Abstract
Maternal alcohol consumption during critical periods of fetal brain development leads to devastating long-term consequences on adult reproductive physiology, cognitive function, and social behaviors. However, very little is known about the long-term consequences of alcohol consumption during puberty, which is perhaps an equally dynamic and critical period of brain development. Alcohol abuse during adulthood has been linked with an increase in clinically diagnosed anxiety disorders, yet the etiology and neurochemical mechanisms of alcohol-induced anxiety behavior is unknown. In this study, we determined the effects of binge ethanol exposure during puberty on two critical central regulators of stress and anxiety behavior: corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP). Our results showed that ethanol increased plasma corticosterone (CORT) levels in both sexes, yet binge-treated animals had significantly lower CORT levels than animals exposed to a single dose, suggesting that the hypothalamo-pituitary-adrenal (HPA) axis habituated to the repeated stressful stimuli of ethanol. Binge ethanol exposure also significantly increased CRH and AVP gene expression in the paraventricular nucleus of males, but not females. Overall, our results demonstrate that binge ethanol exposure during puberty changes the central expression of stress-related genes in a sex-specific manner, potentially leading to permanent dysregulation of the HPA axis and long-term behavioral consequences.
Keywords: hypothalamus, puberty, arginine vasopressin, corticotrophin-releasing hormone, corticosterone, hypothalamo-pituitary-adrenal axis
alcohol abuse during adolescence is a growing fundamental heath concern in the United States. According to the US Department of Health and Human Services, boys on average have had their first drink before age 11, and girls before age 13, with the overall statistics showing that 41% of teenagers have had their first drink by age 14. Underage drinkers typically adopt a “binge” pattern of alcohol consumption, defined by the National Institute on Alcohol Abuse and Alcoholism as heavy, episodic drinking in which enough alcohol is consumed in one sitting to bring the blood alcohol concentration (BAC) >0.08 g/100 g (55). During adolescence, significant neural remodeling occurs as evidenced by changes in cortical gray matter (22, 29, 37), neurogenesis (40), and increased synaptic connectivity (14, 49, 57), raising the possibility that alcohol consumption during this critical period can lead to long-term neurobiological and behavioral defects.
One neurological system that undergoes extensive plasticity during pubertal development is the hypothalamo-pituitary-adrenal (HPA) axis (46). Under normal physiological conditions, an acute psychological or physical stressor activates the HPA axis. Hypothalamic corticotrophin-releasing hormone (CRH) stimulates adrenocorticotropic hormone (ACTH) release from the anterior pituitary, which, in turn, causes the release of adrenal glucocorticoids. This sequence of events sets up a negative feedback system whereby increased circulating glucocorticoid levels serve to inhibit the additional release of hypothalamic CRH. Importantly, alcohol consumption can alter the expression of genes involved in mediating the stress response of the HPA axis. For instance, Rivier and Lee (44) demonstrated that acute ethanol (EtOH) administration to adult rats increased the transcriptional activity of hypothalamic CRH and arginine vasopressin (AVP) neurons, as measured by increased CRH and AVP heteronuclear (hn) RNA following a single dose of EtOH. In addition, the release of ACTH from the pituitary gland in response to EtOH was blocked when CRH and AVP antagonists were used concomitantly (44) . These results show that acute EtOH exposure activates the HPA axis and the effects of EtOH are likely mediated by both CRH and AVP.
AVP is a multifunctional neuropeptide critical for mediating both stress and anxiety responses (17, 56). First discovered as antidiuretic hormone, AVP is synthesized in the paraventricular nucleus (PVN), supraoptic nucleus (SON), bed nucleus of stria terminalis (BST), amygdala (AMY), and suprachiasmatic nucleus (SCN). Neurons that synthesize AVP in the parvocellular division of the PVN project to the median eminence and facilitate the synergistic actions of AVP and CRH following stressful stimuli by enhancing ACTH and glucocorticoid release (15). Several lines of evidence suggest that acute or chronic alcohol exposure might lead to increased displays of anxiety behavior. Correlative studies have demonstrated that over 50% of patients with alcohol dependency also have anxiety-related or depression-related psychiatric disorders (13), and these types of disorders are often associated with an abnormal stress response. Interestingly, women alcoholics have a higher incidence of clinically diagnosed anxiety disorders compared with men alcoholics (19, 59), possibly because of the inherent sex differences in the stress responses of the HPA axis (3, 25). Furthermore, repeated episodes of acute EtOH exposure increased anxiety behaviors in peripubertal animals, as measured by increased latency to explore a novel object in the retention-based passive avoidance task (38). Direct effects of EtOH on AVP gene expression in the hypothalamus is one possible mechanism by which EtOH could alter anxiety behaviors, and, in fact, acute EtOH administration has been shown to increase the amount of AVP hnRNA in adult rats (44) . Taken together, these studies show that AVP is an important modulator of normal HPA axis function that can be disrupted by alcohol exposure.
Our current understanding of how EtOH exposure, especially a binge pattern of EtOH exposure, affects the brain during pubertal development is very limited. In this study, we tested the hypothesis that a binge pattern of EtOH exposure during pubertal development stimulates the stress response by increasing circulating levels of corticosterone (CORT) and upregulating CRH and AVP mRNA levels in the PVN of the hypothalamus in male and female rats. Our results showed a striking sex difference in the response to binge EtOH exposure for most parameters measured. Overall, the results from our study confirm that a binge pattern of EtOH exposure during puberty significantly alters the expression of genes important for regulating the HPA axis and might lead to a higher risk of developing anxiety disorders in adulthood.
MATERIALS AND METHODS
Animals.
Male and female Wistar rats were purchased from Charles River Laboratories (Wilmington, MA) at weaning [postnatal day (PND) 23] and allowed to acclimate to the new environment for 7 days after arrival. This study used the well-established model for periadolescence in rodents (28). All treatments began on PND 37 and continued until PND 44, which is defined as peripubertal (28). Animals were housed on a 12:12-h light-dark cycle with lights on at 0700. Food and water were available ad libitum, and all procedures were approved by the Loyola University Medical Center Institutional Animal Care and Use Committee.
