Abstract
We have used Western blot analysis and immunocytochemistry to determine the effect of dietary K intake on the expression of protein kinase C (PKC) isoforms in the kidney. Western blot has demonstrated that conventional PKC isoforms (α and β), novel PKC isoforms (δ, ε, and η), and atypical PKC isoforms (ζ) are expressed in the renal cortex and outer medulla. Moreover, a low K intake significantly increases the expression of PKC-ε in the renal cortex and outer medulla but does not change the expression of PKC-α, PKC-β, PKC-δ, PKC-η, and PKC-ζ. Also, immunocytochemistry shows that PKC-ε isoform is expressed in the cortical collecting duct (CCD) and outer medullary collecting duct (OMCD) and that the intensity of PKC-ε staining is higher in the kidney from rats on a K-deficient diet than those on a control diet. Also, we used the patch-clamp technique to study the role of PKC in mediating internalization of ROMK (Kir 1.1)-like small-conductance K (SK) channels induced by phenylarsine oxide (PAO), an agent that inhibits protein tyrosine phosphatase and has been shown to stimulate the internalization of the SK channel in the CCD (Sterling H, Lin DH, Qu RM, Dong K, Herbert SC, and Wang WH. J Biol Chem 277: 4317–4323, 2002). Inhibition of PKC with calphostin C and GF-109203x had no significant effect on channel activity but abolished the inhibitory effect of PAO on SK channels. In conclusion, a low K intake increases the expression of PKC-ε isoform in the renal cortex and outer medulla, and PKC is involved in mediating the internalization of SK channels in the CCD induced by stimulation of protein tyrosine kinase activity.
Keywords: protein tyrosine kinase, ROMK (Kir 1.1), endocytosis, hypokalemia, potassium secretion
Dietary k intake plays an important role in the regulation of K secretion: a high K intake stimulates, whereas a low K intake suppresses, renal K secretion (15–17, 22). The effect of K intake on K secretion is partially achieved by changing the number of ROMK (Kir 1.1)-like small-conductance K (SK) channels in the plasma membrane of the cortical collecting duct (CCD; see Refs. 19, 20, 25): a high K intake increases, whereas a low K intake decreases, the activity of the SK channels in the apical membrane of the CCD. The effect of low K intake on SK channels is mediated by the protein tyrosine kinase (PTK)-dependent pathway. Our laboratory has previously demonstrated that a low K intake increases not only the activity of PTK but also the expression of Src family PTK (31). As a consequence of increase in PTK activity, the tyrosine phosphorylation of ROMK is enhanced (14), and it leads to the internalization of ROMK-like SK channels in the CCD (18, 24).
Although PTK has been shown to play an important role in the regulation of ROMK channel trafficking in the CCD, it is possible that the PTK-induced effect on ROMK trafficking may require the interaction of PTK with a variety of signal transduction pathways (21). Stimulation of PTK has been shown to regulate protein kinase C (PKC) activity by stimulating the tyrosine phosphorylation of phospholipase C (PLC) or by tyrosine phosphorylation of PKC (5, 12). Also, it has been demonstrated that PKC participation is required for the PTK-induced stimulation of GLUT4 trafficking (23). ROMK1 has three putative PKC phosphorylation sites (11), and serine residues 4 at the NH2-terminus and 201 at the COOH-terminus are two main sites for PKC-induced phosphorylation (13). Also, we have previously demonstrated that stimulation of PKC inhibits the ROMK-like SK channel in the CCD (27). However, whether PKC is involved in mediating the internalization of ROMK in the CCD has not been explored. Thus the present study was done to investigate whether dietary K intake regulates the expression of PKC isoforms and explore the role of PKC in mediating the internalization of ROMK-like SK channels induced by stimulation of PTK activity.
METHODS
Tissue Preparations
We used Sprague-Dawley rats (6 wk, either sex; Taconic Farms, Germantown, NY) for experiments. Rats were kept on the following different K diets (Harlan Teklad, Madison, WI): K-deficient (KD; <0.01%); high K (HK; 10%, wt/wt); and normal K (1%) diet for 1 wk before use. For immunocytochemistry, rats were anesthetized with isoflurane, and the abdomens were cut open for perfusion of kidneys with 50 ml PBS containing heparin (40 U/ml) followed by 200 ml of 4% paraformaldehyde. After perfusion, the kidneys were removed and subjected to postfixation with 4% paraformaldehyde for 12 h. The kidneys were dehydrated and cut to 8- to 10-μm slices with a Leica1900 cryostat (Leica). The tissue slices were dried at 42°C for 1 h. The protocol of animal use has been approved by the NYMC Vertebrate animal review committee.
For Western blot analysis, the renal cortex and outer medulla were dissected and suspended (1:8, wt/vol) in RIPA buffer [1× PBS, 1% Igepal CA-630 (Sigma), 0.5% sodium deoxycholate, and 0.1% SDS] containing 10 μl PMSF (10 mg/ml solution in isopropanol) and 10 μl each of protease and phosphatase inhibitor cocktail/ml lysis buffer. The tissues were left on ice for 15 min, homogenized, and incubated in the presence of 1 μl DNase (11.6 U/μl) for 1 h at 4°C. After centrifugation at 3,000 rpm for 10 min at 4°C, the supernatants were removed, and the protein concentrations were determined using the Bio-Rad DC protein assay kit (Bio-Rad, Hercules, CA).
