cannabinoid receptor agonist WIN 55,212-2 on spontaneous object and place recognition in rats. Behav Brain Res. 2008;190:248–252. doi: 10.1016/j.bbr.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Suenaga T, Kaku M, Ichitani Y. Effects of intrahippocampal cannabinoid receptor agonist and antagonist on radial maze and T-maze delayed alternation performance in rats. Pharmacol Biochem Behav. 2008;91:91–96. doi: 10.1016/j.pbb.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Anum E, Niyuhire F, Wise LE, Lichtman AH. Delta(9)-THC-induced cognitive deficits in mice are reversed by the GABA(A) antagonist bicuculline. Psychopharmacology (Berl) 2004a doi: 10.1007/s00213-004-1988-2. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Cichewicz DL, Lichtman AH. Interactions Between Cannabinoids and Opioids. In: Wenger T, editor. Recent Advances on Pharmacology and Physiology of Cannabinoids. Research Signpost; Keraka, India: 2004b. pp. 157–182. [Google Scholar]
- Varvel SA, Hamm RJ, Martin BR, Lichtman AH. Differential effects of delta9-THC on spatial reference and working memory in mice. Psychopharmacology (Berl) 2001;157:142–150. doi: 10.1007/s002130100780. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Lichtman AH. Evaluation of CB1 receptor knockout mice in the Morris water maze. J Pharmacol Exp Ther. 2002;301:915–924. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- Wegener N, Kuhnert S, Thuns A, Roese R, Koch M. Effects of acute systemic and intra-cerebral stimulation of cannabinoid receptors on sensorimotor gating, locomotion and spatial memory in rats. Psychopharmacology (Berl) 2008;198:375–385. doi: 10.1007/s00213-008-1148-1. [DOI] [PubMed] [Google Scholar]
- Yim TT, Hong NS, Ejaredar M, McKenna JE, McDonald RJ. Post-training CB1 cannabinoid receptor agonist activation disrupts long-term consolidation of spatial memories in the hippocampus. Neuroscience. 2008;151:929–936. doi: 10.1016/j.neuroscience.2007.08.037. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]