Binge exposure paradigm and treatment design.
After 1 wk of daily handling, the animals were randomly assigned to either 1) untreated control (n = 16), 2) saline-treated control (n = 16), 3) acute EtOH (n = 16, 3 g · kg−1 · day−1), or 4) binge EtOH (n = 16, 3 g · kg−1 · day−1) groups. The animals in the binge-pattern EtOH exposure received an intraperitoneal EtOH injection every morning for three consecutive days, followed by 2 days of saline injections, and then injected for an additional 3 days with EtOH. This binge-pattern exposure paradigm has been used previously to mimic the pattern of binge alcohol consumption in adolescents (27), and it has been shown that this intraperitoneal route of alcohol administration in juvenile animals does not yield statistically different BAC compared with oral gavage (54). Importantly, the animals were given alcohol in the morning. It has been previously shown that morning alcohol administration does not interfere with normal feeding behavior and does not result in body weight differences between alcohol-treated and control animals, thus eliminating the need for pair-fed controls (7, 8). All other groups were treated as follows: untreated = daily handling only; saline = one intraperitoneal saline injection each day for 8 consecutive days; acute EtOH = one intraperitoneal saline injection each day for 7 consecutive days, followed by one intraperitoneal EtOH (3 g/kg) injection on day 8. On the last day of treatment (day 8), animals were killed by rapid decapitation 60 min after the final injection.
BAC measurements.
Trunk blood samples were collected in heparinized tubes and centrifuged at 3,000 rpm for 10 min at 4°C, and plasma was stored at −20°C. Blood alcohol levels were determined by measuring the change in absorbance at 340 nm following enzymatic oxidation of EtOH to acetylaldehyde (Point Scientific Alcohol Reagent Kit). The assay range is 0–400 mg/dl with inter- and intra-assay coefficients of variation (CV) of 1.78 and 3.34%, respectively.
Hormone measurements.
Plasma levels of testosterone and 17β-estradiol were measured using commercially available EIA kits (Cayman Chemical, Ann Arbor, MI) according to manufacturer's instructions. CORT was measured using radioimmunoassay (RIA). Briefly, blood samples were collected in heparinized tubes and centrifuged at 3,000 rpm for 10 min at 4°C, and plasma was stored at −20°C. For the testosterone EIA kit, the range of detection was between 3.9 and 500 pg/ml, and for the estradiol kit the range of detection was 8.6–4,000 pg/ml. Intra-assay CVs were 2.2 and 6.1% for testosterone and estradiol, respectively. To measure CORT, [3H]CORT (PerkinElmer, Waltham, MA) and rabbit CORT antiserum (MP Biomedicals, Solon, OH) were used to perform RIA. On the first day of the procedure, a standard curve was prepared using CORT-standards (4-pregnen, 11b, 21-diol-3,20-dione; Steraloids). Appropriate sample tubes were filled with 100 μl of a sample, 100 μl of antibody solution (in 0.1% gel PBS), and 100 μl diluted radioactive tracer (10–12,000 cpm). Tubes were incubated overnight at 4°C. On the 2nd day, all tubes were filled with 100 μl of 0.5% gel PBS, 1 ml dextran-coated charcoal, and 1 ml 0.01 M PBS. Samples were centrifuged at 3,000 rpm at 4°C for 15 min, and the supernatant was collected in scintillation vials. Radioactivity was measured in each vial for 1 min using a Packard liquid scintillation counter. Levels of CORT in unknown samples were interpolated based on the standard curve. The assay range is 0–500 ng/ml with inter- and intra-assay CV of 2.98 and 5.96%, respectively.
Tissue collection and quantitative RT-PCR.
After death, brains were rapidly collected and frozen using isopentane and stored at −80°C until further processing. Frozen brains were sectioned at 200 μm on a freezing microtome, and the PVN and SON were microdissected using a 0.75-mm Palkovit‘s brain punch tool (Stoelting, Wood Dale, IL) Total RNA isolation was performed on sonicated tissue samples using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s directions. Following RNA isolation, samples were treated with DNA-Free (Applied Biosystems/Ambion, Austin, TX) according to manufacturer's instructions to eliminate genomic DNA contamination. Subsequently, 0.4 μg total RNA was reverse transcribed using the First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen). Total RNA samples subjected to first-strand cDNA synthesis reaction minus reverse transcriptase enzyme were used as controls for transcript dependency of the PCR reaction. Roche FastStart SYBR Green Master Mix was added to AVP-specific upper and lower AVP primer (0.25 μM final concentration; 5-CGCAGTGCCCACCTATGCTC; 3-AGGAAGCAGCCCAGCTCGTC), CRH primer (0.25 μM final concentration; 5-CTGGGGAACCTCAACAGAAG; 3-GGTGGAAGGTGAGATCCAGA). Next, 2 μl cDNA templates were added to triplicate reactions performed in a 96-well plate. Thermal reaction conditions were 95°C for 10 min and then a repeated cycle of 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s with SYBR green fluorescence detection at the end of each 72°C step. Samples were then subjected to thermal melting curve analysis to verify that the melting temperature (Tm) of the product is consistent with the calculated Tm based on a sequence with continuous fluorescence detection to 95°C. Quantification of the target gene expression was achieved by extrapolating from standard curve of known concentrations ran simultaneously in the same plate. Before extrapolation of absolute numbers using the standard curve, all samples were normalized to the hypoxanthine guanine phosphoribosyl transferase 1 (HPRT) housekeeping gene. The HPRT gene was used as an internal control in these studies because of its constitutive expression in all cells and its relatively low expression levels. The low levels of expression make it an ideal internal control for highly sensitive quantification of genes that might be expressed at low levels within the cell. In addition, our preliminary survey of several different housekeeping genes showed that HPRT was unaffected by EtOH treatments both in vivo and in cell culture (unpublished observation). Amplification efficiency of all target and reference genes were equivalent as determined by measuring the change (Δ) in the cycle threshold (CT) (CT target − CT ref) over a range of dilutions and calculating the absolute value of the slope (log cDNA dilution vs. ΔCT). Total copies were determined by generating an HPRT standard curve within each PCR reaction, using 10-fold serial dilutions ranging from 1 × 10 to 1 × 107 copies of pcDNA3.1 vector plasmids containing rat HPRT gene (plasmid constructs generously provided by Dr. Phong Le, Loyola University Chicago). Samples without cDNA acted as negative controls. Expression of CRH and AVP was compared by determining the ratios of copies per microliter of the transcript with copies per microliter of HPRT in individual samples.