Western blot
Protein samples extracted from the renal cortex and outer medulla were separated by electrophoresis on 8% SDS-poly-acrylamide gels and transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline (TBS), rinsed, and washed with 1% milk in Tween-TBS. Anti-PKC (α, δ, ε, ξ,) antibodies were purchased from Upstate USA (Charlottesville, VA), whereas PKC-β1 and actin antibody were obtained from Santa Cruz (Santa Cruz, CA). PKC-η was from Oxford Biomedical Research (Oxford, MI). The protein concentration used for immunoblot was 50–100 μg. The change in PKC band density was normalized with the corresponding actin band. The density of the band was determined by Alpha DigiDoc 1000 (Alpha Innotech, San Leandro, CA).
Immunocytochemical Staining
The slides were washed with 1× PBS for 15 min and permeabilized with 0.4% Triton dissolved in 1× PBS buffer containing 1% BSA and 0.1% lysine (pH = 7.4) for 15 min. Kidney slices were blocked with 2% goat serum for 1 h at room temperature and then incubated with an anion exchanger antibody as a marker of collecting duct (gift from Dr. D. Biemesderfer, Yale University) and PKC antibodies for 12 h at 4°C. Slides were thoroughly washed with 1× PBS followed by addition of second antibody mixture in 0.4% Triton dissolved in 1× PBS for 2 h at room temperature.
Preparation of CCDs for patch-clamp study
Single CCDs were isolated, placed on a 5 × 5-mm coverglass coated with polylysine, and transferred to a chamber (1,000 μl) mounted on an inverted Nikon microscope. The CCDs were superfused with HEPES-buffered NaCl solution containing (in mM) 140 NaCl, 5 KCl, 1.8 CaCl2, 1.8 MgCl2, and 5 HEPES (pH = 7.4). The temperature of the chamber was maintained at 37 ± 1°C by circulating warm water around the chamber. The CCD was cut open with a sharpened micropipette to expose the apical membrane.
Patch-clamp technique
An Axon200A patch-clamp amplifier was used to record channel current. The current was low-pass filtered at 1 kHz by an eight-pole Bessel filter (902LPF; Frequency Devices, Haverhill, MA) and digitized by an Axon interface (Digitada1200). Data were acquired by an IBM-compatible Pentium computer (Gateway 2000) at a rate of 4 KHz and analyzed using the pClamp software system 6.04 (Axon Instruments, Burlingame, CA). Channel activity was defined as the number of channels with an open probability (NPo), which was calculated from data samples of 60-s duration in the steady state as follows
| (1) |
where ti is the fractional open time spent at each of the observed current levels.
Experimental solutions and statistics
The pipette solution contained (in mM) 140 KCl, 1.8 MgCl2, and 10 HEPES (pH = 7.4). The bath solution was composed of (in mM) 140 NaCl, 5 KCl, 1.8 CaCl2, 1.8 MgCl2, 5 glucose, and 10 HEPES (pH = 7.4). Calphostin C and GF-109203x were purchased from Biomol (Plymouth Meeting, PA), and phenylarsine oxide (PAO) was obtained from Sigma.
Data are shown as means ± SE, and paired or unpaired Student’s t-test was used to determine the significance between the two groups. Statistical significance was taken as P < 0.05.
RESULTS
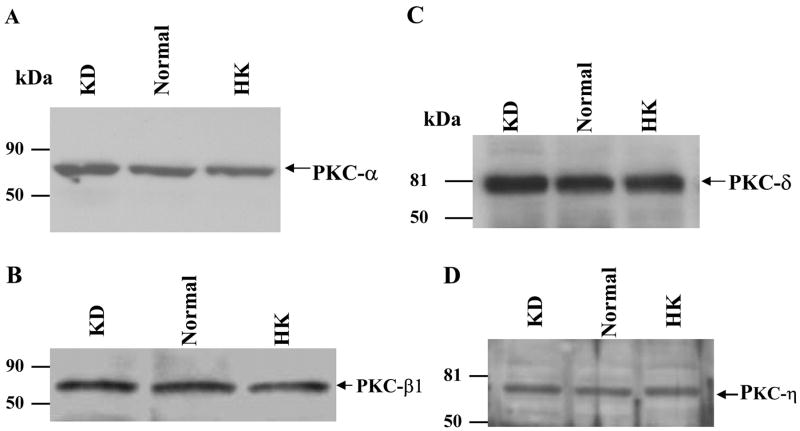
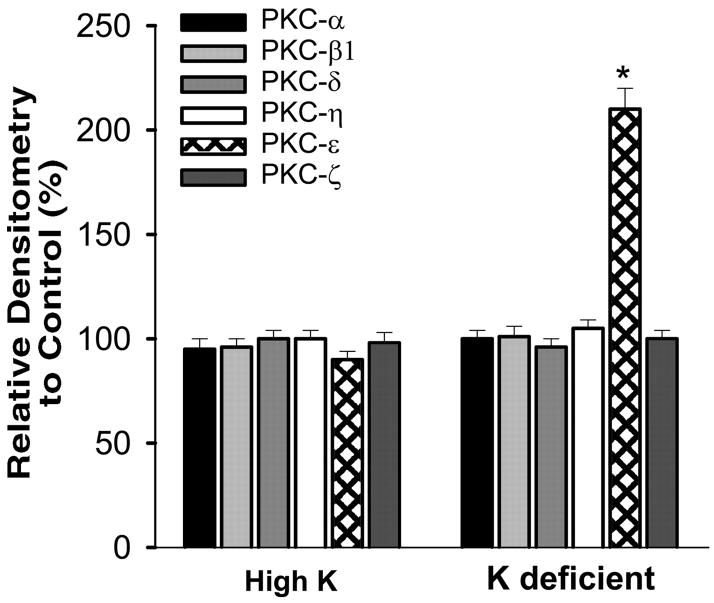
To study the effect of K intake on PKC expression in the renal cortex and outer medulla, we selected two conventional PKC isoforms (α and β1), three novel PKC isoforms (δ, ε, and η), and one atypical PKC isoform (ζ). The reason for the selection of these six PKC isoforms is because of the fact that all except PKC-β have been shown to be expressed in the isolated CCDs (32). We first examined the effect of dietary K intake on the expression of the conventional PKC isoforms, α and β1. Figure 1 is a Western blot analysis showing the effect of K diet on the expression of PKC-α and PKC-β. Data summarized in Fig. 2 show that the renal expression of PKC-α in rats on a HK diet and on a KD diet was 95 ± 5 and 100 ± 4% of the control (normal K diet), respectively (2–3 experiments for each rat; n = 4 rats). Also, the change in K diet has no effect on the expression of PKC-β (HK, 96 ± 4% of the control value; KD, 101 ± 5% of the control value; 2–3 experiments for each rat, n = 4 rats).