Statistical analysis.
One-way ANOVA was used to test for differences between treatment groups within sexes followed by Dunett‘s t-test if one-way achieved significance. Untreated or saline groups were each chosen as controls in subsequent Dunett’s t-tests. Between-sex variations were determined using two-way ANOVA followed by Tukey's post hoc analyses. One-way ANOVA with repeated measures was used to analyze body weight data throughout the course of treatments. A P value <0.05 was considered to be significant.
RESULTS
EtOH exposure did not alter final body weight or growth pattern during puberty.
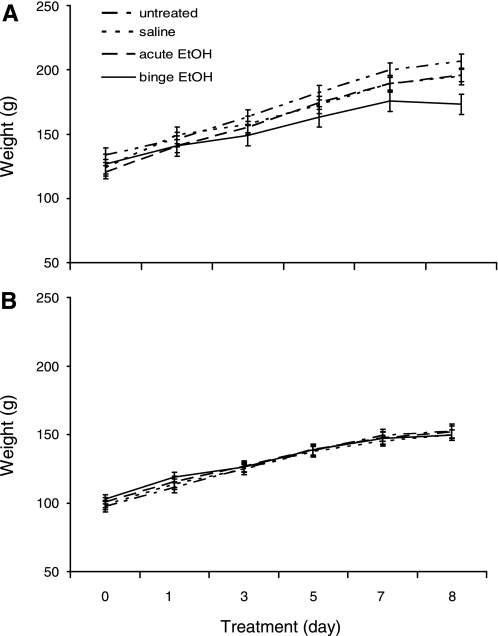
Body weight was measured every other day during the duration of treatments to determine whether EtOH altered normal growth patterns during pubertal development. There was no significant difference in body weight among groups for males or females at any point during the 8-day treatment regiment (Fig. 1).
Fig. 1.
Effects of ethanol (EtOH) treatment on body weight during pubertal development. Mean body weights of male (A) and female (B) animals untreated or treated with daily ip inejections of saline, saline + 1 day EtOH (acute), or binge EtOH paradigm.
BAC following acute and binge EtOH exposure.
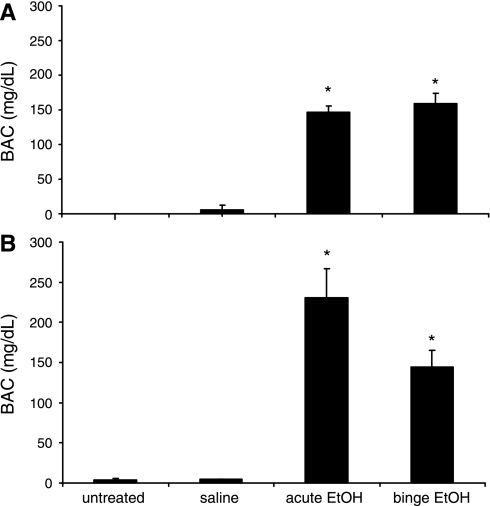
BAC were measured on the final day of treatment (day 8) 60 min following injections. There were no statistically significant differences in BAC levels between acute and binge-treated animals in either sex (Fig. 2), and, as expected, untreated and saline-treated groups were below the limit of detection for the assay. These values are consistent with the defined BAC threshold of binge drinking. In females, there was a strong trend toward lower BAC for the binge-pattern EtOH exposure groups compared with acute, suggesting that the metabolism of EtOH was altered with repeated dosing (Fig. 2B). However, since this result was not statistically significant, more studies would need to be performed to verify that this occurred.
Fig. 2.
Effects of EtOH treatments on blood alcohol and corticosterone levels in male and female rats. A: blood alcohol concentrations (BAC) 1.0 h postinjection in males (A) and females (B) treated with saline, acute EtOH, or binge EtOH. Data expressed as mean alcohol mg/dl. *Statistically significant difference (P < 0.05).
Effects of binge alcohol exposure on gonadal steroid hormone levels.
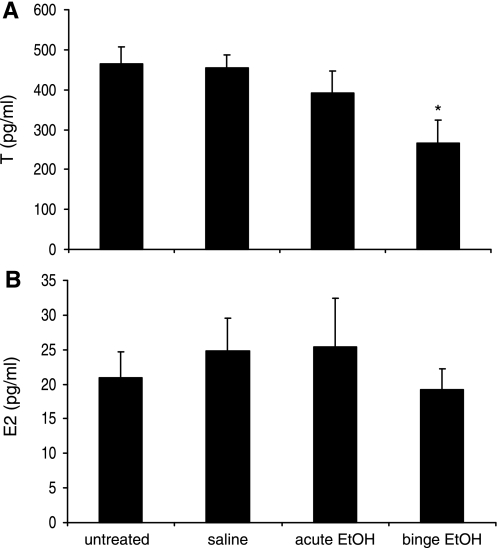
To determine the effects of binge EtOH exposure during puberty on circulating gonadal steroid hormone levels, testosterone and 17β-estradiol were measured on the last day of treatment. Binge, but not acute, EtOH treatment significantly decreased plasma testosterone levels in males compared with untreated and saline groups (P < 0.001; Fig. 3A). Testosterone levels in the untreated, saline, and acute groups were consistent with normal levels for peripubertal rats (11). On the other hand, EtOH had no effect on 17β-estradiol levels in females (Fig. 3B). Average 17β-estradiol levels were similar among females in all groups, indicating that they were in similar stages of sexual maturity (24.76 ± 4.84, 25.34 ± 7.11, and 19.25 ± 2.95 pg/ml in saline, acute EtOH, and binge EtOH groups, respectively). Pubertal onset was also verified in female rats by monitoring vaginal introitus. The first day of vaginal introitus was 33.84 ± 0.17 days of age for all groups, verifying that all female animals had achieved pubertal onset at least 4 days before the first day of EtOH administration.
Fig. 3.
Effects of EtOH treatment on gonadal steroid hormone levels. Plasma concentrations of testosterone (T) in male rats (A) and 17β-estradiol (E2) in female rats (B) 1.0 h after ip injection of saline, acute EtOH, or binge EtOH treatments. Untreated animals received no injection. Data expressed as T or E2 pg/ml. *Statistically significant difference (P < 0.05).
Effects of acute and binge EtOH exposure on circulating CORT levels.
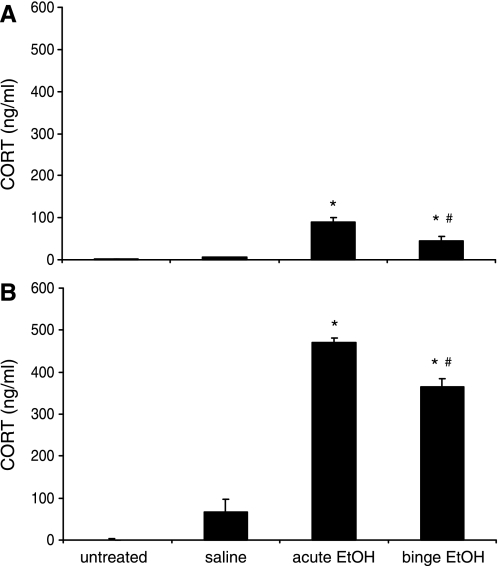
To determine whether EtOH treatment activated the stress response, circulating CORT levels were measured using RIA. As expected from previous studies, the overall stress-induced CORT levels were significantly higher in females compared with males (P < 0.001; Fig. 4). Moreover, in both males and females, EtOH exposure significantly increased plasma CORT levels in both acute and binge-pattern EtOH treatment groups compared with saline and untreated animals (Fig. 4). Interestingly, CORT levels were lower in the animals that were administered the binge-pattern EtOH paradigm compared with those that received a single does of EtOH (acute, P < 0.05). This habituation effect was similar in both males and females (Fig. 4, A and B).
Fig. 4.
Effects of EtOH treatment on circulating corticosterone (CORT) levels. Plasma CORT levels in peripubertal male (A) and female (B) rats 1.0 h after ip injection of saline, acute, or binge EtOH treatments. Untreated animals received no injection. Data expressed as mean CORT pg/ml of blood. Different symbols indicate statistically significant difference among groups (P < 0.05)
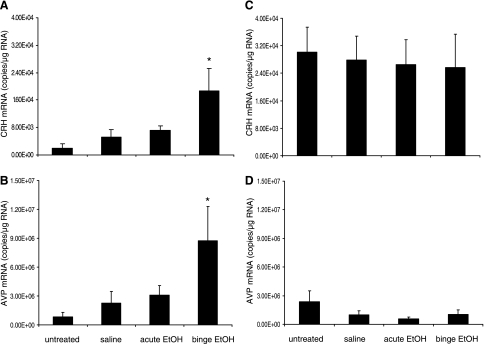
Sex-dependent effects of binge alcohol exposure on CRH and AVP gene expression in the PVN.
To investigate the effects of EtOH exposure during puberty on the expression of genes involved in stress and anxiety responses in males and females, CRH and AVP mRNA expression was measured using real-time quantitative RT-PCR. Overall, females had significantly higher basal levels (untreated groups) of CRH mRNA than males (P < 0.001; Fig. 5). However, binge, but not acute, EtOH exposure significantly increased the expression of CRH (P < 0.05; Fig. 5A) and AVP mRNA (P < 0.05; Fig. 5B) in the PVN of male animals only. There were no differences between the expression of CRH and AVP mRNA in the acute EtOH exposure group compared with the saline-treated group, indicating that the effect observed in the binge EtOH group is the result of repeated EtOH exposure. By stark contrast, in females there were no significant differences among treatment groups in the expression of CRH and AVP mRNA in the PVN (Fig. 5, C and D).
Fig. 5.
Effects of EtOH treatments on corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) mRNA in the paraventricular nucleus (PVN). CRH and AVP mRNA expression in the PVN of peripubertal male (A and B) and female (C and D) rats treated with saline, acute, or binge EtOH. Data expressed as mRNA copies/μg total RNA. *Statistically significant difference (P < 0.05).
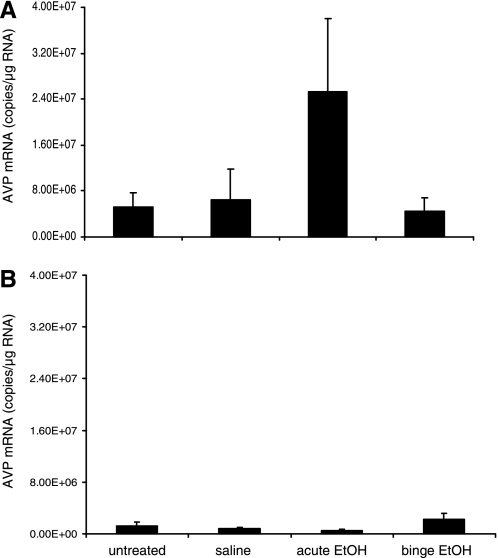
EtOH exposure does not change the expression of AVP in the SON.