Fig. 1.
Effect of K intake on the expression of protein kinase C (PKC)- α (A), PKC-β (B), PKC-δ (C), and PKC-η (D) in the renal cortex and outer medulla. KD, K-deficient diet; HK, high-K (10%, wt/wt) diet; normal, 1% (wt/wt) K diet.
Fig. 2.
Relative changes in the expression of PKC isoforms in response to HK or KD diet compared with the PKC level observed in the kidney from a rat on a normal K diet. *Difference is significant from corresponding PKC from rats on normal K, P < 0.001.
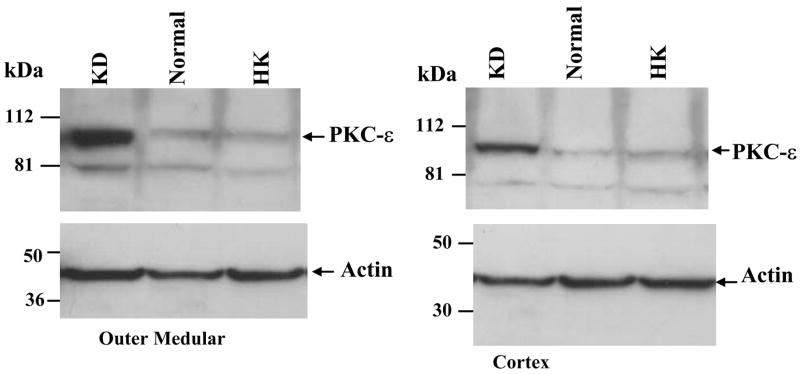
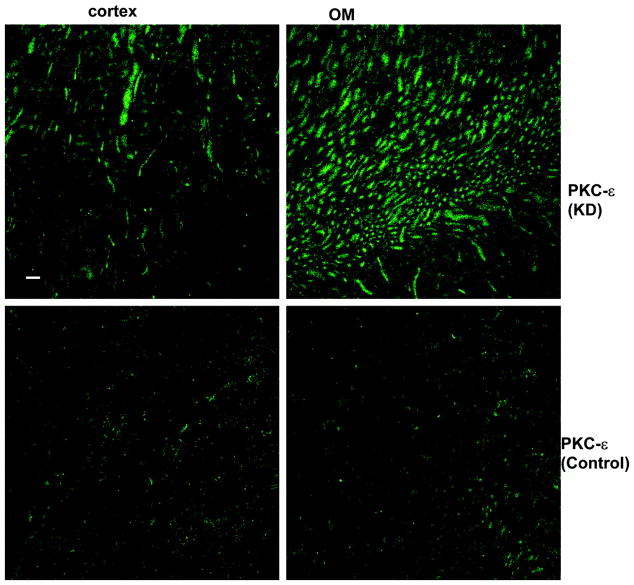
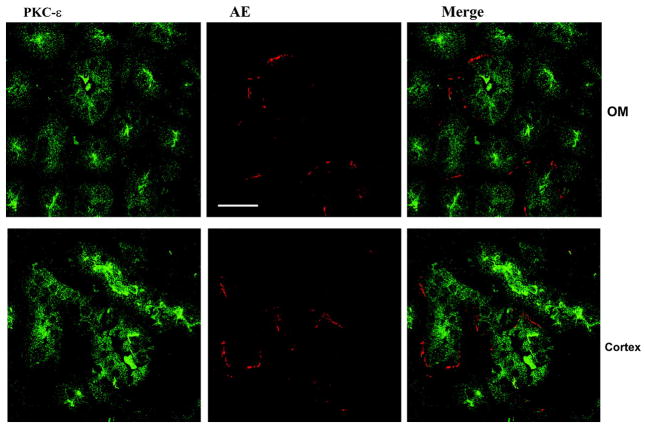
We next examined the effect of dietary K intake on the expression of novel PKC isoforms, δ, ε, and η, because their mRNA have been identified in the dissected rabbit CCD tubules (32). Figure 1, C and D, is a Western blot demonstrating the effect of K intake on the expression of PKC-δ (C) and PKC-η (D). Data summarized in Fig. 2 show that changes in K intake have no effect on the expression of PKC-δ (HK, 100 ± 4% of the control value; KD, 96 ± 4% of the control value) and PKC-η (HK, 100 ± 4% of the control value; KD, 105 ± 4% of the control value; n = 4 rats). In contrast, the expression of PKC-ε significantly increased in the kidney from rats on a KD diet. Figure 3 is a typical Western blot showing the expression of PKC-ε in the renal cortex and outer medulla. The band intensity of PKC-ε from the tissue of rats on a KD diet is 210 ± 10% of those on the normal K diet (Fig. 2, n = 4 rats). However, increase in K intake did not significantly alter the expression of PKC-ε (90 ± 4% of the control value) compared with those on a normal K diet (Figs. 2 and 3). The finding that low K intake increases PKC-ε expression has also been confirmed by immunostaining results. Figure 4 is a confocal image from six experiments (n = 3 rats) showing the PKC-ε staining in the renal cortex and outer medulla from a rat on a KD diet and from a rat on a normal K diet. Both kidney slices were on the same slide and were processed with the identical protocol. From inspection of Fig. 4, it is apparent that the intensity of the PKC-ε staining in the renal cortex and outer medulla is