To confirm that the observed changes in the expression of AVP mRNA in PVN of male rats were not exclusively the result of the reported diuretic effect of EtOH, we measured the expression of the AVP mRNA in the SON, a region of the brain involved in regulating fluid homeostasis. There were no differences between treatment groups in the expression of AVP mRNA in the SON in males (Fig. 6A) or in females (Fig. 6B), suggesting that the change in the AVP mRNA observed in the PVN reflected the change in the population of AVP-expressing cells responsible for mediating stress and anxiety. Interestingly, baseline levels of AVP mRNA were significantly lower in females compared with males in the SON (P < 0.001; Fig. 6B).
Fig. 6.
Effects of EtOH treatments on AVP gene expression in the supraoptic nucleus (SON) of male and female rats. AVP mRNA expression in SON of peripubertal male (A) and female (B) rats treated with saline, acute, or binge EtOH. Data expressed as AVP mRNA copies/μg total RNA.
DISCUSSION
Our current understanding of how alcohol consumption affects the developing postnatal brain is severely limited. To date, specific molecular and neuroendocrine markers that are activated by alcohol during puberty have not been identified. Elucidating the neurobiological targets of alcohol resulting from a binge pattern of consumption during adolescence is critical for understanding the long-term behavioral consequences and potential development of mental health disorders. Therefore, the goal of this study was to investigate the effects of binge-pattern EtOH exposure during puberty on the expression of specific neuroendocrine factors that mediate stress and anxiety, including CRH and AVP. Our data reveal the novel findings that peripubertal binge-pattern, but not acute, EtOH exposure increased the expression of CRH and AVP mRNA in the PVN of peripubertal male rats, yet had no effect in peripubertal females. Data presented here indicate that the effects of alcohol on the HPA axis are sex-specific and dependent on repeated high-dose exposures. Moreover, CORT levels were increased significantly in response to both acute and binge EtOH exposure paradigms, suggesting that EtOH is a potent activator of the HPA axis during pubertal development. Notably, there was no sex difference in the CORT response to EtOH, and, under binge-pattern exposure conditions, the animals appeared to habituate to the effects of the stressor. Last, we have shown that binge-pattern EtOH exposure decreased sex steroid hormone levels in male, but not female, rats and that the observed effects of EtOH on AVP gene expression were unlikely because of the diuretic effects of EtOH but, instead, specific for the population of AVP-expressing neurons that regulate stress and anxiety.
Acute and binge-pattern EtOH exposure increased plasma CORT levels in our study, confirming previous reports that EtOH is a potent activator of the stress response in adults. Ogilvie and Rivier (35) showed that acute EtOH administration increased CORT levels, which peaked at 15 min post-EtOH injection and were sustained until 1.0 h after the injection. However, we showed that plasma CORT levels in the binge EtOH groups were significantly lower compared with the acute EtOH exposure groups, suggesting that the HPA axis habituated to the stressful stimuli of multiple doses of EtOH. Also, females had higher plasma CORT levels after EtOH exposure compared with males, indicating that females were more sensitive to EtOH administration than males. These data are in agreement with multiple studies demonstrating that there are gender differences in responsiveness to alcohol, with females tending to achieve higher CORT levels compared with males given the same dose of EtOH (35, 42).
Acute alcohol consumption, chronic alcohol consumption, and withdrawal from alcohol dependency have all been associated with dysregulation of the HPA axis in adults (5, 26, 30, 32, 34, 43–45, 52). For instance, studies in adult rats have shown that acute alcohol administration increased circulating levels of ACTH (43). This effect was dependent on the synergistic action of CRH and AVP, since pharmacological antagonism of the CRH and AVP receptors abolished the response (34, 45). Similarly, chronic alcohol treatment resulted in a 33% decrease in the number of AVP-expressing neurons in the SCN (30) and altered the diurnal rhythm in circulating glucocorticoids (52). AVP neurons in the SCN convey circadian information to the PVN and other extrahypothalamic brain nuclei. Moreover, the circadian expression of AVP in the SCN modulates the diurnal rhythm of circulating glucocorticoids (26). Our data showing that EtOH administration alters CRH and AVP mRNA in peripubertal male rats are consistent with the observations in adult animals. Notably, in our study, a single dose of acute EtOH failed to increase CRH and AVP mRNA in the PVN despite inducing a dramatic increase in circulating CORT levels. These data suggest that the EtOH-induced upregulation of these genes occurs as a result of repeated EtOH exposure, as would be achieved with a binge pattern of alcohol consumption. It remains to be determined whether there are long-lasting behavioral or physiological deficiencies associated with these changes in gene expression. Nevertheless, there is a growing body of evidence to suggest that neurite remodeling, neurogenesis, and synaptic connectivity are prominent developmental events during puberty (see Ref. 49). Accordingly, it is logical to assume that repeated and excessive alcohol use during this critical stage of development could lead to permanent alterations in the morphological and/or neurochemical circuitry that might be organized during this time. These changes could, in turn, have negative consequences on normal adult behavior patterns, since brain morphology and neurochemistry are intimately linked with behavior.
The neuropeptide AVP is a compelling candidate for mediating the underlying neuroendocrine basis of the sexually dimorphic effects of alcohol. First, central release of AVP from the PVN, BST, and AMY mediates a broad range of social behaviors and participates in modulation of the stress response. In both males and females, AVP augments the stress response by acting synergistically with CRH, which can be altered by a variety of factors, including gonadal steroid hormones and alcohol (1, 4, 18, 20, 24, 31, 41). Moreover, AVP and the AVP receptors are differentially regulated by gonadal steroid hormones in a sex- and age-dependent manner (2, 36, 39, 50, 58). For example, AVP expression in the BST is greater in males than females (33), and this sex difference is abolished by castration and restored with testosterone replacement, an effect that is accomplished mainly by testosterone-derived 17β-estradiol (23). To date, little is known about the maturation of the central AVP system, including the temporal, sex-specific, and hormonal influences that occur during pubertal development. Previously, we showed that AVP mRNA expression in the BST and AMY was differentially regulated in pre- and postpubertal animals (36). Here, we have demonstrated prominent sex-dependent effects of binge-pattern EtOH administration on AVP mRNA levels during the pubertal transition. Our study showed that, in males, there was a significant increase in AVP mRNA following binge-pattern, but not acute, EtOH administration. Conversely, there were no effects on AVP in females using the same binge-pattern paradigm. It is important to note that, although the binge-pattern EtOH treatments significantly reduced testosterone levels, there was no effect on estradiol levels, suggesting that the EtOH effects on gonadal steroid hormones alone are not sufficient to explain these sex differences.
Developmental changes in HPA axis reactivity have been well documented. Most striking is the observation that CORT and ACTH levels take much longer to return to baseline in juvenile compared with adult animals subjected to a variety of stressful stimuli (46, 53). It is well known that males and females achieve sexual maturity at different rates with respect to reproductive function, but whether there are sex differences in the rate of HPA axis maturation during puberty is unknown. The majority of studies investigating the maturation of the HPA axis during pubertal development have focused strictly on comparisons between the pre- and postpubertal states, but not at time points in between (46). However, one study in human adolescents showed that there were sex differences in the cortisol response to a CRH challenge (51). In general, girls achieved peak cortisol levels later and had a slower return to baseline following a CRH challenge compared with boys (51), a difference that persisted throughout all Tanner stages of development. These data suggest that sex differences in HPA axis reactivity might not necessarily be because of a differential timing in HPA axis maturation between males and females but rather because of inherent differences that are perhaps organized before pubertal onset. Recent evidence from Evuarherhe and colleagues (21) support this possibility. In their study, 17β-estradiol inhibited the CORT-induced response to restraint stress in prepubertal female rats but enhanced the response postpubertal. Importantly, the adult response to restraint stress in the presence of 17β-estradiol was still enhanced regardless of whether the animals were ovariectomized before, or following, puberty. These data indicate that the maturation of the HPA axis in females might be programmed before pubertal onset and that it is independent of circulating gonadal steroid hormones during pubertal development. At this time, there are no empirical data to suggest that the HPA axis matures at different rates in males compared with females, and, in fact, the evidence available from humans would predict that they likely mature at similar rates. However, we cannot rule out the possibility that the differences we observed might be because of discrepancies in the relative state of HPA axis maturation between males and females. Nevertheless, our data demonstrate a clear distinction between the effects of repeated EtOH exposure during the pubertal transition that might lend some insight into the time course for the development of the HPA axis between males and females. Furthermore, our data have demonstrated that repeated EtOH exposure during the pubertal transition results in a dysregulation of the HPA axis akin to that of a chronic stressor in adults.
In this study, both males and females began receiving injection treatments on day 37, which were completed on day 44, falling within the defined peripubertal period for both males and females (30–45 days of age; see Ref. 28). In general, female rats undergo pubertal onset (as measured by vaginal introitus) at 32–35 days of age, with regular estrous cyclicity and mature ova first occurring between 43 and 47 days of age (48). Unlike females, in which puberty is defined as the 1st day of vaginal introitus, males do not have a reliable external benchmark that defines pubertal onset, since the commonly used external marker of preputial separation is dependent upon increased testosterone levels. The binge pattern alcohol exposure paradigm employed in our experiments did not inhibit feeding behavior or normal growth patterns that occur during pubertal development. Moreover, it allowed us to distinguish the pharmacological effects of EtOH from the effects of other nonspecific stressors, such as handling and injections. This binge EtOH exposure paradigm has been previously shown to be reliable for testing the effects of alcohol using an exposure pattern that is typical for adolescents (27). In addition, it has been show that the intraperitoneal injection of EtOH, employed in our paradigm, does not result in significantly different BAC compared with oral gavage in adolescent Wistar rats (54).
Although it was not possible to separate the parvocellular and magnocellular divisions of the PVN in our tissue punch sample preparations, we are confident that the EtOH-induced changes in AVP expression accurately reflect changes in the AVP-expressing neurons associated with regulation of the HPA axis, and not in those responsible for osmoregulation for the following reasons. First, we showed that there were no changes in the expression of the AVP mRNA in the SON, a region where AVP is primarily responsible for regulating fluid homeostasis. Second, there was no effect of EtOH in the acute-treated group, and, since fluid homeostasis is an immediate and not cumulative physiological response, any diuretic effect of EtOH would have been observed in the acutely treated groups. Third, if the effects of EtOH on AVP expression were because of an osmoregulatory effect, we would have expected to see a significant increase in the females, as well as the males. Finally, our data are in agreement with previous studies showing that there were no changes in AVP expression in the magnocellular division of the PVN 3.0 h after an acute EtOH administration (34).
Alcohol is a potent neurotoxic agent that induces widespread cell death in the developing brain (12, 47). Exciting new data have revealed that AVP has an important neuroprotective role in the PVN. Chen and colleagues (9, 10) have shown that AVP prevented apoptosis in H32 cells, a cell line derived from the PVN, following serum deprivation. These effects were mediated by mitogen-activated protein kinase activated Bad phosphorylation and by protein kinase Cα and -β. These data raise the possibility that the physiological role of increased AVP following binge EtOH exposure in males is, in part, to provide cellular protection against repeated EtOH toxicity. In females, on the other hand, 17β-estradiol is known to be neuroprotective in addition to playing roles in modulating reproductive functions and anxiety responses (6, 16). Our study showed that there was no increase in AVP mRNA after binge EtOH exposure in females, possibly because there are different mechanisms for neuroprotection in males compared with females.