higher in the kidney slice from rats on a KD diet than that on a normal K diet. Furthermore, double staining of the kidney slice with PKC-ε antibody and the anion exchanger antibody, which is specifically expressed in the basolateral membrane of the collecting duct (1), has demonstrated that PKC-ε is expressed in the CCD and outer medullary collecting duct (OMCD; Fig. 5). In addition, PKC-ε is also expressed in the thick ascending limb (TAL; data not shown).
Fig. 3.
Effect of K diet on the expression of PKC-ε in the renal outer medulla (left) and cortex (right). The position of PKC is indicated by an arrow, and the change in PKC expression was normalized by comparing the corresponding actin content.
Fig. 4.
Immunostaining with PKC-ε antibody in the renal cortex and outer medulla (OM) from rats on a KD diet or on a normal K diet. Bar = 50 M. The kidney slices were placed in the same slide and treated with the same protocol. Also, the confocal images were prepared under identical conditions.
Fig. 5.
Confocal images show that PKC-ε (green) is expressed in renal tubules, which have positive staining of anion exchanger (AE; red), a marker of collecting duct. Top and bottom show the immunostaining in the renal outer medullary (OM) and renal cortex, respectively. Bar = 10 μM.
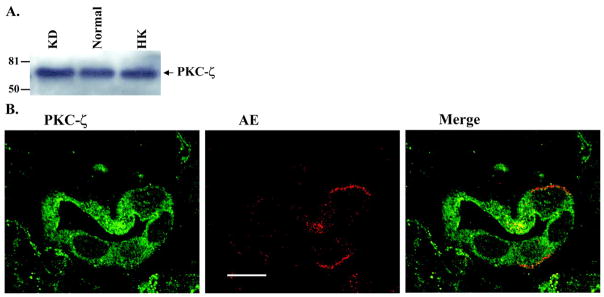
After demonstrating that low K intake increases PKC-ε expression in the renal cortex and outer medulla, we examined the effect of low K on the expression of atypical PKC-ζ (Fig. 6), which has been shown to be expressed in the CCD (32). Figure 6A is a Western blot showing that neither high K nor low K intake alters the expression of PKC-ζ (n = 4 rats) because the renal PKC-ζ levels in rats on HK and KD diets were 98 ± 5 and 100 ± 4% of the control value, respectively (Fig. 2). Figure 6B is a confocal image (6 experiments from 3 rats) demonstrating that PKC-ζ is expressed in the CCD.
Fig. 6.
A: effect of K intake on the expression of PKC-ζ in the renal cortex and outer medulla. B: confocal image showing PKC-ζ (green) and AE (red) staining in the renal cortex to demonstrate that PKC-ζ is expressed in the cortical collecting duct (CCD). Bar = 10 μM.
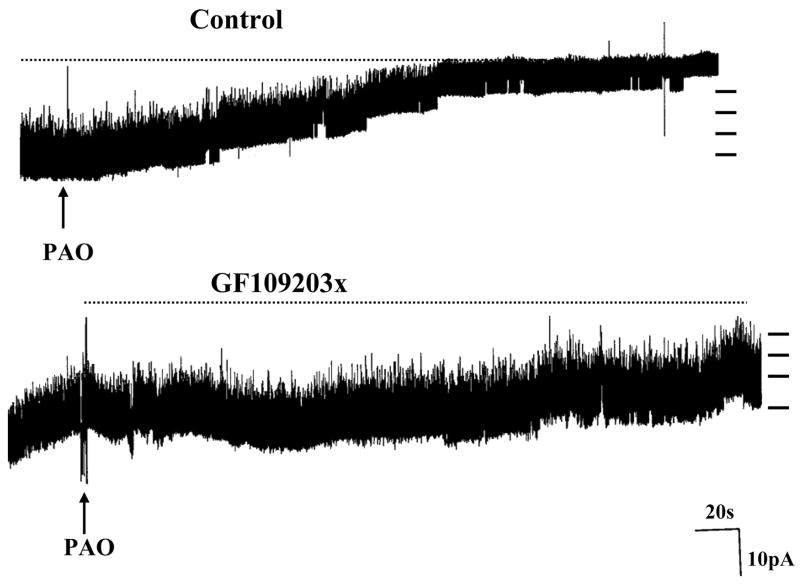
We next examined the role of PKC in the regulation of ROMK-like SK channel trafficking because stimulation of PKC has previously been shown to inhibit the ROMK-like SK channels in the CCD (27). To determine whether PKC is involved in suppressing channel activity in the CCD under control conditions, we studied the effect of inhibiting PKC on the SK channel activity. Data summarized in Fig. 7 show that inhibition of PKC with either 100 nM calphostin C or 10 μM GF-109203x did not affect the channel activity (n = 9 patches). This suggests that PKC does not determine the basal level of SK channel activity. We next investigated whether PKC is involved in mediating the internalization of ROMK-like SK channels induced by inhibition of protein tyrosine phosphatase (PTP). We confirmed the previous finding that inhibition of PTP with PAO (1 μM) decreases the SK channel activity by 95 ± 8% (Fig. 7) and that NPo decreased from 3.1 ± 0.5 to 0.2 ± 0.1 (n = 5 patches; see Ref. 30). However, the inhibitory effect of PAO was absent in the presence of PKC inhibitors (Fig. 7) because NPo remained unchanged (before PAO, 3.2 ± 0.4 and after PAO, 3.0 ± 0.3; n = 7 patches). Figure 8 is a channel recording showing the effect of PAO on the SK channel activity in the presence of GF-109203x (10 μM). It is apparent that PAO (1 μM) did not inhibit SK channels in the CCD. Thus results strongly suggest that PKC is involved in mediating the internalization of the SK channel induced by PTK.
Fig. 7.
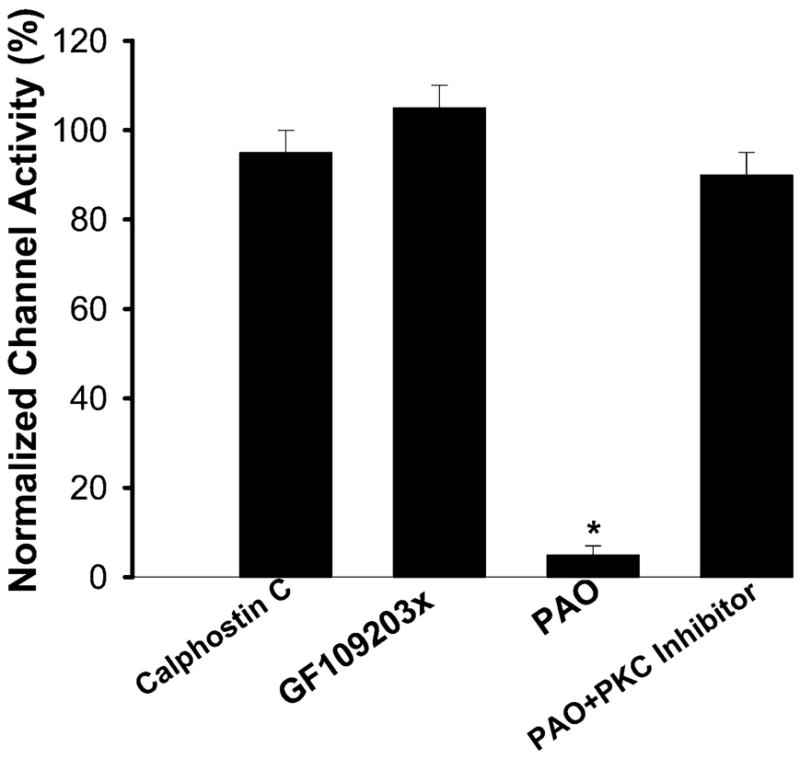
Effect of calphostin C (100 nM), GF-109203x (10 μM), phenylarsine oxide (PAO, 1 μM), and PAO ± PKC inhibitor on the normalized channel activity in the CCD. *Difference is significant compared with the control value (without PAO, P < 0.001).
Fig. 8.
Channel recording showing the effect of PAO on the ROMK (Kir 1.1)-like small-conductance K channel in the absence of PKC inhibitor (Control) and in the presence of 10 μM GF-109203x. The channel closed level is indicated by dotted line, and short bars on right indicate the channel current level. The experiments were conducted in cell-attached patches.
DISCUSSION
The main findings of the present study are that low K diet increases the expression of PKC-ε in renal tubules, such as the CCD, and that PKC is involved in mediating the internalization of ROMK-like SK channels induced by stimulation of PTK in the CCD. It has been reported that PKC-ε and -ζ are two major PKC isoforms in the rabbit CCD (7). This view has also been supported by experiments in which isolated rabbit CCDs and cultured rabbit CCD cells have been used to demonstrate the presence of PKC-α, -ε, and -ζ in the CCD (32). In the present study, we used immunocytochemical staining to confirm that PKC-ε and -ζ are present in the rat CCD. Moreover, we demonstrated that low K intake increases only the expression of PKC-ε but has no effect on the expression of PKC-α, -β1, -δ, -η, and -ζ in the kidney. This suggests that PKC-ε may be involved in the regulation of K secretion. This view is also supported by the finding that stimulation of PKC inhibited the net Na absorption and K secretion in microperfused CCD (10).