Taken together, we have identified specific neuroendocrine targets of EtOH in the adolescent hypothalamus following a binge pattern of EtOH exposure. Furthermore, we have demonstrated that the effects of EtOH administration on genes critical for the developing HPA axis are sex specific. Sexually dimorphic patterns of addictive behavior emerge during adolescence and often persist in adulthood. Patterns of alcohol use during this developmental time period can also lead to permanent changes in brain function that often manifest in adulthood as psychological disorders, such as depression and anxiety. Overall, these data have identified potential genes involved in increased anxiety responses after EtOH exposure and might lead to a better understanding of the mechanisms involved in increased prevalence of anxiety disorders in females compared with males.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grants T32AA-013527 and R21AA-018398.
REFERENCES
- 1.Albeck DS, McKittrick CR, Blanchard DC, Blanchard RJ, Nikulina J, McEwen BS, Sakai RR. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. J Neurosci 17: 4895–4903, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bale TL, Dorsa DM. Regulation of oxytocin receptor messenger ribonucleic acid in the ventromedial hypothalamus by testosterone and its metabolites. Endocrinology 136: 5135–5138, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Becker JB, Monteggia LM, Perrot-Sinal TS, Romeo RD, Taylor JR, Yehuda R, Bale TL. Stress and disease: is being female a predisposing factor? J Neurosci 27: 11851–11855, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanchard RJ, Griebel G, Farrokhi C, Markham C, Yang M, Blanchard DC. AVP V1b selective antagonist SSR149415 blocks aggressive behaviors in hamsters. Pharmacol Biochem Behav 80: 189–194, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, Neumann ID. Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur J Neurosci 27: 1947–1956, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Bodo C, Rissman EF. New roles for estrogen receptor beta in behavior and neuroendocrinology. Front Neuroendocrinol 27: 217–232, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Callaci JJ, Juknelis D, Patwardhan A, Sartori M, Frost N, Wezeman FH. The effects of binge alcohol exposure on bone resorption and biomechanical and structural properties are offset by concurrent bisphosphonate treatment. Alcohol Clin Exp Res 28: 182–191, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callaci JJ, Juknelis D, Patwardhan A, Wezeman FH. Binge alcohol treatment increases vertebral bone loss following ovariectomy: compensation by intermittent parathyroid hormone. Alcohol Clin Exp Res 30: 665–672, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Liu Y, Soh JW, Aguilera G. Antiapoptotic effects of vasopressin in the neuronal cell line H32 involve protein kinase Calpha and beta. J Neurochem 110: 1310–1320, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Volpi S, Aguilera G. Anti-apoptotic actions of vasopressin in H32 neurons involve MAP kinase transactivation and Bad phosphorylation. Exp Neurol 211: 529–538, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowen JA, Garcia-Segura LM, Gonzalez-Parra S, Argente J. Sex steroid effects on the development and functioning of the growth hormone axis. Cell Mol Neurobiol 16: 297–310, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu J, Tong M, de la Monte SM. Chronic ethanol exposure causes mitochondrial dysfunction and oxidative stress in immature central nervous system neurons. Acta Neuropathol 113: 659–673, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry 67: 247–257, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol 453: 116–130, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Dickstein G, DeBold CR, Gaitan D, DeCherney GS, Jackson RV, Sheldon WR, Jr, Nicholson WE, Orth DN. Plasma corticotropin and cortisol responses to ovine corticotropin-releasing hormone (CRH), arginine vasopressin (AVP), CRH plus AVP, and CRH plus metyrapone in patients with Cushing's disease. J Clin Endocrinol Metab 81: 2934–2941, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci USA 98: 1952–1957, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egashira N, Tanoue A, Matsuda T, Koushi E, Harada S, Takano Y, Tsujimoto G, Mishima K, Iwasaki K, Fujiwara M. Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav Brain Res 178: 123–127, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Engelmann M, Wotjak CT, Ebner K, Landgraf R. Behavioural impact of intraseptally released vasopressin and oxytocin in rats. Exp Physiol 85: 125S–130S, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Epstein EE, Fischer-Elber K, Al-Otaiba Z. Women, aging, and alcohol use disorders. J Women Aging 19: 31–48, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Erkut ZA, Pool C, Swaab DF. Glucocorticoids suppress corticotropin-releasing hormone and vasopressin expression in human hypothalamic neurons. J Clin Endocrinol Metab 83: 2066–2073, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Evuarherhe O, Leggett J, Waite E, Kershaw Y, Lightman S. Reversal of the hypothalamo-pituitary-adrenal response to oestrogens around puberty. J Endocrinol 202: 279–285, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, Molloy EA, Blumenthal JD, Tossell JW, Stayer C, Samango-Sprouse CA, Shen D, Davatzikos C, Merke D, Chrousos GP. Puberty-related influences on brain development. Mol Cell Endocrinol 254–255: 154–162, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Han TM, De Vries GJ. Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. J Neurobiol 54: 502–510, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Handa RJ, Corbier P, Shryne JE, Schoonmaker JN, Gorski RA. Differential effects of the perinatal steroid environment on three sexually dimorphic parameters of the rat brain. Biol Reprod 32: 855–864, 1985 [DOI] [PubMed] [Google Scholar]