In the present study, we have shown that inhibition of PKC abolished the effect of PAO on the ROMK-like SK channels in the CCD. We have previously shown that the effect of PAO is the result of inhibiting PTP, which leads to an increase in the tyrosine phosphorylation of ROMK-like SK channels and internalization (30). Therefore, the fact that inhibition of PKC abolished the effect of PAO suggests that PKC is involved in mediating the PTK-induced internalization of ROMK in the CCD. However, it is not known which PKC isoform is involved in the regulation of the internalization of SK channels in the CCD. Although low K diet increases only PKC-ε, it is possible that PKC isoforms other than PKC-ε may be involved in mediating the internalization induced by stimulation of PTK because low K intake also increases the expression of PLC-γ (unpublished observation), which stimulates the formation of diacyglycerol and inositol trisphosphate. Also, it has been shown that PTK stimulates the activity of PLC-γ (29). However, the role of PKC-ε in the regulation of transport function in the CCD has been supported by observations that suppression of PKC-ε expression with antisense DNA stimulates Na reabsorption in the CCD (7). Thus we need further experiments to confirm the role of PKC-ε in the regulation of SK channel trafficking in the CCD. In addition, low K intake stimulates the expression of PKC-ε in the OMCD and TAL. This suggests that PKC-ε may also be involved in the regulation of membrane transport in both TAL and OMCD. It has been reported that K restriction increases the activity of gastric H-K-ATPase in OMCD (33). Moreover, PKC-induced phosphorylation of H-K-ATPase α-subunit stimulates the activity of the H-K-ATPase (6). Thus it is possible that an increase in PKC activity may be involved in enhancing the K absorption via H-K-ATPase in the OMCD.
The CCD is responsible for Na absorption and K secretion. K secretion is a two-step process: K enters the cell via the basolateral Na-K-ATPase and leaves the cell across the apical membrane through ROMK-like SK channels (8). Aldosterone is the most important hormone to regulate K secretion. However, it is likely that the effect of aldosterone on K secretion could be the result of increasing the electrochemical driving force for K secretion rather than direct stimulation of SK channel activity (26). Dietary K intake has been shown to play an important role in the regulation of K secretion: a high K stimulates whereas a low K inhibits K secretion in the CCD (15–17, 22). Also, low K intake increases the K absorption by stimulation of H-K-ATPase in the collecting duct (33).
Several lines of evidence indicate that the inhibitory effect of low K intake on K secretion is mediated by a PTK-dependent signal transduction pathway. First, low K intake stimulates not only the activity of PTK but also the expression of PTK in the kidney (31). Second, low K increases the tyrosine phosphorylation of ROMK (14). Third, inhibition of PTK increases the number of ROMK-like SK channels in the apical membrane of the CCD (28). However, the observation that inhibition of PTK did not increase the number of SK channels in the plasma membrane in the TAL suggests that tyrosine phosphorylation of ROMK alone is not sufficient to initiate the endocytosis (9). ROMK isoforms expressed in the CCD are ROMK1 and -2, whereas they are ROMK2 and ROMK3 in the TAL (2). The difference among the three ROMK isoforms is their NH2 terminus: ROMK1 has a longer (19 amino acids) NH2 terminus, including the PKC phosphorylation site, than ROMK2, whereas ROMK3 has a unique NH2 terminus containing no PKC site. It is possible that tyrosine phosphorylation of ROMK1 induces the internalization, whereas tyrosine phosphorylation of ROMK2 and -3 did not result in endocytosis. Thus it is speculated that PKC may be involved in mediating the internalization of ROMK1 after tyrosine phosphorylation. PKC has been shown to interact with PTK to mediate the GLUT4 translocation induced by insulin (23).
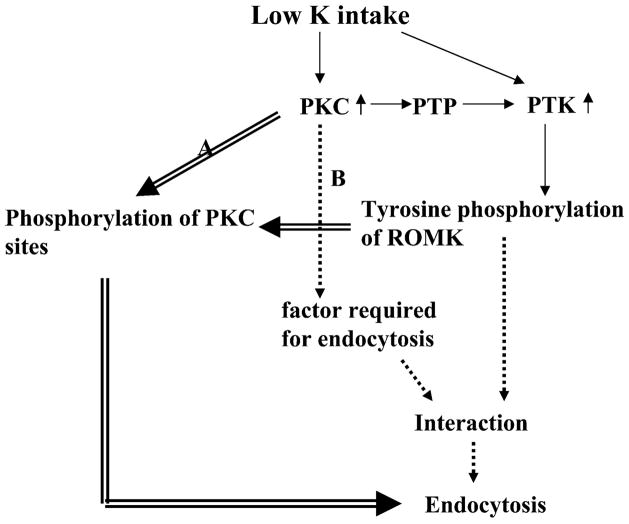
We have demonstrated that PKC is required for initiating the internalization of ROMK-like SK channels in the CCD induced by stimulation of PTK activity. However, the mechanism by which PKC is involved in facilitating endocytosis is not known. Figure 9 is a scheme showing two possibilities by which PKC could be involved in the regulation of endocytosis induced by PTK. Because a PKC isoform other than PKC-ε can also be involved in the regulation of ROMK endocytosis, we use a general terminology of PKC in the scheme. First, the internalization of ROMK-like SK channels induced by stimulating PTK requires phosphorylation of SK channels by PKC and tyrosine phosphorylation by PTK. Thus inhibition of PKC abolished the internalization. Second, PKC may be involved in stimulation of phosphorylation of unidentified factors that are required in mediating the internalization of ROMK-like SK channels induced by inhibiting PTP in the CCD. Relevant to this possibility is the report that stimulation of PKC induces actin reorganization in aortic smooth muscle cells and that the effect of PKC also depends on the Src family PTK (3). We need further experiments to determine the role of PKC in mediating the internalization of ROMK induced by stimulation of PTK. Also, low K intake not only increases the expression of PKC-ε but also stimulates the activity of PTK (28); it raises an interesting question whether an increase in PKC expression is required for the effect of low K intake on PTK activity. It has been shown that PKC can activate Src family PTK by stimulation of PTP, which causes the dephosphorylation of tyrosine residue 527 of c-Src and activates the enzyme (4).
Fig. 9.
Scheme showing two possible mechanisms (indicated by double line arrow or dotted line arrow, respectively) by which PKC is involved in the regulation of ROMK endocytosis induced by stimulation of protein tyrosine kinase (PTK).
We conclude that low K intake increases the expression of PKC-ε and PLC-γ in the kidney and that PKC is involved in mediating the internalization of ROMK-like SK channels in the CCD.
Acknowledgments
We thank Dr. D. Biemesderfer at Yale University School of Medicine for the generous gift of anion exchanger antibody.
GRANTS
The work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-54983 and DK-47402 (to W.-H. Wang). D.-H. Lin was supported by Chinese National Science Foundation Project no. 30370734.
References
- 1.Alvarez de la Rosa D, Coric T, Todorovic N, Shao D, Wang T, Canessa CM. Distribution and regulation of expression of serum- and glucocorticoid-induced kinase-1 in the rat kidney. J Physiol. 2003;551:455–466. doi: 10.1113/jphysiol.2003.042903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boim MA, Ho K, Schuck ME, Bienkowski MJ, Block JH, Slightom JL, Yang Y, Brenner BM, Hebert SC. ROMK inwardly rectifying ATP-sensitive K channel. II. Cloning and distribution of alternatively spliced forms. Am J Physiol Renal Fluid Electrolyte Physiol. 1995;268:F1132–F1140. doi: 10.1152/ajprenal.1995.268.6.F1132. [DOI] [PubMed] [Google Scholar]
- 3.Brandt D, Gimona M, Hillmann M, Haller H, Mischak H. Protein kinase C induces actin reorganization via a Src- and Rho-dependent pathway. J Biol Chem. 2002;277:20903–20910. doi: 10.1074/jbc.M200946200. [DOI] [PubMed] [Google Scholar]
- 4.Brandt DT, Goerke A, Heuer M, Gimona M, Leitges M, Kremmer E, Lammers R, Haller H, Mischak H. Protein kinase Cδ induces Src kinase activity via activation of the protein tyrosine phospatase PTPα. J Biol Chem. 2003;278:30473–30478. doi: 10.1074/jbc.M211650200. [DOI] [PubMed] [Google Scholar]
- 5.Copalarishna R, Anderson WB. Ca2+ and phopholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Natl Acad Sci USA. 1989;86:6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornelius F, Mahmmoud YA. Direct activation of gastric H,K-ATPase by N-terminal protein kinase C phosphorylation. Biophys J. 2003;84:1690–1700. doi: 10.1016/S0006-3495(03)74977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decoy DL, Snapper JR, Breyer MD. Anti sense DNA down-regulates protein kinase C-ε and enhances vasopressin-stimulated Na absorption in rabbit cortical collecting duct. J Clin Invest. 1995;95:2749–2756. doi: 10.1172/JCI117978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giebisch G. Renal potassium transport:mechanisms and regulation. Am J Physiol Renal Physiol. 1998;274:F817–F833. doi: 10.1152/ajprenal.1998.274.5.F817. [DOI] [PubMed] [Google Scholar]
- 9.Gu RM, Wei Y, Falck JR, Krishna UM, Wang WH. Effects of protein tyrosine kinase and protein tyrosine phosphatase on the apical K channels in the thick ascending limb. Am J Physiol Cell Physiol. 2001;281:C1185–C1195. doi: 10.1152/ajpcell.2001.281.4.C1188. [DOI] [PubMed] [Google Scholar]
- 10.Hays SR, Baum M, Kokko JP. Effect of protein kinase C activation on sodium, potassium, chloride, and total CO2 transport in the rabbit cortical collecting tubule. J Clin Invest. 1987;80:1561–1570. doi: 10.1172/JCI113242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- 12.Konish H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci USA. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin DH, Sterling H, Lerea KM, Giebisch G, Wang WH. Protein kinase C (PKC)-induced phosphorylation of ROMK1 is essential for the surface expression of ROMK1 channels. J Biol Chem. 2002;277:44332–44338. doi: 10.1074/jbc.M203702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin DH, Sterling H, Lerea KM, Welling P, Jin L, Giebisch G, Wang WH. K depletion increases the protein tyrosine-mediated phosphorylation of ROMK. Am J Physiol Renal Physiol. 2002;283:F671–F677. doi: 10.1152/ajprenal.00160.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linas SL, Peterson LN, Anderson RJ, Aisenbrey GA, Simon FR, Berl T. Mechanism of renal Potassium conservation in the rat. Kidney Int. 1979;15:601–611. doi: 10.1038/ki.1979.79. [DOI] [PubMed] [Google Scholar]