- 25.Handa RJ, Zoeller RT, McGivern RF. Changes in vasoactive intestinal peptide and arginine vasopressin expression in the suprachiasmatic nucleus of the rat brain following footshock stress. Neurosci Lett 425: 99–104, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isobe Y, Isobe M. Circadian rhythm of Arg-vasopressin contents in the suprachiasmatic nucleus in relation to corticosterone. Brain Res 800: 78–85, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Lauing K, Himes R, Rachwalski M, Strotman P, Callaci JJ. Binge alcohol treatment of adolescent rats followed by alcohol abstinence is associated with site-specific differences in bone loss and incomplete recovery of bone mass and strength. Alcohol 42: 649–656, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laviola G, Adriani W, Morley-Fletcher S, Terranova ML. Peculiar response of adolescent mice to acute and chronic stress and to amphetamine: evidence of sex differences. Behav Brain Res 130: 117–125, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev 30: 718–729, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Madeira MD, Andrade JP, Lieberman AR, Sousa N, Almeida OF, Paula-Barbosa MM. Chronic alcohol consumption and withdrawal do not induce cell death in the suprachiasmatic nucleus, but lead to irreversible depression of peptide immunoreactivity and mRNA levels. J Neurosci 17: 1302–1319, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy MM, McDonald CH, Brooks PJ, Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol Behav 60: 1209–1215, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci 15: 5439–5447, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller MA, Vician L, Clifton DK, Dorsa DM. Sex differences in vasopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization. Peptides 10: 615–619, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Ogilvie KM, Lee S, Rivier C. Role of arginine vasopressin and corticotropin-releasing factor in mediating alcohol-induced adrenocorticotropin and vasopressin secretion in male rats bearing lesions of the paraventricular nuclei. Brain Res 744: 83–95, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Ogilvie KM, Rivier C. Gender difference in hypothalamic-pituitary-adrenal axis response to alcohol in the rat: activational role of gonadal steroids. Brain Res 766: 19–28, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Pak TR, Chung WC, Hinds LR, Handa RJ. Arginine vasopressin regulation in pre- and postpubertal male rats by the androgen metabolite 3β-diol. Am J Physiol Endocrinol Metab 296: E1409–E1413, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science 283: 1908–1911, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Popovic M, Caballero-Bleda M, Puelles L, Guerri C. Multiple binge alcohol consumption during rat adolescence increases anxiety but does not impair retention in the passive avoidance task. Neurosci Lett 357: 79–82, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Quadros PS, Pfau JL, Goldstein AY, De Vries GJ, Wagner CK. Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology 143: 3727–3739, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Raymond AD, Kucherepa NN, Fisher KR, Halina WG, Partlow GD. Neurogenesis of oxytocin-containing neurons in the paraventricular nucleus (PVN) of the female pig in 3 reproductive states: puberty gilts, adult gilts and lactating sows. Brain Res 1102: 44–51, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 185: 218–225, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Rivier C. Female rats release more corticosterone than males in response to alcohol: influence of circulating sex steroids and possible consequences for blood alcohol levels. Alcohol Clin Exp Res 17: 854–859, 1993 [DOI] [PubMed] [Google Scholar]
- 43.Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF). J Pharmacol Exp Ther 229: 127–131, 1984 [PubMed] [Google Scholar]
- 44.Rivier C, Lee S. Acute alcohol administration stimulates the activity of hypothalamic neurons that express corticotropin-releasing factor and vasopressin. Brain Res 726: 1–10, 1996 [PubMed] [Google Scholar]
- 45.Rivier C, Rivier J, Mormede P, Vale W. Studies of the nature of the interaction between vasopressin and corticotropin-releasing factor on adrenocorticotropin release in the rat. Endocrinology 115: 882–886, 1984 [DOI] [PubMed] [Google Scholar]
- 46.Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology 147: 1664–1674, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Silva SM, Madeira MD, Ruela C, Paula-Barbosa MM. Prolonged alcohol intake leads to irreversible loss of vasopressin and oxytocin neurons in the paraventricular nucleus of the hypothalamus. Brain Res 925: 76–88, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Sisk CL, Richardson HN, Chappell PE, Levine JE. In vivo gonadotropin-releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology 142: 2929–2936, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol 26: 163–174, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Sladek CD, Swenson KL, Kapoor R, Sidorowicz HE. The role of steroid hormones in the regulation of vasopressin and oxytocin release and mRNA expression in hypothalamo-neurohypophysial explants from the rat. Exp Physiol 85: 171S–177S, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Stroud LR, Papandonatos GD, Williamson DE, Dahl RE. Sex differences in the effects of pubertal development on responses to a corticotropin-releasing hormone challenge: the Pittsburgh psychobiologic studies. Ann NY Acad Sci 1021: 348–351, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Tabakoff B, Jafee RC, Ritzmann RF. Corticosterone concentrations in mice during ethanol drinking and withdrawal. J Pharm Pharmacol 30: 371–374, 1978 [DOI] [PubMed] [Google Scholar]
- 53.Vazquez DM, Akil H. Pituitary-adrenal response to ether vapor in the weanling animal: characterization of the inhibitory effect of glucocorticoids on adrenocorticotropin secretion. Pediatr Res 34: 646–653, 1993 [DOI] [PubMed] [Google Scholar]
- 54.Walker BM, Ehlers CL. Age-related differences in the blood alcohol levels of Wistar rats. Pharmacol Biochem Behav 91: 560–565, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White AM, Kraus CL, Swartzwelder H. Many college freshmen drink at levels far beyond the binge threshold. Alcohol Clin Exp Res 30: 1006–1010, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Wigger A, Sanchez MM, Mathys KC, Ebner K, Frank E, Liu D, Kresse A, Neumann ID, Holsboer F, Plotsky PM, Landgraf R. Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharmacology 29: 1–14, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Woo TU, Pucak ML, Kye CH, Matus CV, Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience 80: 1149–1158, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Yue C, Mutsuga N, Scordalakes EM, Gainer H. Studies of oxytocin and vasopressin gene expression in the rat hypothalamus using exon- and intron-specific probes. Am J Physiol Regul Integr Comp Physiol 290: R1233–R1241, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Zilberman ML, Tavares H, Blume SB, el-Guebaly N. Substance use disorders: sex differences and psychiatric comorbidities. Can J Psychiatry 48: 5–13, 2003. [DOI] [PubMed] [Google Scholar]