- 16.Malnic G, Klose RM, Giebisch G. Micropuncture study of renal potassium excretion in the rat. Am J Physiol. 1964;206:674–686. doi: 10.1152/ajplegacy.1964.206.4.674. [DOI] [PubMed] [Google Scholar]
- 17.Malnic G, Klose RM, Giebisch G. Micropuncture study of distal tubular potassium and sodium transport in rat nephron. Am J Physiol. 1966;211:529–547. doi: 10.1152/ajplegacy.1966.211.3.529. [DOI] [PubMed] [Google Scholar]
- 18.Moral Z, Deng K, Wei Y, Sterling H, Deng H, Ali S, Gu RM, Huang XY, Hebert SC, Giebisch G, Wang WH. Regulation of ROMK1 channels by protein tyrosine kinase and tyrosine phosphatase. J Biol Chem. 2001;276:7156–7163. doi: 10.1074/jbc.M008671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer LG. Potassium secretion and the regulation of distal nephron K channels. Am J Physiol Renal Physiol. 1999;277:F821–F825. doi: 10.1152/ajprenal.1999.277.6.F821. [DOI] [PubMed] [Google Scholar]
- 20.Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol. 1994;105:693–710. doi: 10.1085/jgp.104.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–579. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 22.Rabinowich L, Sarason RL, Yamauchi H, Yamanaka KK, Tzendzalian PA. Time course of adaptation to altered K intake in rats and sheep. Am J Physiol Renal Fluid Electrolyte Physiol. 1984;247:F607–F617. doi: 10.1152/ajprenal.1984.247.4.F607. [DOI] [PubMed] [Google Scholar]
- 23.Standaert ML, Bandyopadhyay G, Perez L, Price D, Galloway L, Poklepovic A, Sajan MP, Cenni V, Sirri A, Moscat J, Toker A, Farese R. Insulin activates protein kinase C-ζ and C-λ by an autophosphorylation-dependent mechanism and stimulates their translocation to GLUT4 vesicles and other membrane fractions in rat adipocytes. J Biol Chem. 1999;274:25308–25316. doi: 10.1074/jbc.274.36.25308. [DOI] [PubMed] [Google Scholar]
- 24.Sterling H, Lin DH, Gu RM, Dong K, Hebert SC, Wang WH. Inhibition of protein-tyrosine phosphatase stimulates the dynamin-dependent endocytosis of ROMK1. J Biol Chem. 2002;277:4317–4323. doi: 10.1074/jbc.M109739200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang W, Schwab A, Giebisch G. Regulation of small-conductance K channel in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol. 1990;259:F494–F502. doi: 10.1152/ajprenal.1990.259.3.F494. [DOI] [PubMed] [Google Scholar]
- 26.Wang WH. Regulation of Renal K transport by dietary K intake. Annu Rev Physiol. 2004;66:1–23. doi: 10.1146/annurev.physiol.66.032102.112025. [DOI] [PubMed] [Google Scholar]
- 27.Wang WH, Giebisch G. Dual modulation of renal ATP-sensitive K+ channel by protein kinases A and C. Proc Natl Acad Sci USA. 1991;88:9722–9725. doi: 10.1073/pnas.88.21.9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang WH, Lerea KM, Chan M, Giebisch G. Protein tyrosine kinase regulates the number of renal secretory K channel. Am J Physiol Renal Physiol. 2000;278:F165–F171. doi: 10.1152/ajprenal.2000.278.1.F165. [DOI] [PubMed] [Google Scholar]
- 29.Wang X-T, McCullough KD, Wang X-J, Carpenter G, Holbrook NJ. Oxidative stress-induced phospholipase C-γ1 activation enhances cell survival. J Biol Chem. 2001;276:28364–28371. doi: 10.1074/jbc.M102693200. [DOI] [PubMed] [Google Scholar]
- 30.Wei Y, Bloom P, Gu RM, Wang WH. Protein-tyrosine phosphatase reduces the number of apical small conductance K channels in the rat cortical collecting duct. J Biol Chem. 2000;275:20502–20507. doi: 10.1074/jbc.M000783200. [DOI] [PubMed] [Google Scholar]
- 31.Wei Y, Bloom P, Lin DH, Gu RM, Wang WH. Effect of dietary K intake on the apical small-conductance K channel in the CCD: role of protein tyrosine kinase. Am J Physiol Renal Physiol. 2001;281:F206–F212. doi: 10.1152/ajprenal.2001.281.2.F206. [DOI] [PubMed] [Google Scholar]
- 32.Wilborn TW, Schafer JA. Differential expression of PKC isoforms in fresh and cultured rabbit CCD. Am J Physiol Renal Fluid Electrolyte Physiol. 1996;270:F766–F775. doi: 10.1152/ajprenal.1996.270.5.F766. [DOI] [PubMed] [Google Scholar]
- 33.Wingo CS, Cain BD. The renal H-K-ATPase: physiological significance and role in potassium homeostasis. Annu Rev Physiol. 1993;55:323–347. doi: 10.1146/annurev.ph.55.030193.001543. [DOI] [PubMed] [Google Scholar]